Redox-Sensitive Nanocomplex for Targeted Delivery of Melittin
Abstract
1. Introduction
2. Results and Discussion
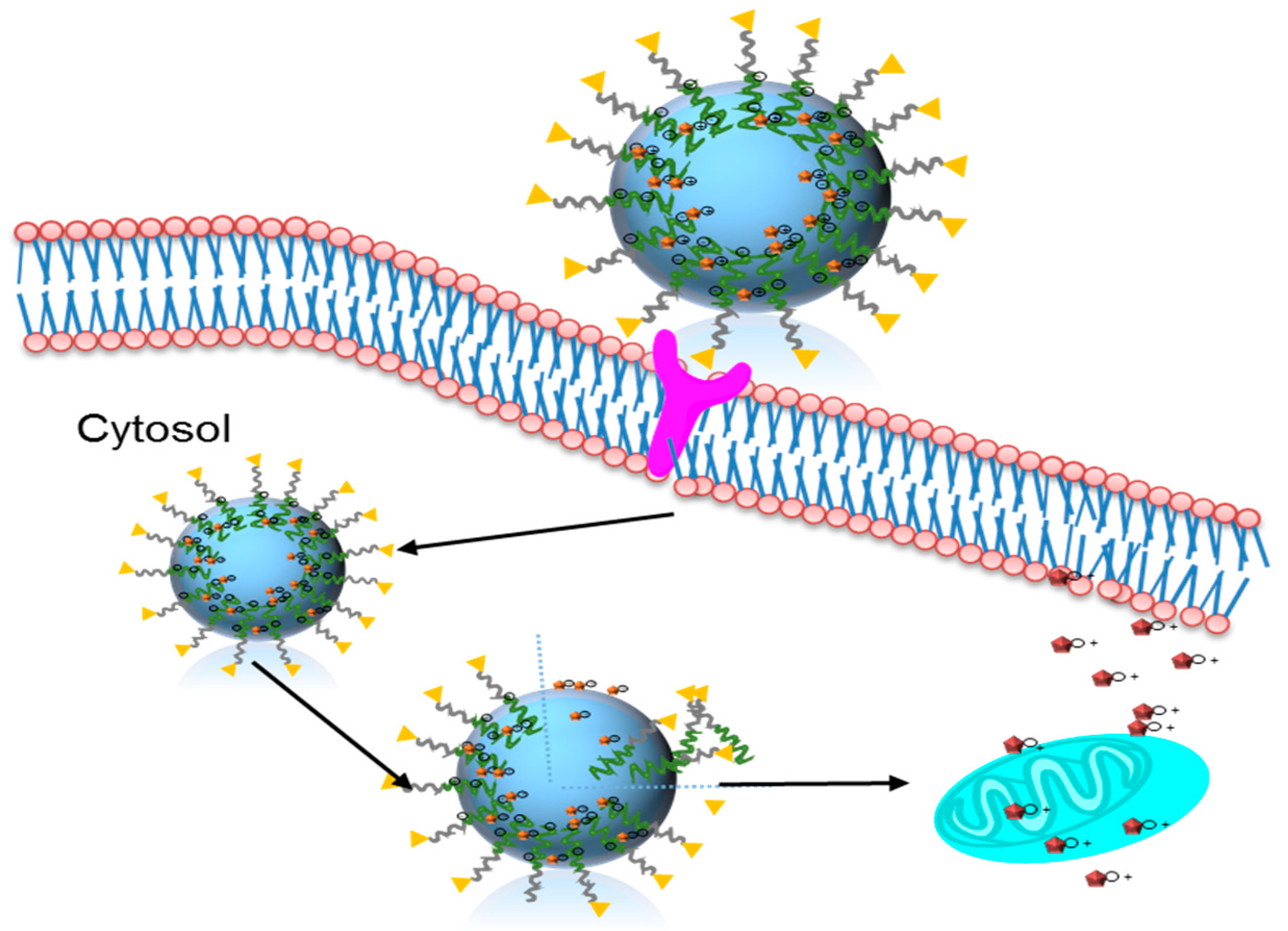
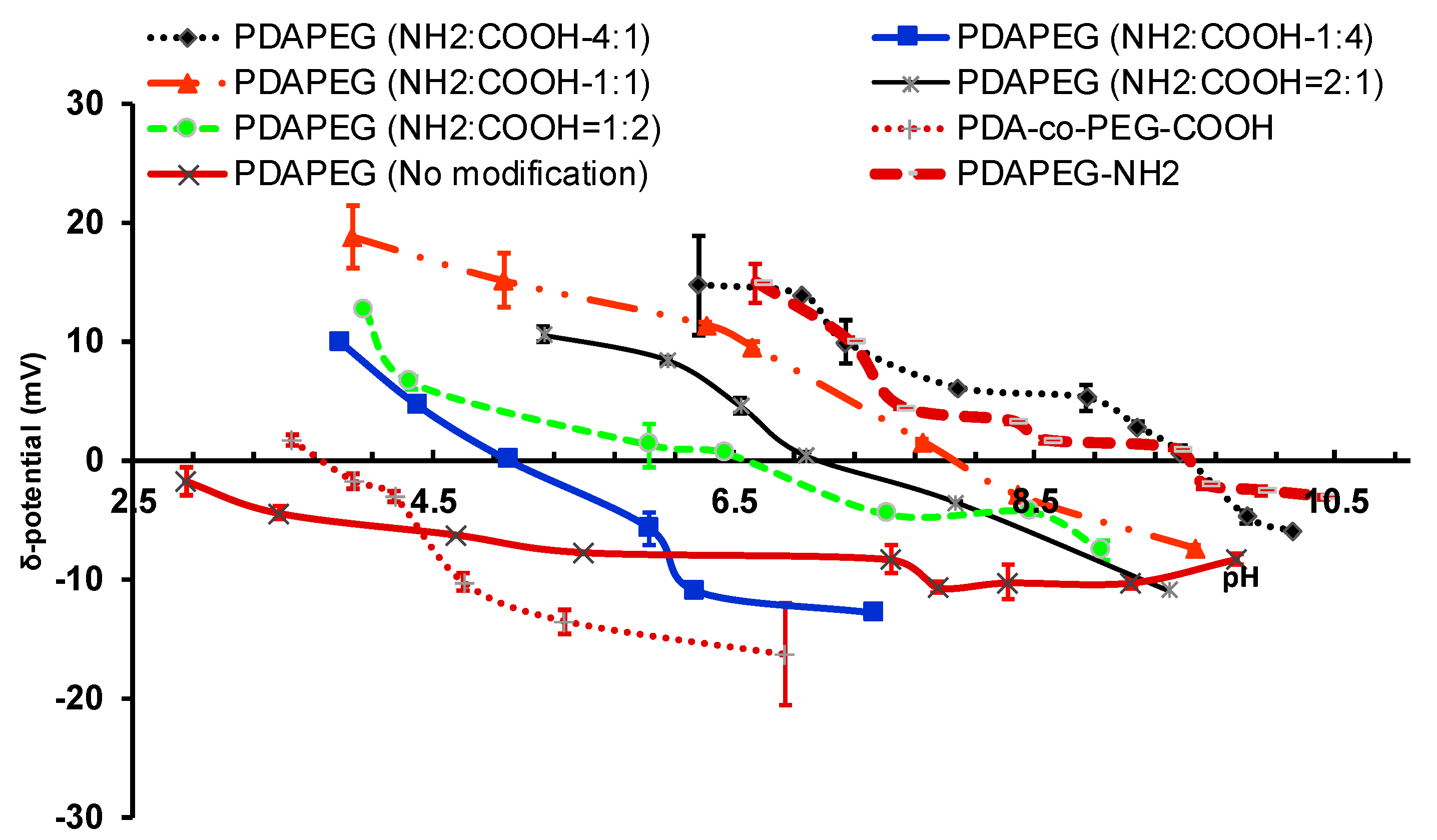
2.1. Design and Synthesis of Polymer LBA-PDAPEG
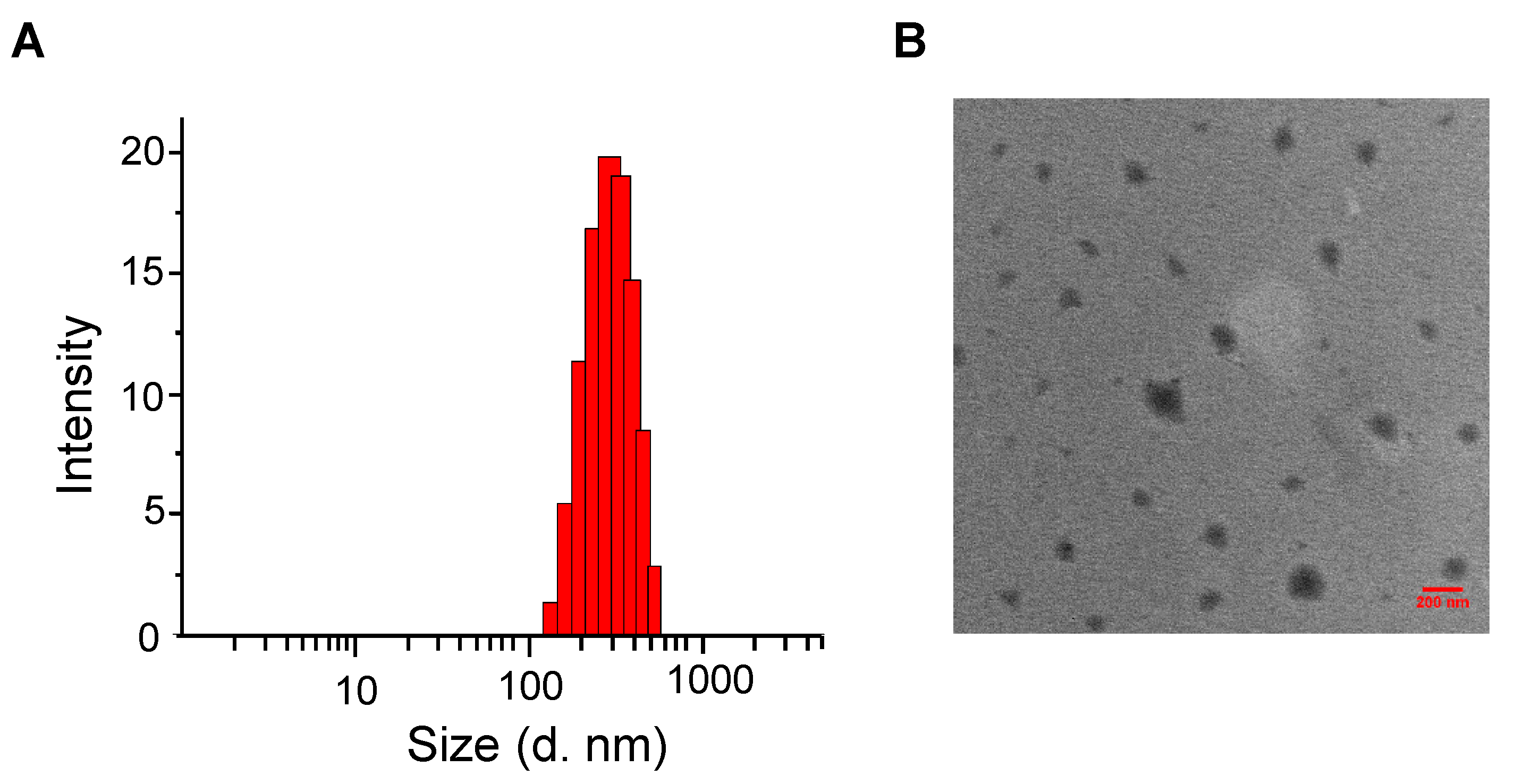
2.2. Particle Characterization
2.3. High Redox Potential Environment Could Trigger the Release of Melittin
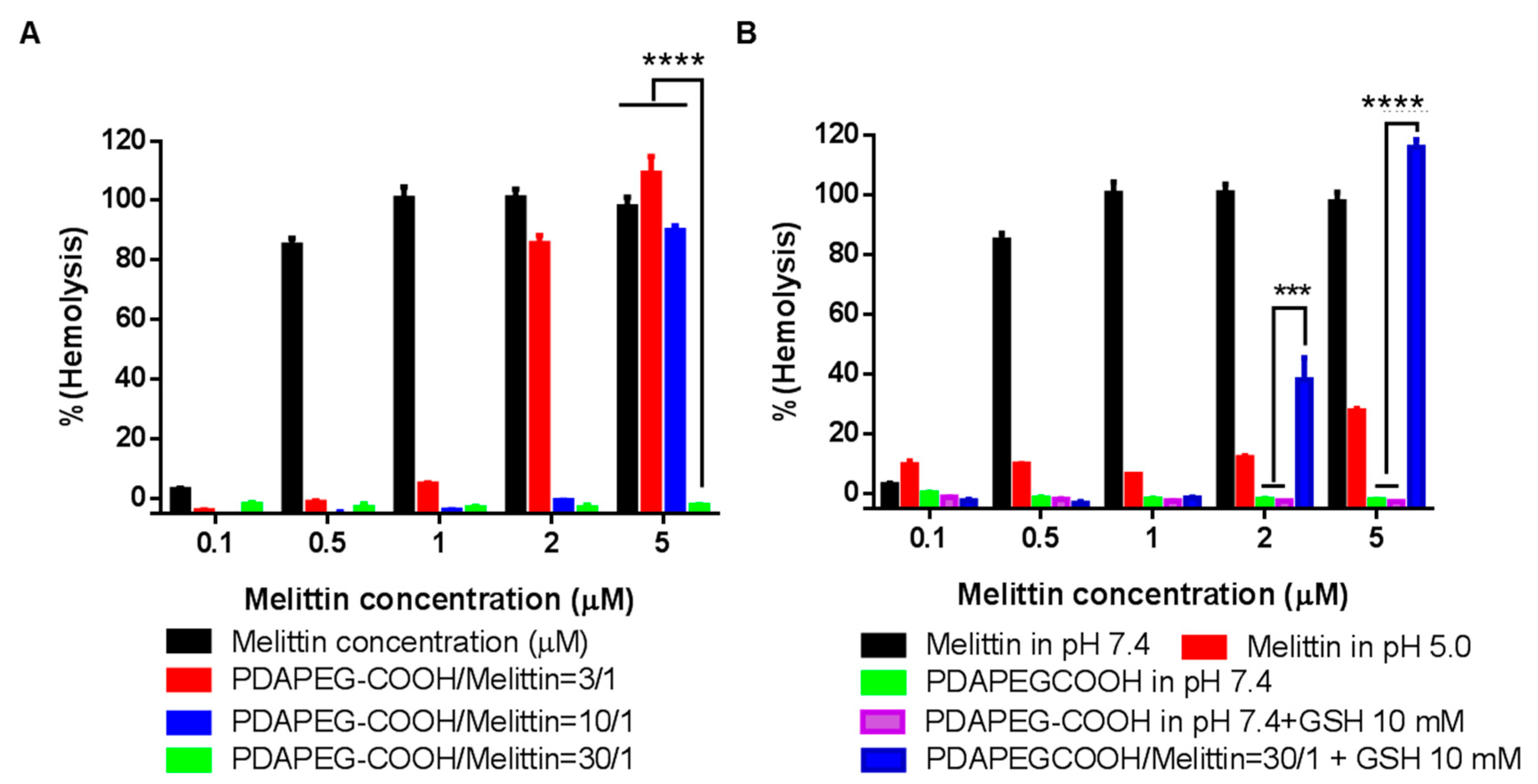
2.4. Hemolytic Assay
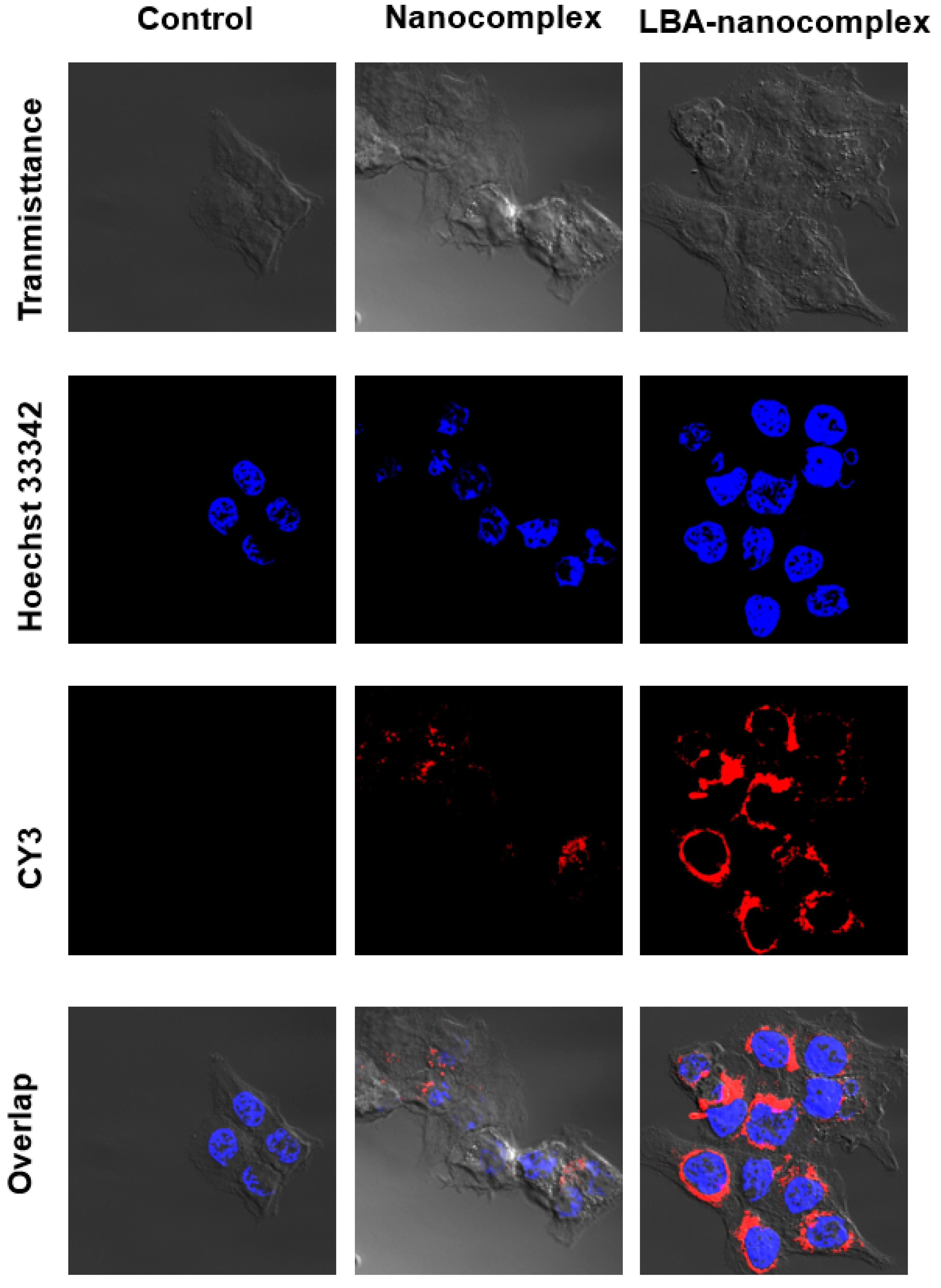
2.5. In vitro Cellular Uptake
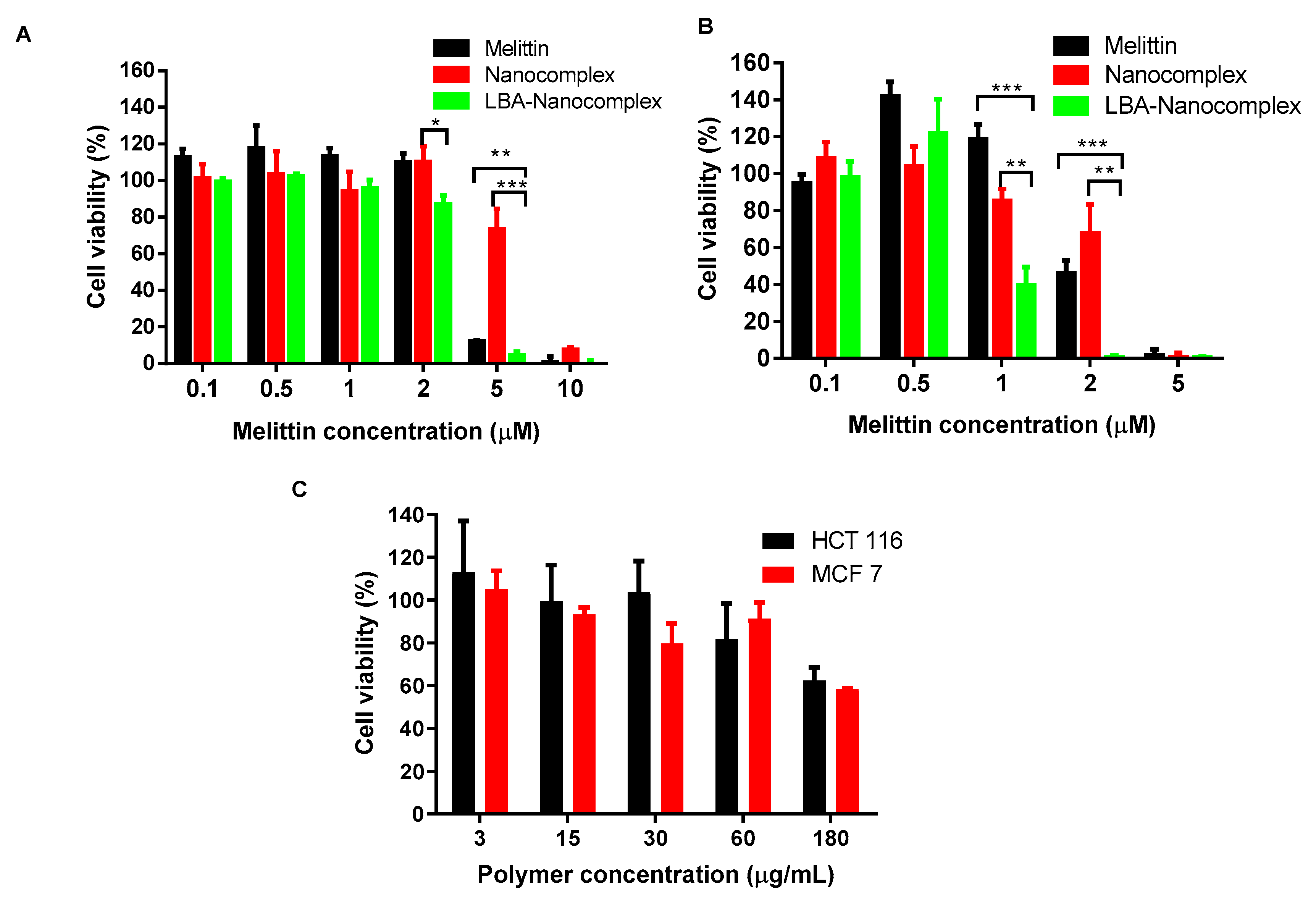
2.6. In vitro Anticancer Activities
2.7. In Vivo Experiment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Melittin Polymer
4.3. FRET Measurement
4.4. Hemolysis Experiment
4.5. Cellular Uptake
4.6. In Vitro Cytotoxicity
4.7. Tumor Growth Retardation Study
4.8. Statistics Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Midtvedt, K. Therapeutic drug monitoring of cyclosporine. Transpl. Proc. 2004, 36, 430S–433S. [Google Scholar] [CrossRef]
