Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin
Abstract
1. Introduction
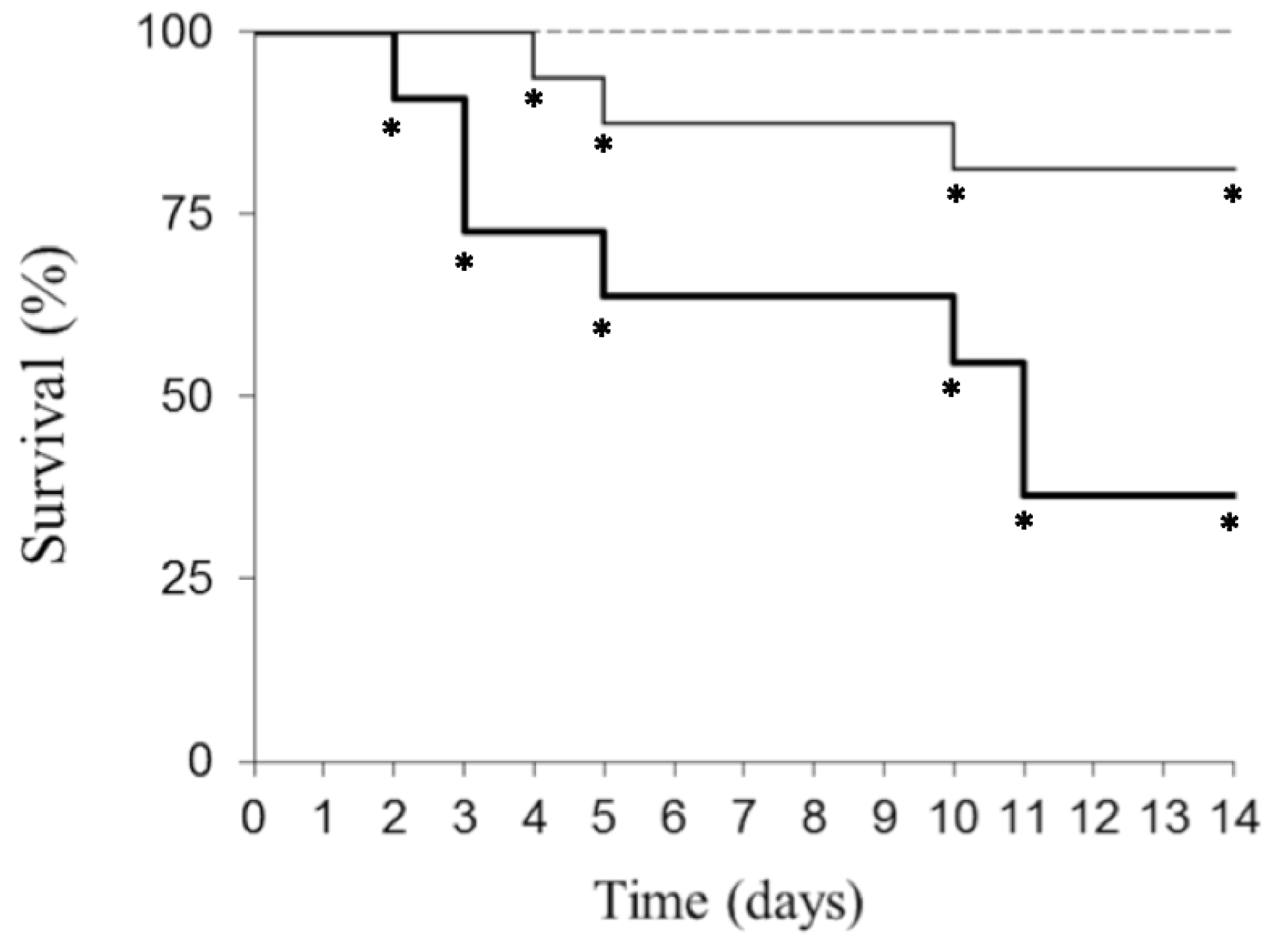
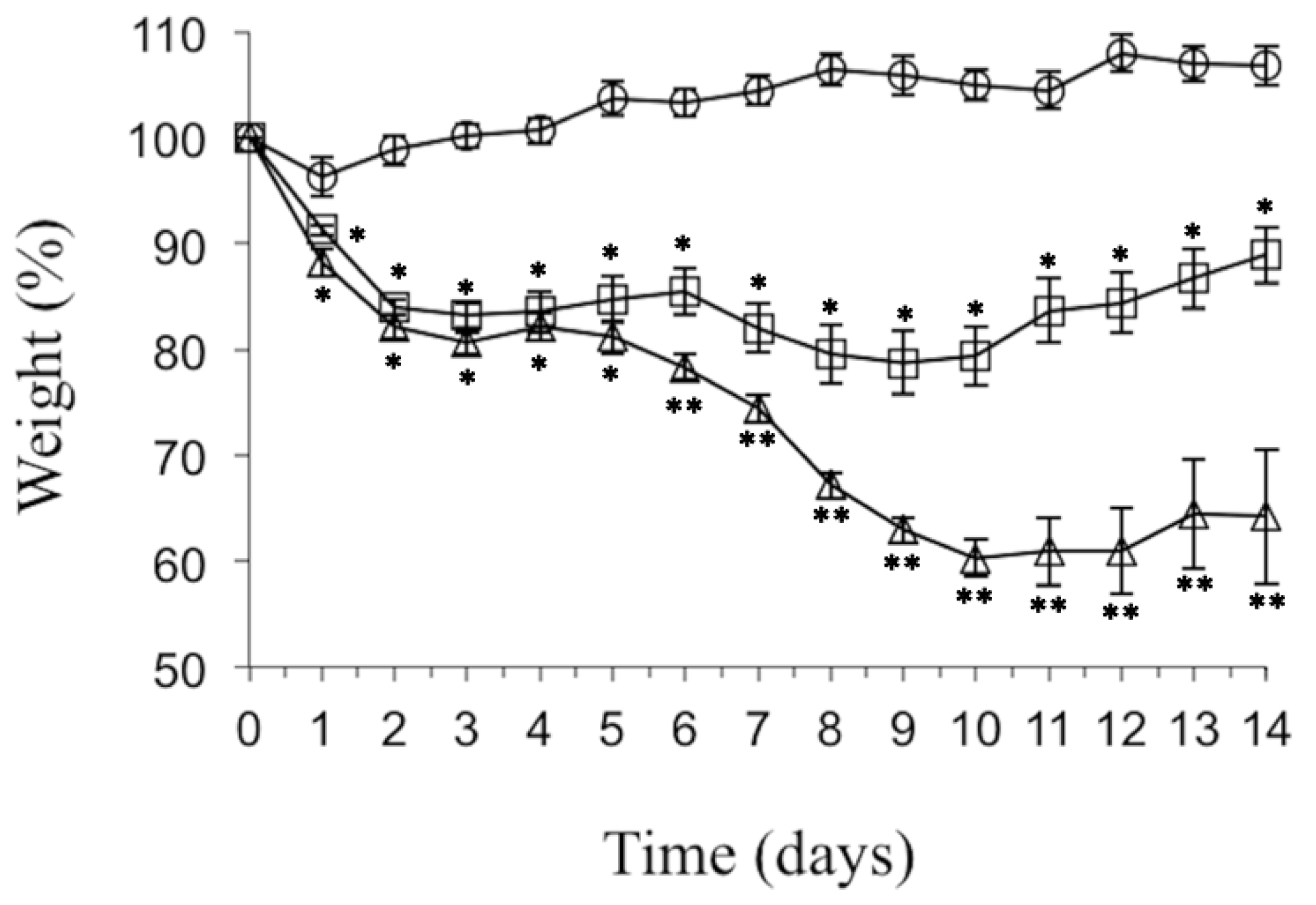
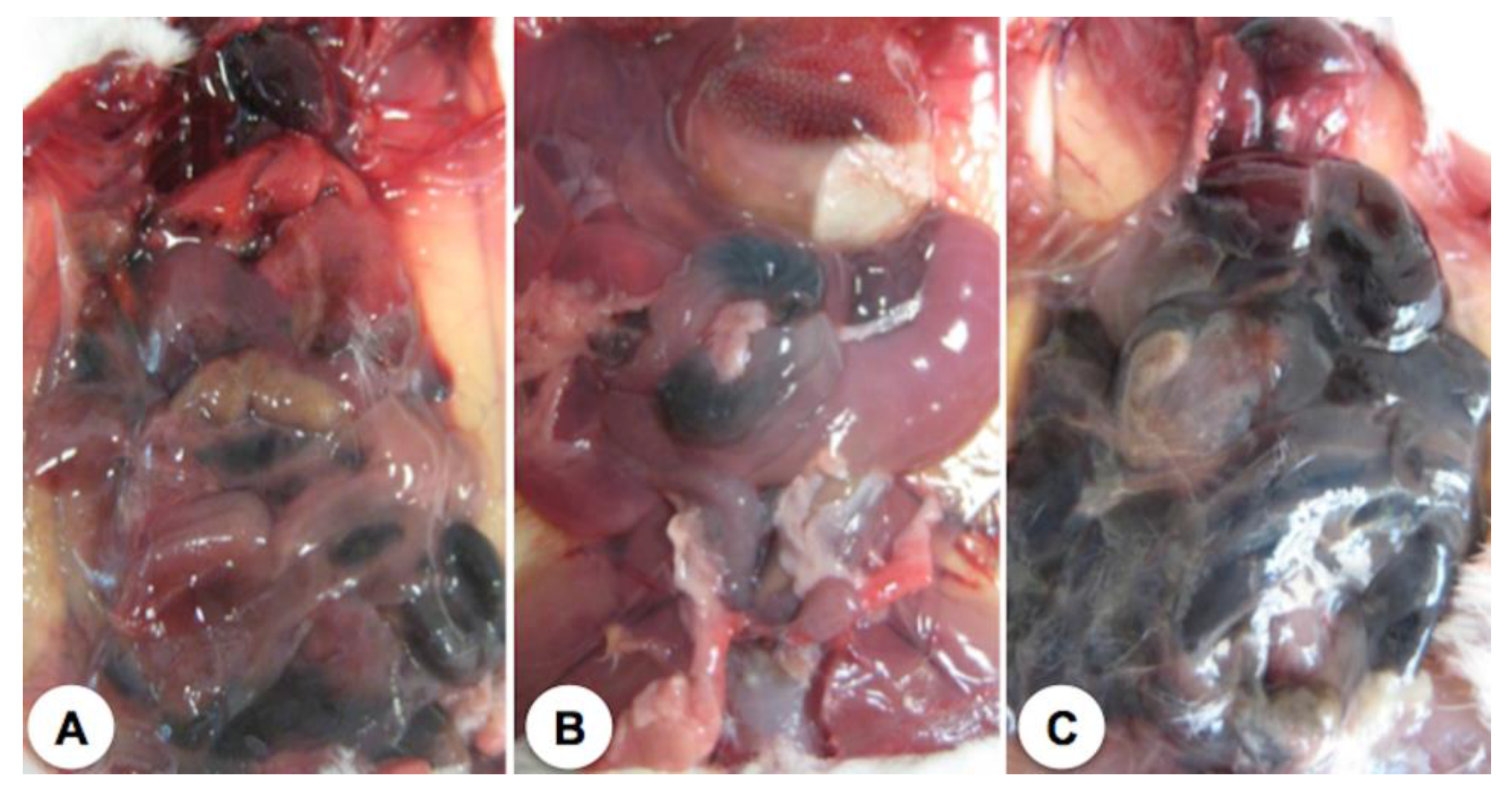
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Isolation of Ebulin F
5.3. Green Tea Aqueous Extract Preparation
5.4. Subjects
5.5. Treatment
5.6. Phenolic Analysis
5.7. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DNBS | dinitrobenzene sulfonic acid |
| DSS | dextran sulfate sodium |
| RIP | ribosome-inactivating protein |
| IBD | inflammatory bowel diseases |
| EGCG | epigallocatechin gallate |
| GTPs | green tea polyphenols |
| p.o. | per os |
| i.p. | intraperitoneal |
| Pol60 | Polyphenon 60 |
| RILs | ribosome-inactivating lectins |
References
- Cooper, R. Green tea and theanine: Health benefits. Int. J. Food Sci. Nutr. 2012, 63, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Hügel, H.M.; Jackson, N. Redox chemistry of green tea polyphenols: Therapeutic benefits in neurodegenerative diseases. Mini-Rev. Med. Chem. 2012, 12, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. (Elite Ed.) 2012, 4, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Danesi, F.; Di Nunzio, M.; Boschetti, E.; Bordoni, A. Green tea extract selectively activates peroxisome-proliferator-activated receptor beta/delta in cultured cardiomyocytes. Br. J. Nutr. 2009, 101, 1736–1739. [Google Scholar] [CrossRef]
- Yang, C.S.; Zhang, J. Studies on the Prevention of Cancer and Cardiometabolic Diseases by Tea: Issues on Mechanisms, Effective Doses, and Toxicities. J. Agric. Food Chem. 2019, 67, 5446–5456. [Google Scholar] [CrossRef]
