Copepod Prey Selection and Grazing Efficiency Mediated by Chemical and Morphological Defensive Traits of Cyanobacteria
Abstract
1. Introduction
2. Results
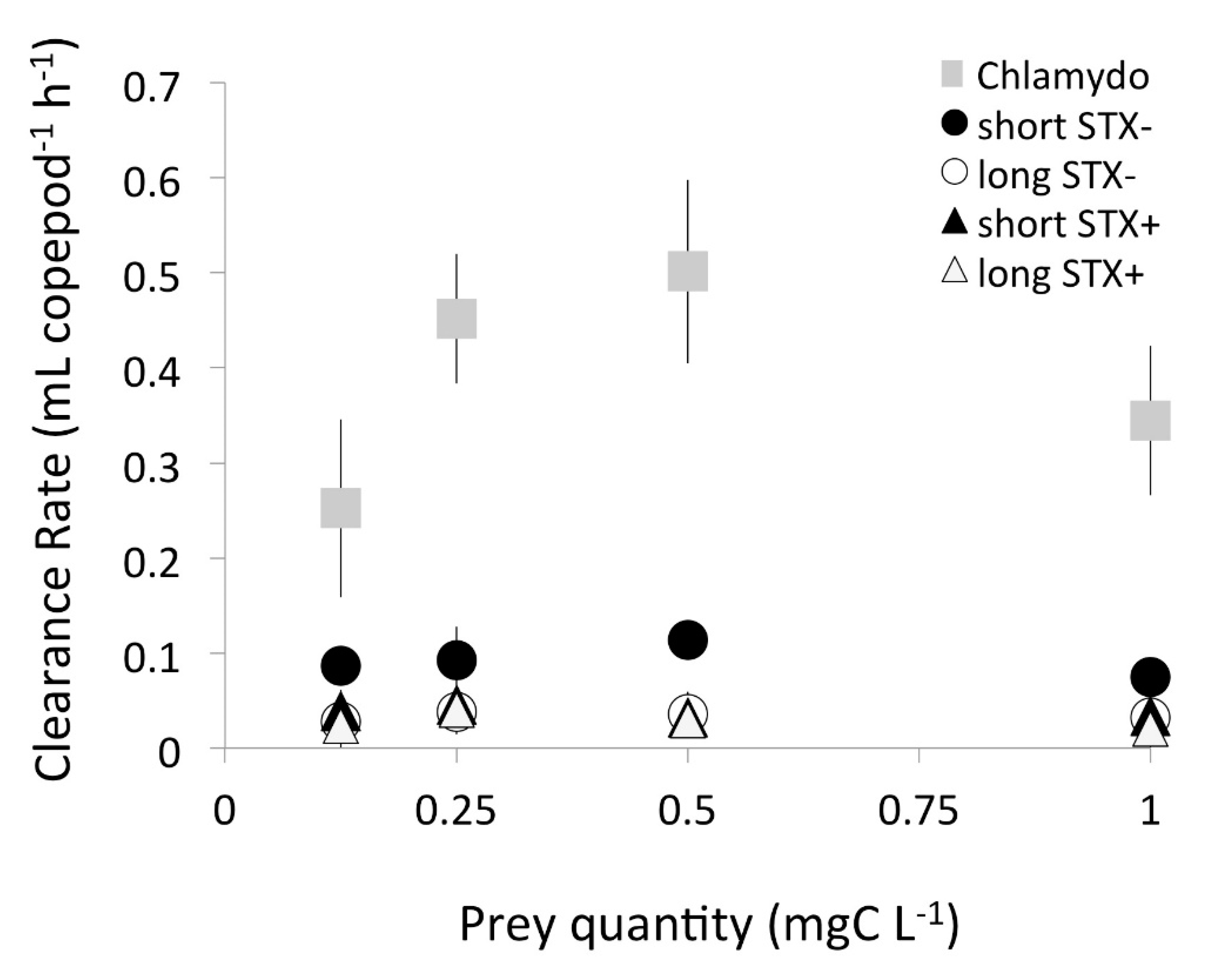
2.1. Functional Response
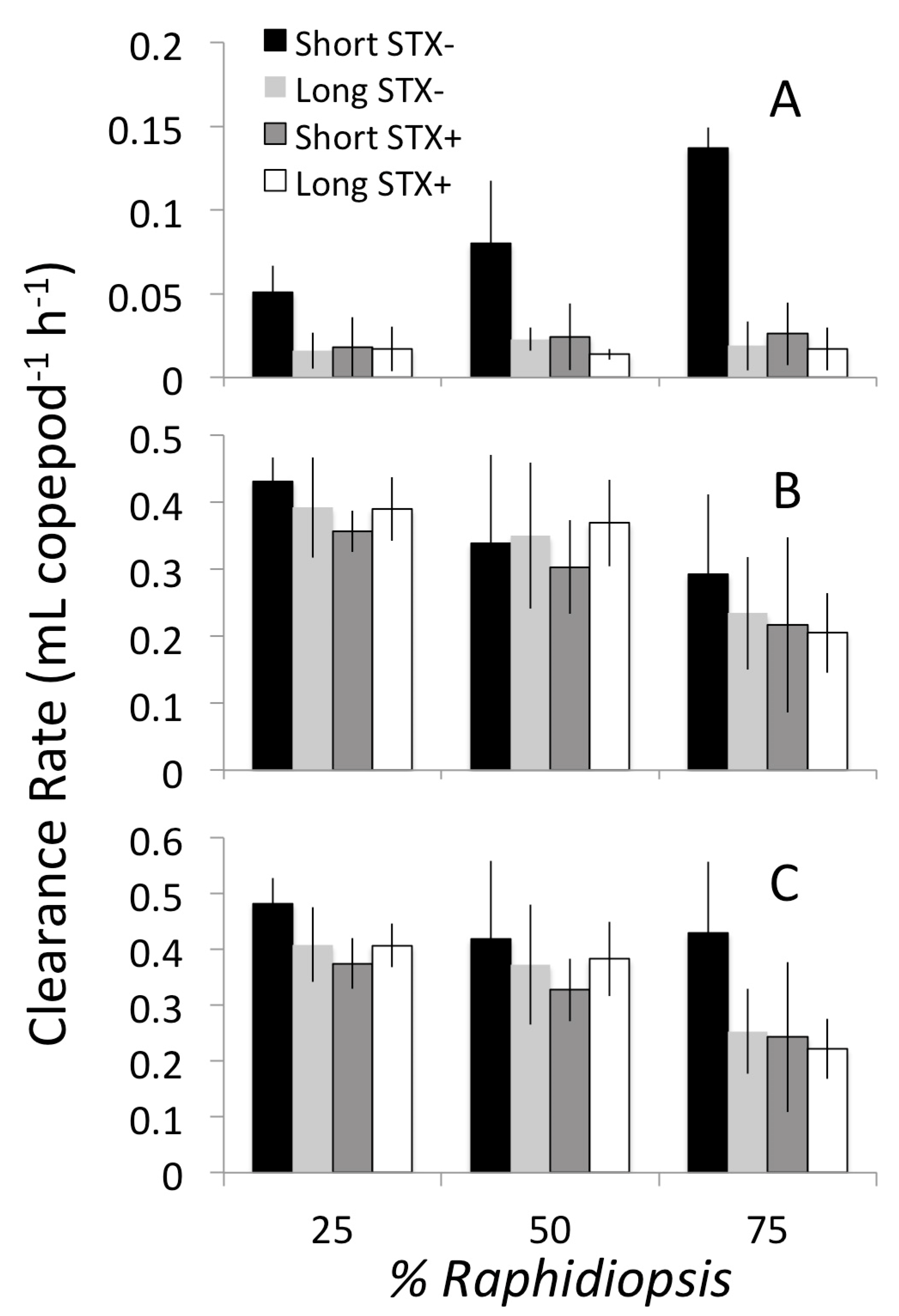
2.2. Grazing on Mixed Diets