- Koie, T.; Mitsuzuka, K.; Yoneyama, T.; Narita, S.; Kawamura, S.; Kaiho, Y.; Tsuchiya, N.; Tochigi, T.; Habuchi, T.; Arai, Y.; et al. Neoadjuvant luteinizing-hormone-releasing hormone agonist plus low-dose estramustine phosphate improves prostate-specific antigen-free survival in high-risk prostate cancer patients: A propensity score-matched analysis. Int. J. Clin. Oncol. 2015. [Google Scholar] [CrossRef]
- Merseburger, A.S.; Bjork, T.; Whitehouse, J.; Meani, D. Treatment costs for advanced prostate cancer using LHRH agonists: A solid biodegradable leuprorelin implant versus other formulations. J. Comp. Effect. Res. 2014, 1–7. [Google Scholar] [CrossRef]
- Tsomaia, N. Peptide therapeutics: Targeting the undruggable space. Eur. J. Med. Chem. 2015, 94, 459–470. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Y.; Qi, Y.; Chen, L.; Ma, Y.; Li, Y. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014, 351, 13–22. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Anti-inflammatory applications of Melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef]
- Park, H.J.; Son, D.J.; Lee, C.W.; Choi, M.S.; Lee, U.S.; Song, H.S.; Lee, J.M.; Hong, J.T. Melittin inhibits inflammatory target gene expression and mediator generation via interaction with IkappaB kinase. Biochem. Pharmacol. 2007, 73, 237–247. [Google Scholar] [CrossRef]
- Aufschnaiter, A.; Kohler, V.; Khalifa, S.; Abd El-Wahed, A.; Du, M.; El-Seedi, H.; Buttner, S. Apitoxin and its components against cancer, neurodegeneration and rheumatoid arthritis: Limitations and possibilities. Toxins 2020, 12, 66. [Google Scholar] [CrossRef]
- He, S.D.; Tan, N.; Sun, C.X.; Liao, K.H.; Zhu, H.J.; Luo, X.G.; Zhang, J.Y.; Li, D.Y.; Huang, S.G. Treatment with melittin induces apoptosis and autophagy of fibroblastlike synoviocytes in patients with rheumatoid arthritis. Curr. Pharm. Biotechnol. 