- Rahman, S.U.; Li, Y.; Huang, Y.; Zhu, L.; Feng, S.; Wu, J.; Wang, X. Treatment of inflammatory bowel disease via green tea polyphenols: Possible application and protective approaches. Inflammopharmacol 2018, 26, 319–330. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M.; Tsurumi, H.; Hara, Y.; Tanaka, T.; Moriwaki, H. EGCG and Polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol. Med. Rep. 2008, 1, 355–361. [Google Scholar] [CrossRef]
- Erba, D.; Riso, P.; Bordoni, A.; Foti, P.; Biagi, P.L.; Testolin, G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J. Nutr. Biochem. 2005, 16, 144–149. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Kishimoto, Y.; Miura, N.; Shiota, G.; Kohri, T.; Hara, Y.; Hasegawa, J.; Isemura, M. Synergistic effects of (-)-epigallocatechin gallate with sulindac against colon carcinogenesis of rats treated with azoxymethane. Cancer Lett. 2002, 177, 49–56. [Google Scholar] [CrossRef]
- Shimizu, M.; Adachi, S.; Masuda, M.; Kozawa, O.; Moriwaki, H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol. Nutr. Food Res. 2011, 55, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Targeting Bacterial Biofilms by the Green Tea Polyphenol EGCG. Molecules 2019, 24, 2403. [Google Scholar] [CrossRef] [PubMed]
- Toschi, T.G.; Bordoni, A.; Hrelia, S.; Bendini, A.; Lercker, G.; Biagi, P.L. The protective role of different green tea extracts after oxidative damage is related to their catechin composition. J. Agric. Food Chem. 2000, 48, 3973–3978. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Tang, S.N.; Fu, J.; Shankar, S.; Srivastava, R.K. EGCG enhances the therapeutic potential of gemcitabine and CP690550 by inhibiting STAT3 signaling pathway in human pancreatic cancer. PLoS ONE 2012, 7, e31067. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Tsai, C.F.; Chen, W.K.; Huang, C.F.; Yen, C.C. A subacute toxicity evaluation of green tea (Camellia sinensis) extract in mice. Food Chem. Toxicol. 2011, 49, 2624–2630. [Google Scholar] [CrossRef]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef]