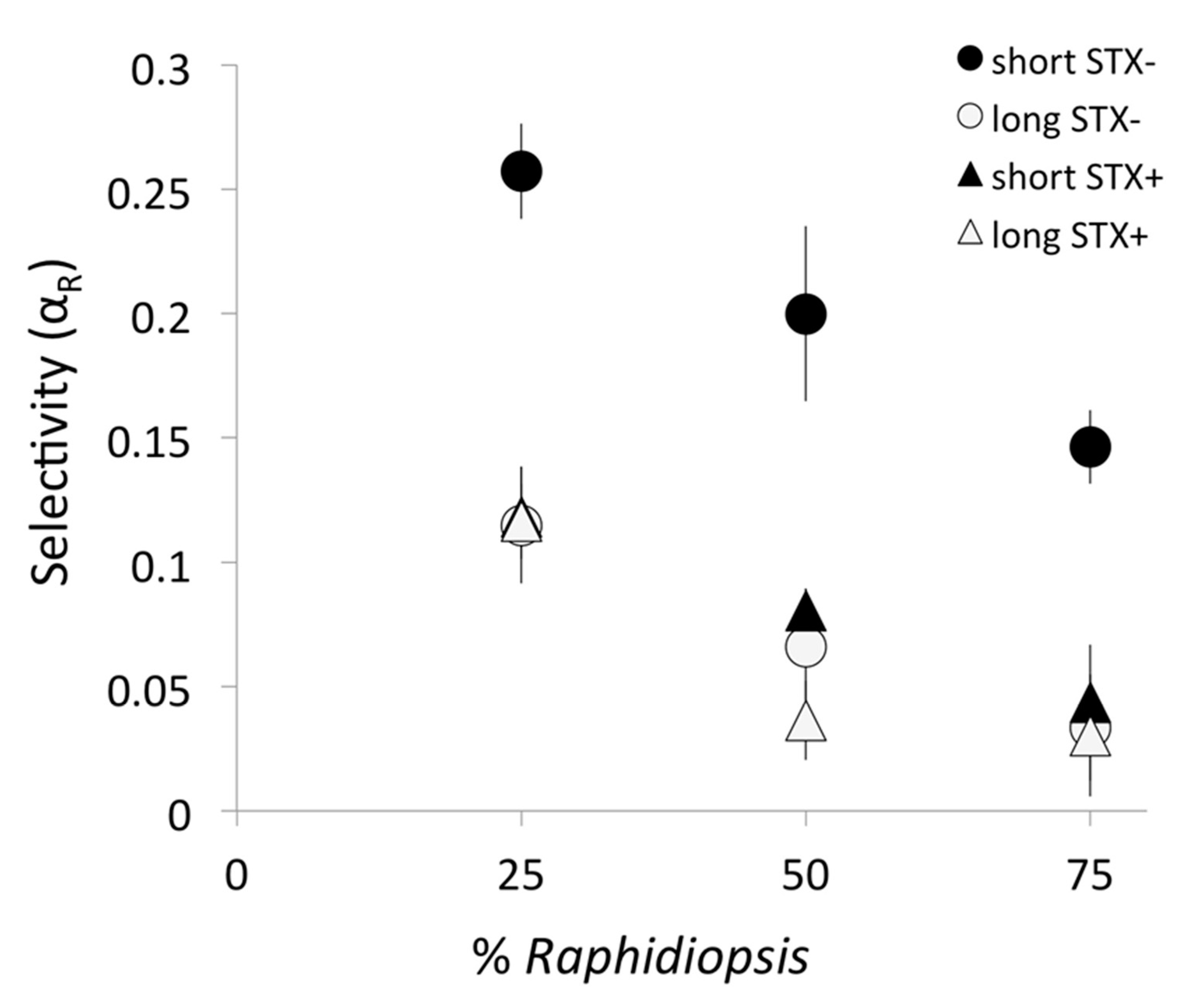
2.3. Copepod Prey Selection: The Selectivity Coefficient for Raphidiopsis (αR)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Phytoplankton Cultivation
5.2. Establishment of Short and Long Filament Cyanobacterial Morphotypes
5.3. Toxin Analyses of the STX+ and STX- Strains
5.4. Copepods Used for Grazing Experiments
5.5. Copepod Functional Response to Different Prey
5.6. Copepod Selective Grazing in Mixed Prey Diets
- 25% Raphidiopsis + 75% Chlamydomonas;
- 50% Raphidiopsis + 50% Chlamydomonas;
- 75% Raphidiopsis + 25% Chlamydomonas.
