Botulinum Toxin Effects on Sensorimotor Integration in Focal Dystonias
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. STDT Testing
4.3. Electromyographic Recordings
4.4. Kinematic Recordings
4.5. Experimental Paradigm
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Artieda, J.; Pastor, M.A.; Lacruz, F.; Obeso, J.A. Temporal discrimination is abnormal in Parkinson’s disease. Brain 1992, 115, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Defazio, G.; Hallett, M.; Fabbrini, G.; Berardelli, A. The role of sensory information in the pathophysiology of focal dystonias. Nat. Rev. Neurol. 2019, 15, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Leodori, G.; Formica, A.; Zhu, X.; Conte, A.; Belvisi, D.; Cruccu, G.; Hallett, M.; Berardelli, A. The third-stimulus temporal discrimination threshold: Focusing on the temporal processing of sensory input within primary somatosensory cortex. J. Neurophysiol. 2017, 118, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Belvisi, D.; Manzo, N.; Bologna, M.; Barone, F.; Tartaglia, M.; Upadhyay, N.; Berardelli, A. Understanding the link between somatosensory temporal discrimination and movement execution in healthy subjects. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef]
- Conte, A.; Belvisi, D.; De Bartolo, M.I.D.; Manzo, N.; Cortese, F.N.; Tartaglia, M.; Ferrazzano, G.; Fabbrini, G.; Berardelli, A. Abnormal sensory gating in patients with different types of focal dystonias. Mov. Disord. 2018, 33, 1910–1917. [Google Scholar] [CrossRef]
- Tinazzi, M.; Fiorio, M.; Stanzani, C.; Moretto, G.; Smania, N.; Fiaschi, A.; Bhatia, K.P.; Rothwell, J.C. Temporal discrimination of two passive movements in writer’s cramp. Mov. Disord. 2006, 21, 1131–1135. [Google Scholar] [CrossRef]
- Hutchinson, M.; Kimmich, O.; Molloy, A.; Whelan, R.; Molloy, F.; Lynch, T.; Healy, D.G.; Walsh, C.; Edwards, M.J.; Ozelius, L.; et al. The endophenotype and the phenotype: Temporal discrimination and adult-onset dystonia. Mov. Disord. 2013, 28, 1766–1774. [Google Scholar] [CrossRef]
- Patel, N.; Jankovic, J.; Hallett, M. Sensory aspects of movement disorders. Lancet Neurol. 2014, 13, 100–112. [Google Scholar] [CrossRef]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef]
- Conte, A.; Defazio, G.; Mascia, M.; Belvisi, D.; Pantano, P.; Berardelli, A. Advances in the pathophysiology of adult-onset focal dystonias: Recent neurophysiological and neuroimaging evidence. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Currà, A.; Berardelli, A. Do the unintended actions of botulinum toxin at distant sites have clinical implications? Neurology 2009, 72, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Currà, A.; Buccolieri, A.; Suppa, A.; Abbruzzese, G.; Berardelli, A. Botulinum toxin changes intrafusal feedback in dystonia: A study with the tonic vibration reflex. Mov. Disord. 2006, 21, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M.; GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke upper limb spasticity. Curr. Med. Res. Opin. 2010, 26, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Gilio, F.; Currà, A.; Lorenzano, C.; Modugno, N.; Manfredi, M.; Berardelli, A. Effects of botulinum toxin type A on intracortical inhibition in patients with dystonia. Ann. Neurol. 2000, 48, 20–26. [Google Scholar] [CrossRef]