- Schönthal, A.H. Adverse effects of concentrated green tea extracts. Mol. Nutr. Food Res. 2011, 55, 874–885. [Google Scholar] [CrossRef]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Akiyama, S.; Maeda-Yamamoto, M.; Nesumi, A.; Tanaka, T.; Murakami, A. High-dose green tea polyphenols induce nephrotoxicity in dextran sulfate sodium-induced colitis mice by down-regulation of antioxidant enzymes and heat-shock protein expressions. Cell Stress Chaperon. 2011, 16, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Van Damme, E.J. Lectins as plant defense proteins. Plant Physiol. 1995, 109, 347–352. [Google Scholar] [CrossRef]
- Girbes, T.; de Torre, C.; Iglesias, R.; Ferreras, J.M.; Méndez, E. RIP for viruses. Nature 1996, 379, 777–778. [Google Scholar] [CrossRef]
- Girbés, T.; Ferreras, J.M.; Arias, F.J.; Stirpe, F. Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini-Rev. Med. Chem. 2004, 4, 461–476. [Google Scholar] [CrossRef]
- Ng, T.B.; Wong, J.H.; Wang, H. Recent progress in research on ribosome inactivating proteins. Curr. Protein Pept. Sci. 2010, 11, 37–53. [Google Scholar] [CrossRef]
- Puri, M.; Kaur, I.; Perugini, M.A.; Gupta, R.C. Ribosome-inactivating proteins: Current status and biomedical applications. Drug Discov. Today 2012, 13, 774–783. [Google Scholar] [CrossRef]
- Stirpe, F. Ribosome-inactivating proteins. Toxicon 2004, 44, 371–383. [Google Scholar] [CrossRef]
- Stirpe, F. Ribosome-inactivating proteins: From toxins to useful proteins. Toxicon 2013, 67, 12–16. [Google Scholar] [CrossRef]
- Barbieri, L.; Ciani, M.; Girbés, T.; Liu, W.Y.; Van Damme, E.J.; Peumans, W.J.; Stirpe, F. Enzymatic activity of toxic and non-toxic type 2 ribosome-inactivating proteins. FEBS Lett. 2004, 563, 219–222. [Google Scholar] [CrossRef]