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pohnert, G.; Steinke, M.; Tollrian, R. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends Ecol. Evol. 2007, 22, 198–204. [Google Scholar] [CrossRef]
- Pančić, M.; Kiørboe, T. Phytoplankton defence mechanisms: Traits and trade-offs. Biol. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Smayda, T.J. Complexity in the eutrophication–harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae 2008, 8, 140–151. [Google Scholar] [CrossRef]
- Azevedo, S.M.F.O.; Carmichael, W.W.; Jochimsen, E.M.; Rinehart, K.L.; Lau, S.; Shaw, G.R.; Eaglesham, G.K. Human intoxication by microcystins during renal dialysis treatment in Caruaru—Brazil. Toxicology 2002, 181–182, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; Meester, L.D.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Heathcote, A.J.; Filstrup, C.T.; Kendall, D.; Downing, J.A. Biomass pyramids in lake plankton: Influence of Cyanobacteria size and abundance. Inland Waters 2016, 6, 250–257. [Google Scholar] [CrossRef]
- Dickman, E.M.; Newell, J.M.; González, M.J.; Vanni, M.J. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proc. Natl. Acad. Sci. USA 2008, 105, 18408–18412. [Google Scholar] [CrossRef] [PubMed]
- Ger, K.A.; Faassen, E.J.; Pennino, M.G.; Lürling, M. Effect of the toxin (microcystin) content of Microcystis on copepod grazing. Harmful Algae 2016, 52, 34–45. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef]
- Wilson, A.E.; Sarnelle, O.; Tillmanns, A.R. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: Meta-analyses of laboratory experiments. Limnol. Oceanogr. 2006, 51, 1915–1924. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Why do cladocerans fail to control algal blooms? Hydrobiologia 1990, 200–201, 83–97. [Google Scholar] [CrossRef]
- Pearson, L.A.; Dittmann, E.; Mazmouz, R.; Ongley, S.E.; D’Agostino, P.M.; Neilan, B.A. The genetics, biosynthesis and regulation of toxic specialized metabolites of cyanobacteria. Harmful Algae 2016, 54, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Fewer, D.P.; Neilan, B.A. Cyanobacterial toxins: Biosynthetic routes and evolutionary roots. FEMS Microbiol. Rev. 2013, 37, 23–43. [Google Scholar] [CrossRef]
- Lürling, M. Effects of microcystin-free and microcystin-containing strains of the cyanobacterium Microcystis aeruginosa on growth of the grazer Daphnia magna. Environ. Toxicol. 2003, 18, 202–210. [Google Scholar] [CrossRef]
- Wilson, A.E.; Hay, M.E. A direct test of cyanobacterial chemical defense: Variable effects of microcystin-treated food on two Daphnia pulicaria clones. Limnol. Oceanogr. 2007, 52, 1467–1479. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Lürling, M.; Huszar, V.L.M. Growth and temperature-related phenotypic plasticity in the cyanobacterium Cylindrospermopsis raciborskii. Phycol. Res. 2013, 61, 61–67. [Google Scholar] [CrossRef]
- Willis, A.; Chuang, A.W.; Woodhouse, J.N.; Neilan, B.A.; Burford, M.A. Intraspecific variation in growth, morphology and toxin quotas for the cyanobacterium, Cylindrospermopsis raciborskii. Toxicon 2016, 119, 307–310. [Google Scholar] [CrossRef]
- Pimentel, J.S.M.; Giani, A. Microcystin production and regulation under nutrient stress conditions in toxic microcystis strains. Appl. Environ. Microbiol. 2014, 80, 5836–5843. [Google Scholar] [CrossRef]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.-A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef]
- Litchman, E.; Ohman, M.D.; Kiørboe, T. Trait-based approaches to zooplankton communities. J. Plankton Res. 2013, 35, 473–484. [Google Scholar] [CrossRef]
- Ger, K.A.; Naus-Wiezer, S.; Meester, L.D.; Lürling, M. Zooplankton grazing selectivity regulates herbivory and dominance of toxic phytoplankton over multiple prey generations. Limnol. Oceanogr. 2019, 64, 1214–1227. [Google Scholar] [CrossRef]