2020, 21, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Kim, J.M.; Park, K.K.; Chang, Y.C.; Pak, S.C. Neuroprotective effects of melittin on hydrogen peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. BMC Complement Altern. Med. 2014, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Lee, K.W.; Choi, S.M.; Yang, E.J. Bee venom protects against rotenone-induced cell death in NSC34 motor neuron cells. Toxins 2015, 7, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Hung, W.C.; Chen, F.Y.; Huang, H.W. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 5087–5092. [Google Scholar] [CrossRef]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef]
- Hou, K.K.; Pan, H.; Ratner, L.; Schlesinger, P.H.; Wickline, S.A. Mechanisms of nanoparticle-mediated siRNA transfection by melittin-derived peptides. ACS Nano 2013, 7, 8605–8615. [Google Scholar] [CrossRef]
- Hou, K.K.; Pan, H.; Lanza, G.M.; Wickline, S.A. Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials 2013, 34, 3110–3119. [Google Scholar] [CrossRef]
- Yu, X.; Dai, Y.; Zhao, Y.; Qi, S.; Liu, L.; Lu, L.; Luo, Q.; Zhang, Z. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat. Commun. 2020, 11, 1110. [Google Scholar] [CrossRef]
- Jallouk, A.P.; Palekar, R.U.; Marsh, J.N.; Pan, H.; Pham, C.T.; Schlesinger, P.H.; Wickline, S.A. Delivery of a protease-activated cytolytic peptide prodrug by perfluorocarbon nanoparticles. Bioconjug. Chem. 2015, 26, 1640–1650. [Google Scholar] [CrossRef]
- Hood, J.L.; Jallouk, A.P.; Campbell, N.; Ratner, L.; Wickline, S.A. Cytolytic nanoparticles attenuate HIV-1 infectivity. Antivir. Ther. 2013, 18, 95–103. [Google Scholar] [CrossRef]
- Bei, C.; Bindu, T.; Remant, K.C.; Peisheng, X. Dual secured nano-melittin for the safe and effective eradication of cancer cells. J. Mater. Chem. B 2015, 3, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Remant, B.K.; Chandrashekaran, V.; Cheng, B.; Chen, H.; Pena, M.M.; Zhang, J.; Montgomery, J.; Xu, P. Redox potential ultrasensitive nanoparticle for the targeted delivery of camptothecin to HER2-positive cancer cells. Mol. Pharm. 