- Govindan, S.V.; Goldenberg, D.M. Designing immunoconjugates for cancer therapy. Exp. Opin. Biol. Ther. 2012, 12, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Arias, Y.; Ferreras, J.M.; Jiménez, P.; Langa, C.; Rojo, M.A.; Gayoso, M.J.; Córdoba-Díaz, D.; Bernabéu, C.; Girbés, T. In vitro and in vivo effects of an anti-mouse endoglin (CD105)-immunotoxin on the early stages of mouse B16MEL4A5 melanoma tumours. Cancer Immunol. Immunother. 2013, 62, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Arias, Y.; Ferreras, J.M.; Rojo, M.A.; Gayoso, M.J.; Nocito, M.; Benitez, J.; Jiménez, P.; Bernabéu, C.; Girbés, T. Targeting a marker of the tumour neovasculature using a novel anti-human CD105-immunotoxin containing the non-toxic type 2 ribosome-inactivating protein nigrin b. Cancer Lett. 2007, 256, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, J.M.; Citores, L.; Iglesias, R.; Souza, A.M.; Jiménez, P.; Gayoso, M.; Girbés, T. Occurrence of the type two ribosome-inactivating protein nigrin b in elderberry (Sambucus nigra L.) bark. Food Res. Int. 2011, 44, 2798–2805. [Google Scholar] [CrossRef]
- Citores, L.; de Benito, F.M.; Iglesias, R.; Ferreras, J.M.; Argüeso, P.; Jimenez, P.; Méndez, E.; Girbés, T. Presence of polymerized and free forms of the non-toxic type 2 ribosome-inactivating protein ebulin and a structurally related new homodimeric lectin in fruits of Sambucus ebulus L. Planta 1998, 204, 310–319. [Google Scholar] [CrossRef]
- Jiménez, P.; Gayoso, M.J.; Tejero, J.; Cabrero, P.; Córdoba-Díaz, D.; Basterrechea, J.E.; Girbés, T. Toxicity in mice of lectin ebulin f present in dwarf elderberry (Sambucus ebulus L.). Toxicon 2013, 61, 26–29. [Google Scholar] [CrossRef]
- Gayoso, M.J.; Muñoz, R.; Arias, Y.; Villar, R.; Rojo, M.A.; Jiménez, P.; Ferreras, J.M.; Aranguez, I.; Girbés, T. Specific dose-dependent damage of Lieberkün crypts promoted by large doses of type 2 ribosome-inactivating protein nigrin b intravenous injection to mice. Toxicol. Appl. Pharmacol. 2005, 207, 138–146. [Google Scholar] [CrossRef]