- DeMott, W.R. Optimal foraging theory as a predictor of chemically mediated food selection by suspension-feeding copepods. Limnol. Oceanogr. 1989, 34, 140–154. [Google Scholar] [CrossRef]
- DeMott, W.R.; Zhang, Q.-X.; Carmichael, W.W. Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnol. Oceanogr. 1991, 36, 1346–1357. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Vardaka, E.; Michaloudi, E.; Kormas, K.A.; Tryfon, E.; Mihalatou, H.; Gkelis, S.; Lanaras, T. Plankton food web structure in a eutrophic polymictic lake with a history of toxic cyanobacterial blooms. Limnol. Oceanogr. 2006, 51, 715–727. [Google Scholar] [CrossRef]
- Bouvy, M.; Pagano, M.; Troussellier, M. Effects of a cyanobacterial bloom (Cylindrospermopsis raciborskii) on bacteria and zooplankton communities in Ingazeira reservoir (northeast Brazil). Aquat. Microb. Ecol. 2001, 25, 215–227. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Lürling, M.; Panosso, R.; Huszar, V. Effects of the cyanobacterium Cylindrospermopsis raciborskii on feeding and life-history characteristics of the grazer Daphnia magna. Ecotoxicol. Environ. Saf. 2009, 72, 1183–1189. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.D.; da Silva, D.A. Saxitoxin-producing Raphidiopsis raciborskii (cyanobacteria) inhibits swimming and physiological parameters in Daphnia similis. Sci. Total Environ. 2020, 706, 135751. [Google Scholar] [CrossRef]
- Schultz, M.; Kiorboe, T. Active prey selection in two pelagic copepods feeding on potentially toxic and non-toxic dinoflagellates. J. Plankton Res. 2009, 31, 553–561. [Google Scholar] [CrossRef]
- Ger, K.A.; Leitao, E.; Panosso, R. Potential mechanisms for the tropical copepod Notodiaptomus to tolerate Microcystis toxicity. J. Plankton Res. 2016, 38, 843–854. [Google Scholar] [CrossRef]
- DeMott, W.R.; Moxter, F. Foraging cyanobacteria by copepods: Responses to chemical defense and resource abundance. Ecology 1991, 72, 1820–1834. [Google Scholar] [CrossRef]
- Mitra, A.; Flynn, K.J. Promotion of harmful algal blooms by zooplankton predatory activity. Biol. Lett. 2006, 2, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Sailley, S.F.; Polimene, L.; Mitra, A.; Atkinson, A.; Allen, J.I. Impact of zooplankton food selectivity on plankton dynamics and nutrient cycling. J. Plankton Res. 2015, 37, 519–529. [Google Scholar] [CrossRef]
- Leitão, E.; Ger, K.A.; Panosso, R. Selective grazing by a tropical copepod (Notodiaptomus iheringi) facilitates microcystis dominance. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Antunes, J.T.; Leão, P.N.; Vasconcelos, V.M. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Burford, M.A.; Beardall, J.; Willis, A.; Orr, P.T.; Magalhaes, V.F.; Rangel, L.M.; Azevedo, S.M.F.O.E.; Neilan, B.A. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 2016, 54, 44–53. [Google Scholar] [CrossRef]
- Kokociński, M.; Gągała, I.; Jasser, I.; Karosienė, J.; Kasperovičienė, J.; Kobos, J.; Koreivienė, J.; Soininen, J.; Szczurowska, A.; Woszczyk, M.; et al. Distribution of invasive Cylindrospermopsis raciborskii in the East-Central Europe is driven by climatic and local environmental variables. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Aguilera, A.; Gómez, E.B.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Saker, M.L.; Neilan, B.A.; Griffiths, D.J. Two morphological forms of Cylindrospermopsis raciborskii (cyanobacteria) isolated from Solomon Dam, Palm Island, Queensland. J. Phycol. 1999, 35, 599–606. [Google Scholar] [CrossRef]
- Komárek, J.; Mareš, J. An update to modern taxonomy (2011) of freshwater planktic heterocytous cyanobacteria. Hydrobiologia 2012, 698, 327–351. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shiah, F.-K. Growth, trichome size and akinete production of Cylindrospermopsis raciborskii (cyanobacteria) under different temperatures: Comparison of two strains isolated from the same pond. Phycol. Res. 2014, 62, 147–152. [Google Scholar] [CrossRef]