2014, 11, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, K.C.R.; Xu, P. Multicompartment intracellular self-expanding nanogel for targeted delivery of drug cocktail. Adv. Mater. 2012, 24, 6479–6483. [Google Scholar] [CrossRef]
- Sui, B.L.; Cheng, C.; Wang, M.M.; Hopkins, E.; Xu, P.S. Heterotargeted nanococktail with traceless linkers for eradicating cancer. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Baek, S.; Singh, R.K.; Khanal, D.; Patel, K.D.; Lee, E.J.; Leong, K.W.; Chrzanowski, W.; Kim, H.W. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale 2015, 7, 14191–14216. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Hama, Y.; Koyama, Y.; Kohn, E.C.; Choyke, P.L.; Kobayashi, H. Targeted optical fluorescence imaging of human ovarian adenocarcinoma using a galactosyl serum albumin-conjugated fluorophore. Cancer Sci. 2007, 98, 1727–1733. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, H.; Su, S.; Wang, T.; Zhang, C.; Fida, G.; Cui, S.; Zhao, J.; Gu, Y. Galactose as broad ligand for multiple tumor imaging and therapy. J. Cancer 2015, 6, 658–670. [Google Scholar] [CrossRef]
- Brevet, D.; Gary-Bobo, M.; Raehm, L.; Richeter, S.; Hocine, O.; Amro, K.; Loock, B.; Couleaud, P.; Frochot, C.; Morere, A.; et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem. Commun. 2009, 1475–1477. [Google Scholar] [CrossRef]
- He, H.; Cattran, A.W.; Nguyen, T.; Nieminen, A.-L.; Xu, P. Triple-responsive expansile nanogel for tumor and mitochondria targeted photosensitizer delivery. Biomaterials 2014, 35, 9546–9553. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Sign. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Soman, N.R.; Baldwin, S.L.; Hu, G.; Marsh, J.N.; Lanza, G.M.; Heuser, J.E.; Arbeit, J.M.; Wickline, S.A.; Schlesinger, P.H. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J. Clin. Investig. 2009, 119, 2830–2842. [Google Scholar] [CrossRef]
- He, H.; Markoutsa, E.; Li, J.; Xu, P. Repurposing disulfiram for cancer therapy via targeted nanotechnology through enhanced tumor mass penetration and disassembly. Acta Biomater. 2018, 68, 113–124. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Xu, P. Redox-Sensitive Nanocomplex for Targeted Delivery of Melittin. Toxins 2020, 12, 582. https://doi.org/10.3390/toxins12090582
Cheng B, Xu P. Redox-Sensitive Nanocomplex for Targeted Delivery of Melittin. Toxins. 2020; 12(9):582. https://doi.org/10.3390/toxins12090582
Chicago/Turabian StyleCheng, Bei, and Peisheng Xu. 2020. "Redox-Sensitive Nanocomplex for Targeted Delivery of Melittin" Toxins 12, no. 9: 582. https://doi.org/10.3390/toxins12090582
APA StyleCheng, B., & Xu, P. (2020). Redox-Sensitive Nanocomplex for Targeted Delivery of Melittin. Toxins, 12(9), 582. https://doi.org/10.3390/toxins12090582