- Scontrini, A.; Conte, A.; Fabbrini, G.; Colosimo, C.; Stasio, F.D.; Ferrazzano, G.; Berardelli, A. Somatosensory temporal discrimination tested in patients receiving botulinum toxin injection for cervical dystonia. Mov. Disord. 2011, 26, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Ozdemir, R.A.; Perez, M.A. Gating of Sensory Input at Subcortical and Cortical Levels during Grasping in Humans. J. Neurosci. 2018, 38, 7237–7247. [Google Scholar] [CrossRef] [PubMed]
- Belvisi, D.; Conte, A.; Cortese, F.N.; Tartaglia, M.; Manzo, N.; Li Voti, P.; Suppa, A.; Berardelli, A. Voluntary Movement Takes Shape: The Link Between Movement Focusing and Sensory Input Gating. Front. Hum. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Thickbroom, G.W.; Byrnes, M.L.; Stell, R.; Mastaglia, F.L. Reversible reorganisation of the motor cortical representation of the hand in cervical dystonia. Mov. Disord. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Byrnes, M.L.; Thickbroom, G.W.; Wilson, S.A.; Sacco, P.; Shipman, J.M.; Stell, R.; Mastaglia, F.L. The corticomotor representation of upper limb muscles in writer’s cramp and changes following botulinum toxin injection. Brain 1998, 121, 977–988. [Google Scholar] [CrossRef]
- Kanovský, P.; Streitová, H.; Dufek, J.; Znojil, V.; Daniel, P.; Rektor, I. Change in lateralization of the P22/N30 cortical component of median nerve somatosensory evoked potentials in patients with cervical dystonia after successful treatment with botulinum toxin A. Mov. Disord. 1998, 13, 108–117. [Google Scholar] [CrossRef]
- Khosravani, S.; Buchanan, J.; Johnson, M.D.; Konczak, J. Effect of Neck Botulinum Neurotoxin Injection on Proprioception and Somatosensory-Motor Cortical Processing in Cervical Dystonia. Neurorehabil. Neural. Repair. 2020, 1545968320905799. [Google Scholar] [CrossRef] [PubMed]
- Brodoehl, S.; Wagner, F.; Prell, T.; Klingner, C.; Witte, O.W.; Günther, A. Cause or effect: Altered brain and network activity in cervical dystonia is partially normalized by botulinum toxin treatment. Neuroimage. Clin. 2019, 22, 101792. [Google Scholar] [CrossRef] [PubMed]
- Opavský, R.; Hluštík, P.; Otruba, P.; Kaňovský, P. Somatosensory cortical activation in cervical dystonia and its modulation with botulinum toxin: An fMRI study. Int. J. Neurosci. 2012, 122, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Delnooz, C.C.S.; Pasman, J.W.; Beckmann, C.F.; van de Warrenburg, B.P.C. Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS ONE 2013, 8, e62877. [Google Scholar] [CrossRef] [PubMed]
- Pelosin, E.; Bove, M.; Marinelli, L.; Abbruzzese, G.; Ghilardi, M.F. Cervical dystonia affects aimed movements of nondystonic segments. Mov. Disord. 2009, 24, 1955–1961. [Google Scholar] [CrossRef]
- Belvisi, D.; Suppa, A.; Marsili, L.; Di Stasio, F.; Parvez, A.K.; Agostino, R.; Fabbrini, G.; Berardelli, A. Abnormal experimentally- and behaviorally-induced LTP-like plasticity in focal hand dystonia. Exp. Neurol. 2013, 240, 64–74. [Google Scholar] [CrossRef]