- Wejnerowski, L.; Cerbin, S.; Wojciechowicz, M.; Jurczak, T.; Glama, M.; Meriluoto, J.; Dziuba, M. Effects of Daphnia exudates and sodium octyl sulphates on filament morphology and cell wall thickness of Aphanizomenon gracile (Nostocales), Cylindrospermopsis raciborskii (Nostocales) and Planktothrix agardhii (Oscillatoriales). Eur. J. Phycol. 2018, 53, 280–289. [Google Scholar] [CrossRef]
- Casali, S.P.; Dos Santos, A.C.A.; de Falco, P.B.; Calijuri, M.D. Influence of environmental variables on saxitoxin yields by Cylindrospermopsis raciborskii in a mesotrophic subtropical reservoir. J. Water Health 2017, 15, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.L.; Pacheco, A.B.F.; de Oliveira e Azevedo, S.M.F. Growth and saxitoxin production by Cylindrospermopsis raciborskii (cyanobacteria) correlate with water hardness. Mar. Drugs 2013, 11, 2949–2963. [Google Scholar] [CrossRef]
- Sousa, W.; Attayde, J.L.; Rocha, E.D.S.; Eskinazi-Sant’Anna, E.M. The response of zooplankton assemblages to variations in the water quality of four man-made lakes in semi-arid northeastern Brazil. J. Plankton Res. 2008, 30, 699–708. [Google Scholar] [CrossRef]
- Hong, Y.; Burford, M.A.; Ralph, P.J.; Doblin, M.A. Subtropical zooplankton assemblage promotes the harmful cyanobacterium Cylindrospermopsis raciborskii in a mesocosm experiment. J. Plankton Res. 2015, 37, 90–101. [Google Scholar] [CrossRef]
- Panosso, R.; Carlsson, P.; Kozlowsky-Suzuki, B.; Azevedo, S.M.F.O.; Granéli, E. Effect of grazing by a neotropical copepod, Notodiaptomus, on a natural cyanobacterial assemblage and on toxic and non-toxic cyanobacterial strains. J. Plankton Res. 2003, 25, 1169–1175. [Google Scholar] [CrossRef]
- Kâ, S.; Mendoza-Vera, J.M.; Bouvy, M.; Champalbert, G.; N’Gom-Kâ, R.; Pagano, M. Can tropical freshwater zooplankton graze efficiently on cyanobacteria? Hydrobiologia 2012, 679, 119–138. [Google Scholar] [CrossRef]
- Hong, Y.; Burford, M.A.; Ralph, P.J.; Udy, J.W.; Doblin, M.A. The cyanobacterium Cylindrospermopsis raciborskii is facilitated by copepod selective grazing. Harmful Algae 2013, 29, 14–21. [Google Scholar] [CrossRef]
- Rangel, L.M.; Ger, K.A.; Silva, L.H.S.; Soares, M.C.S.; Faassen, E.J.; Lürling, M. Toxicity overrides morphology on Cylindrospermopsis raciborskii grazing resistance to the calanoid copepod Eudiaptomus gracilis. Microb. Ecol. 2016, 71, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Engström-Öst, J.; Viitasalo, M.; Jónasdóttir, S.; Repka, S.; Sivonen, K.; Koski, M.; Schmidt, K. Calanoid copepods feed and produce eggs in the presence of toxic cyanobacteria Nodularia spumigena. Limnol. Oceanogr. 2002, 47, 878–885. [Google Scholar] [CrossRef]
- Kiørboe, T. How zooplankton feed: Mechanisms, traits and trade-offs. Biol. Rev. 2011, 86, 311–339. [Google Scholar] [CrossRef]
- Kiørboe, T. A Mechanistic Approach to Plankton Ecology; Princeton University Press: Princeton, NJ, USA, 2008; ISBN 978-0-691-13422-2. [Google Scholar]
- Xu, J.; Kiørboe, T. Toxic dinoflagellates produce true grazer deterrents. Ecology 2018, 99, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Gebrehiwot, M.; Kifle, D.; Triest, L. Grazing and growth rate of a cyclopoid copepod fed with a phytoplankton diet constituted by a filamentous cyanobacterium. Hydrobiologia 2019, 828, 213–227. [Google Scholar] [CrossRef]
- Von Elert, E.; Martin-Creuzburg, D.; Le Coz, J.R. Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc. Biol. Sci. 2003, 270, 1209–1214. [Google Scholar] [CrossRef]
- Müller-Navarra, D.C.; Brett, M.T.; Liston, A.M.; Goldman, C.R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 2000, 403, 74–77. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Selander, E.; Thor, P.; Toth, G.; Pavia, H. Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc. Biol. Sci. 2006, 273, 1673–1680. [Google Scholar] [CrossRef]