- Jimenez, P.; Tejero, J.; Cabrero, P.; Cordoba-Diaz, D.; Girbes, T. Differential sensitivity of D-galactose-binding lectins from fruits of dwarf elder (Sambucus ebulus L.) to a simulated gastric fluid. Food Chem. 2013, 136, 794–802. [Google Scholar] [CrossRef]
- Garrosa, M.; Jimenez, P.; Córdoba-Díaz, D.; García-Recio, V.; Gayoso, S.; Rojo, M.A.; Gayoso, M.J.; Girbés, T. In vivo toxicity of the ribosome-inactivating lectin ebulin f in elderly mice. Histol. Histopathol. 2018, 33, 979–986. [Google Scholar] [CrossRef]
- Jiménez, P.; Cordoba-Diaz, D.; Cabrero, P.; Aracil, M.; Gayoso, M.J.; Garrosa, M.; Cordoba-Diaz, M.; Girbés, T. Plasma accumulations of vitamin B6 from an oral dose in a new reversible model for mouse gut injury and regeneration. Food Nutr. Sci. 2013, 4, 908–917. [Google Scholar] [CrossRef]
- Lavín, L.; Garcia-Recio, V.; Jiménez, P.; Girbés, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Pharmaceutical applications of lectins. J. Drug Deliv. Sci. Technol. 2017, 42, 126–133. [Google Scholar] [CrossRef]
- Jimenez, P.; Cabrero, P.; Tejero, J.; Gayoso, M.J.; Garrosa, M.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Girbes, T. Concentrated Extract of Green Tea Polyphenols Enhances the Toxicity of the Elderberry Lectin Nigrin b to Mice. Food Nutr. Sci. 2014, 5, 466–471. [Google Scholar] [CrossRef]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon 60 in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef] [PubMed]
- Ui, A.; Kuriyama, S.; Kakizaki, M.; Sone, T.; Nakaya, N.; Ohmori-Matsuda, K.; Hozawa, A.; Nishino, Y.; Tsuji, I. Green tea consumption and the risk of liver cancer in Japan: The Ohsaki Cohort study. Cancer Causes Control 2009, 20, 1939–1945. [Google Scholar] [CrossRef]