- Hansen, B.; Bjornsen, P.K.; Hansen, P.J. The size ratio between planktonic predators and their prey. Limnol. Oceanogr. 1994, 39, 395–403. [Google Scholar] [CrossRef]
- Dhanker, R.; Kumar, R.; Hwang, J.-S. Predation by Pseudodiaptomus annandalei (Copepoda: Calanoida) on rotifer prey: Size selection, egg predation and effect of algal diet. J. Exp. Mar. Biol. Ecol. 2012, 414–415, 44–53. [Google Scholar] [CrossRef]
- Frost, B.W. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 1972, 17, 805–815. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Paffenhofer, G.-A.; Liebig, J.R. Diaptomus vs. net phytoplankton: Effects of algal size and morphology on selectivity of a behaviorally flexible, omnivorous copepod. Bull. Mar. Sci. 1988, 43, 377–394. [Google Scholar]
- Bergkvist, J.; Thor, P.; Jakobsen, H.H.; Wängberg, S.-Å.; Selander, E. Grazer-induced chain length plasticity reduces grazing risk in a marine diatom. Limnol. Oceanogr. 2012, 57, 318–324. [Google Scholar] [CrossRef]
- Sterner, R.W. The role of grazers in phytoplankton succession. In Plankton Ecology: Succession in Plankton Communities; Brock/Springer Series in Contemporary Bioscience; Sommer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 107–170. ISBN 978-3-642-74890-5. [Google Scholar]
- Ger, K.A.; Panosso, R.; Lürling, M. Consequences of acclimation to Microcystis on the selective feeding behavior of the calanoid copepod Eudiaptomus gracilis. Limnol. Oceanogr. 2011, 56, 2103–2114. [Google Scholar] [CrossRef]
- Engström, J.; Koski, M.; Viitasalo, M.; Reinikainen, M.; Repka, S.; Sivonen, K. Feeding interactions of the copepods Eurytemora affinis and Acartia bifilosa with the cyanobacteria Nodularia sp. J. Plankton Res. 2000, 22, 1403–1409. [Google Scholar] [CrossRef]
- Lürling, M. Daphnia growth on microcystin-producing and microcystin-free Microcystis aeruginosa in different mixtures with the green alga Scenedesmus obliquus. Limnol. Oceanogr. 2003, 48, 2214–2220. [Google Scholar] [CrossRef]
- Chesson, J. Measuring preference in selective predation. Ecology 1978, 59, 211–215. [Google Scholar] [CrossRef]
- Manly, B.F.J. Tables for the analysis of selective predation experiments. Res. Popul. Ecol. 1972, 14, 74–81. [Google Scholar] [CrossRef]
- Codd, G.A. Cyanobacterial toxins: Occurrence, properties and biological significance. Water Sci. Technol. 1995, 32, 149–156. [Google Scholar] [CrossRef]
- Sivonen, K. Cyanobacterial toxins and toxin production. Phycologia 1996, 35, 12–24. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Chorus, I. Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: A review. Environ. Pollut. 2007, 150, 177–192. [Google Scholar] [CrossRef]
- Rangel, L.M.; Soares, M.C.S.; Paiva, R.; Silva, L.H.S. Morphology-based functional groups as effective indicators of phytoplankton dynamics in a tropical cyanobacteria-dominated transitional river–reservoir system. Ecol. Indic. 2016, 64, 217–227. [Google Scholar] [CrossRef]
- Hogfors, H.; Motwani, N.H.; Hajdu, S.; El-Shehawy, R.; Holmborn, T.; Vehmaa, A.; Engström-Öst, J.; Brutemark, A.; Gorokhova, E. Bloom-forming cyanobacteria support copepod reproduction and development in the Baltic Sea. PLoS ONE 2014, 9, e112692. [Google Scholar] [CrossRef] [PubMed]
- Colina, M.; Calliari, D.; Carballo, C.; Kruk, C. A trait-based approach to summarize zooplankton–phytoplankton interactions in freshwaters. Hydrobiologia 2015, 767, 221–233. [Google Scholar] [CrossRef]
- Dam, H.G. Evolutionary adaptation of marine zooplankton to global change. Annu. Rev. Mar. Sci. 2013, 5, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Engström-Öst, J.; Brutemark, A.; Vehmaa, A.; Motwani, N.H.; Katajisto, T. Consequences of a cyanobacteria bloom for copepod reproduction, mortality and sex ratio. J. Plankton Res. 2015, 37, 388–398. [Google Scholar] [CrossRef]
- Motwani, N.H.; Duberg, J.; Svedén, J.B.; Gorokhova, E. Grazing on cyanobacteria and transfer of diazotrophic nitrogen to zooplankton in the Baltic Sea: Cyanobacteria blooms support zooplankton growth. Limnol. Oceanogr. 2018, 63, 672–686. [Google Scholar] [CrossRef]
- Ger, K.A.; Otten, T.G.; DuMais, R.; Ignoffo, T.; Kimmerer, W. In situ ingestion of Microcystis is negatively related to copepod abundance in the upper San Francisco Estuary. Limnol. Oceanogr. 2018, 63, 2394–2410. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Rocha, O.; Duncan, A. The relationship between cell carbon and cell volume in freshwater algal species used in zooplanktonic studies. J. Plankton Res. 1985, 7, 279–294. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
| A. All Prey | Slope | SE | t Value | p |
| Intercept | 0.399 | 0.017 | 23.157 | <0.001 |
| Long STX- | −0.363 | 0.020 | −17.932 | <0.001 |
| Long STX+ | −0.368 | 0.020 | −18.185 | <0.001 |
| Short STX- | −0.304 | 0.020 | −15.055 | <0.001 |
| Short STX+ | −0.359 | 0.020 | −17.739 | <0.001 |
| Conc | −0.006 | 0.018 | −0.354 | 0.725 |
| B. Only Cyanobacteria | Slope | SE | t Value | p |
| Intercept | 0.037 | 0.006 | 6.078 | <0.001 |
| Long STX+ | −0.005 | 0.007 | −0.713 | 0.478 |
| Short STX- | 0.058 | −0.007 | 8.118 | <0.001 |
| Short STX+ | 0.003 | 0.007 | 0.544 | 0.588 |
| Conc | −0.008 | 0.007 | −1.143 | 0.258 |
| A. | Slope | SE | t Value | p |
| Intercept | 0.010 | 0.011 | 0.907 | 0.369 |
| %R | <0.001 | <0.001 | 2.470 | 0.017 |
| Size | −0.038 | 0.008 | −4.802 | <0.001 |
| Toxin | −0.034 | 0.008 | −4.357 | <0.001 |
| B. | Slope | SE | t Value | p |
| Intercept | 0.495 | 0.0359 | 13.780 | <0.001 |
| %R | −0.003 | <0.001 | −5.295 | <0.001 |
| Size | <0.001 | 0.024 | 0.003 | 0.998 |
| Toxin | −0.0331 | 0.024 | −1.384 | 0.173 |
| C. | Slope | SE | t Value | p |
| Intercept | 0.506 | 0.039 | 12.905 | <0.001 |
| %R | −0.002 | 0.001 | −4.097 | <0.001 |
| Size | −0.038 | 0.026 | −1.466 | 0.149 |
| Toxin | −0.067 | 0.026 | −2.600 | 0.013 |
| D. | Slope | SE | t Value | p |
| Intercept | 0.486 | 0.038 | 12.524 | <0.001 |
| %R | −0.003 | <0.001 | −5.075 | <0.001 |
| Size | 0.028 | 0.031 | 0.914 | 0.368 |
| Toxin | −0.004 | 0.031 | −0.134 | 0.894 |
| A. | Slope | SE | t Value | p |
| Intercept | 0.187 | 0.028 | 6.576 | <0.001 |
| %R | −0.001 | 0.000 | −3.798 | <0.001 |
| Size | −0.074 | 0.018 | −3.943 | <0.001 |
| Toxin | −0.065 | 0.018 | −3.446 | 0.001 |
| B. | Slope | SE | t Value | p |
| Intercept | 0.151 | 0.029 | 5.074 | <0.001 |
| %R | −0.001 | 0.000 | −3.298 | 0.002 |
| Size | −0.019 | 0.024 | −0.807 | 0.425 |
| Toxin | −0.010 | 0.024 | −0.421 | 0.676 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, L.M.; Silva, L.H.S.; Faassen, E.J.; Lürling, M.; Ger, K.A. Copepod Prey Selection and Grazing Efficiency Mediated by Chemical and Morphological Defensive Traits of Cyanobacteria. Toxins 2020, 12, 465. https://doi.org/10.3390/toxins12070465
Rangel LM, Silva LHS, Faassen EJ, Lürling M, Ger KA. Copepod Prey Selection and Grazing Efficiency Mediated by Chemical and Morphological Defensive Traits of Cyanobacteria. Toxins. 2020; 12(7):465. https://doi.org/10.3390/toxins12070465
Chicago/Turabian StyleRangel, Luciana M., Lúcia H. S. Silva, Elisabeth J. Faassen, Miquel Lürling, and Kemal Ali Ger. 2020. "Copepod Prey Selection and Grazing Efficiency Mediated by Chemical and Morphological Defensive Traits of Cyanobacteria" Toxins 12, no. 7: 465. https://doi.org/10.3390/toxins12070465
APA StyleRangel, L. M., Silva, L. H. S., Faassen, E. J., Lürling, M., & Ger, K. A. (2020). Copepod Prey Selection and Grazing Efficiency Mediated by Chemical and Morphological Defensive Traits of Cyanobacteria. Toxins, 12(7), 465. https://doi.org/10.3390/toxins12070465