- Chan, P.C.; Ramot, Y.; Malarkey, D.E.; Blackshear, P.; Kissling, G.E.; Travlos, G.; Nyska, A. Fourteen-week toxicity study of green tea extract in rats and mice. Toxicol. Pathol. 2010, 38, 1070–1084. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Crowell, J.A.; Krishnaraj, R.; Zakharov, A.; Lindeblad, M.; Lyubimov, A. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology 2009, 260, 28–36. [Google Scholar] [CrossRef]
- Javaid, A.; Bonkovsky, H.L. Hepatotoxicity due to extracts of Chinese green tea (Camellia sinensis): A growing concern. J. Hepatol. 2006, 45, 334–335. [Google Scholar] [CrossRef]
- Jin, X.; Zheng, R.H.; Li, Y.M. Green tea consumption and liver disease: A systematic review. Liver Int. 2008, 28, 990–996. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmitz, H.J.; Baumgart, A.; Guédon, D.; Netsch, M.I.; Kreuter, M.H.; Schmidlin, C.B.; Schrenk, D. Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture. Food Chem. Toxicol. 2005, 43, 307–314. [Google Scholar] [CrossRef]
- Kim, M.; Murakami, A.; Miyamoto, S.; Tanaka, T.; Ohigashi, H. The modifying effects of green tea polyphenols on acute colitis and inflammation-associated colon carcinogenesis in male ICR mice. Biofactors 2010, 36, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, Y.J.; Park, H.J.; Chung, J.H.; Leem, K.H.; Kim, H.K. Apoptotic effect of red wine polyphenols on human colon cancer SNU-C4 cells. Food Chem. Toxicol. 2006, 44, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Ozdemir Olgun, F.A.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin-Ciocalteu antioxidant capacity assay for measuring lipophilic antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; Van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-Step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef]
- Xu, H.; Nishida, J.; Ma, W.; Wu, H.; Kobayashi, M.; Otsuka, H.; Takahara, A. Competition between oxidation and coordination in cross-linking of polystyrene copolymer containing catechol groups. ACS Macro Lett. 2012, 1, 457–460. [Google Scholar] [CrossRef]
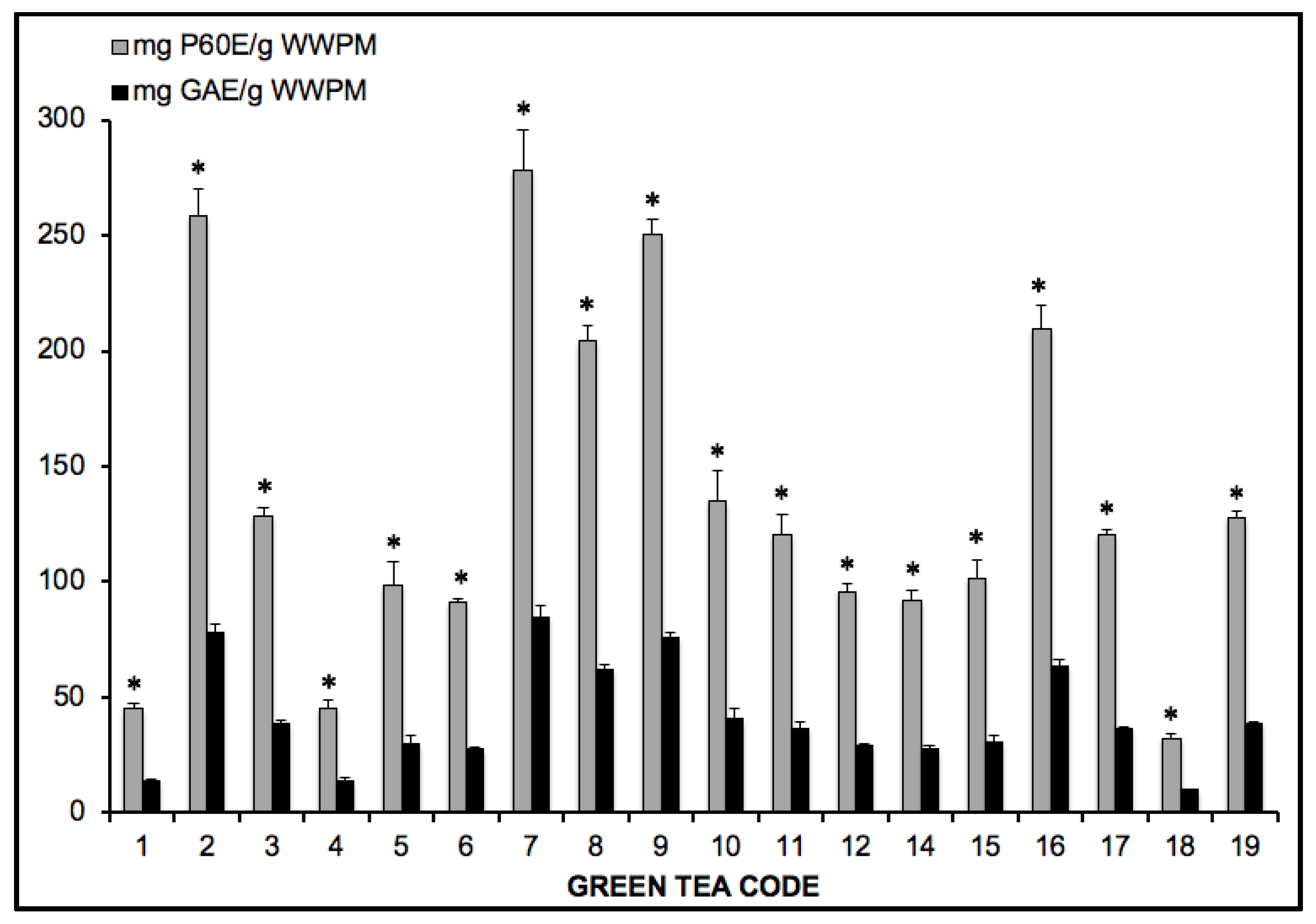

| Time | Tea Name | T (°C) | t (min) |
|---|---|---|---|
| 1 | Kukicha 3 years | 90 | 5 |
| 2 | Sencha | 90 | 2 |
| 3 | Kukicha | 90 | 5 |
| 4 | Hojicha 3 years | 100 | 2 |
| 5 | Bancha | 80 | 3 |
| 6 | Bancha leaf | 80 | 3 |
| 7 | Matcha Second | 100 | 0.5 |
| 8 | Gyokuro | 100 | 2 |
| 9 | Matcha First | 100 | 0.5 |
| 10 | Green Salvage | 90 | 3 |
| 11 | Special Gunpowder | 90 | 3 |
| 12 | Bi Luo Chung | 90 | 3 |
| 13 | Long Jing | 90 | 2 |
| 14 | Tai Ping Hou Kui | 88 | 1 |
| 15 | Chun Mee | 100 | 2 |
| 16 | Jade Rings | 90 | 3 |
| 17 | Mo Li Feng Yan | 85 | 3 |
| 18 | Long Jing Dragon Well | 90 | 3 |
| 19 | Kukicha 3 years | 90 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo, M.Á.; Garrosa, M.; Jiménez, P.; Girbés, T.; Garcia-Recio, V.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin. Toxins 2020, 12, 542. https://doi.org/10.3390/toxins12090542
Rojo MÁ, Garrosa M, Jiménez P, Girbés T, Garcia-Recio V, Cordoba-Diaz M, Cordoba-Diaz D. Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin. Toxins. 2020; 12(9):542. https://doi.org/10.3390/toxins12090542
Chicago/Turabian StyleRojo, M. Ángeles, Manuel Garrosa, Pilar Jiménez, Tomás Girbés, Verónica Garcia-Recio, Manuel Cordoba-Diaz, and Damián Cordoba-Diaz. 2020. "Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin" Toxins 12, no. 9: 542. https://doi.org/10.3390/toxins12090542
APA StyleRojo, M. Á., Garrosa, M., Jiménez, P., Girbés, T., Garcia-Recio, V., Cordoba-Diaz, M., & Cordoba-Diaz, D. (2020). Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin. Toxins, 12(9), 542. https://doi.org/10.3390/toxins12090542