- Bologna, M.; Paparella, G.; Fabbrini, A.; Leodori, G.; Rocchi, L.; Hallett, M.; Berardelli, A. Effects of cerebellar theta-burst stimulation on arm and neck movement kinematics in patients with focal dystonia. Clin. Neurophysiol. 2016, 127, 3472–3479. [Google Scholar] [CrossRef]
- Delrobaei, M.; Rahimi, F.; Jackman, M.E.; Atashzar, S.F.; Shahbazi, M.; Patel, R.; Jog, M. Kinematic and kinetic assessment of upper limb movements in patients with writer’s cramp. J. Neuroeng. Rehabil. 2016, 13, 15. [Google Scholar] [CrossRef]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.C.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef]
- Defazio, G.; Hallett, M.; Jinnah, H.A.; Stebbins, G.T.; Gigante, A.F.; Ferrazzano, G.; Conte, A.; Fabbrini, G.; Berardelli, A. Development and validation of a clinical scale for rating the severity of blepharospasm. Mov. Disord. 2015, 30, 525–530. [Google Scholar] [CrossRef]
- Conte, A.; Modugno, N.; Lena, F.; Dispenza, S.; Gandolfi, B.; Iezzi, E.; Fabbrini, G.; Berardelli, A. Subthalamic nucleus stimulation and somatosensory temporal discrimination in Parkinson’s disease. Brain 2010, 133, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Ferrazzano, G.; Manzo, N.; Leodori, G.; Fabbrini, G.; Fasano, A.; Tinazzi, M.; Berardelli, A. Somatosensory temporal discrimination in essential tremor and isolated head and voice tremors. Mov. Disord. 2015, 30, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; McGovern, E.M.; Narasimham, S.; Beck, R.; Killian, O.; O’Riordan, S.; Reilly, R.B.; Hutchinson, M. Temporal Discrimination: Mechanisms and Relevance to Adult-Onset Dystonia. Front. Neurol. 2017, 8, 625. [Google Scholar] [CrossRef] [PubMed]
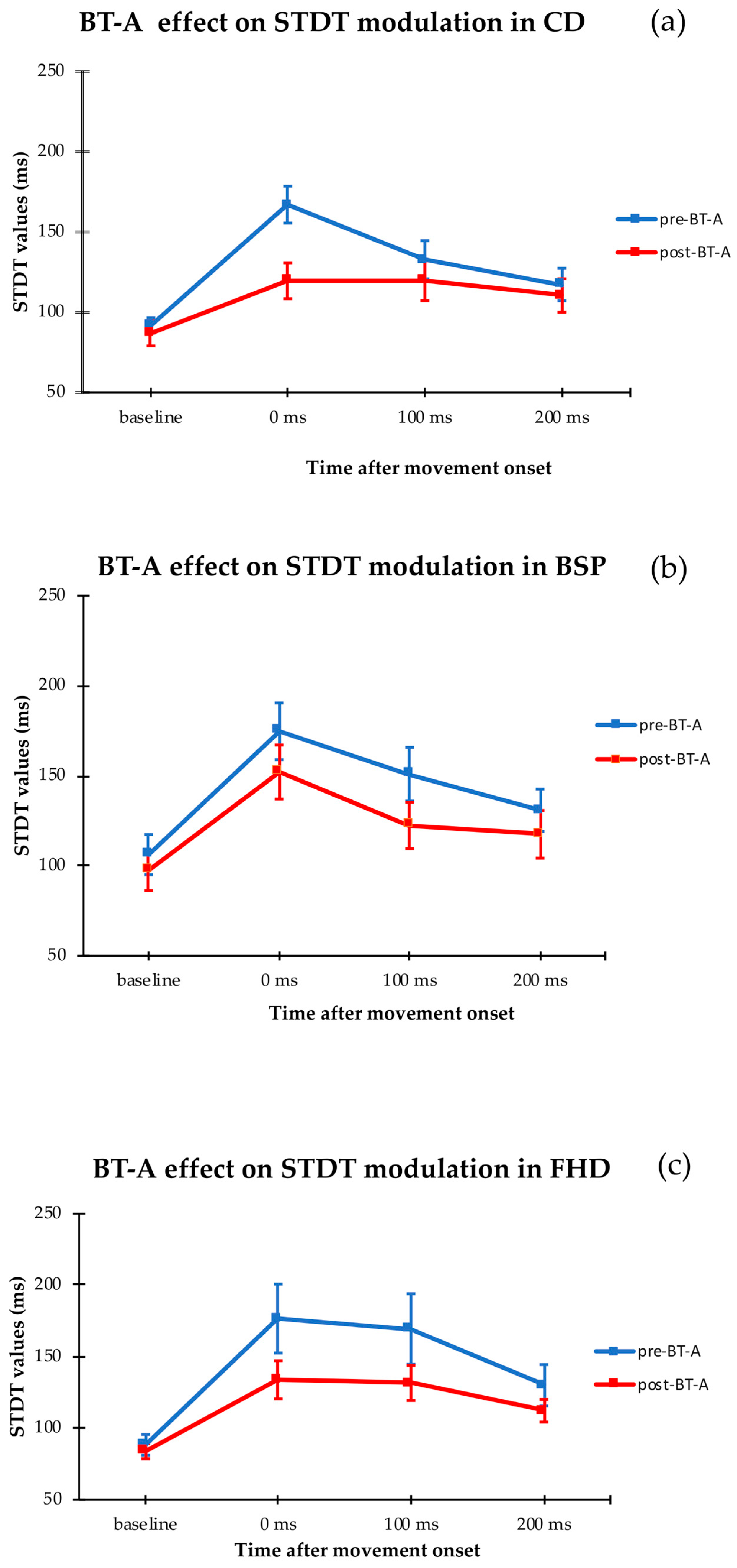
| Subjects | Pre-BT-A | Post-BT-A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rest | 0 ms | 100 ms | 200 ms | Average % Change | Rest | 0 ms | 100 ms | 200 ms | Average % Change | |
| CD | 91 ± 5 | 167 ± 11 | 132 ± 12 | 118 ± 10 | 140 ± 9 | 86 ± 8 | 120 ± 11 | 119 ± 12 | 111 ± 11 | 128 ± 10 |
| FHD | 88 ± 8 | 176 ± 24 | 169 ± 25 | 130 ± 14 | 164 ± 10 | 84 ± 5 | 134 ± 13 | 131 ± 12 | 112 ± 8 | 141 ± 7 |
| BPS | 106 ± 11 | 175 ± 15 | 151 ± 14 | 130 ± 11 | 132 ± 8 | 97 ± 11 | 161 ± 15 | 122 ± 13 | 118 ± 13 | 127 ± 4 |
| HS | 74 ± 4 | 107 ± 5 | 88 ± 5 | 78 ± 4 | 118 ± 5 | - | - | - | - | - |
| PZ | Gender | Age (yrs) | Disease Duration (yrs) | BT-Atreatment Duration (yrs) | Muscles Injected with BT-A | TWSTRS Off | TWSTRS On |
|---|---|---|---|---|---|---|---|
| 1 | M | 45 | 26 | 25 | SPL | 8 | 8 |
| 2 | F | 58 | 16 | 11 | SPL | 14 | 12 |
| 3 | F | 45 | 9 | 3 | SPL | 13 | 10 |
| 4 | F | 36 | 4 | 4 | SCM, SPL | 14 | 8 |
| 5 | F | 46 | 2 | 2 | SCM, SPL, TPZ | 16 | 12 |
| 6 | F | 45 | 4 | 3 | SCM, SPL | 13 | 9 |
| 7 | F | 55 | 13 | 12 | SCM, TPZ | 7 | 6 |
| 8 | F | 60 | 11 | 8 | SCM, SPL | 10 | 10 |
| 9 | F | 65 | 10 | 4 | SPL | 14 | 10 |
| 10 | F | 60 | 20 | 11 | SPL | 14 | 12 |
| 11 | F | 57 | 20 | 13 | SPL | 7 | 7 |
| 12 | F | 64 | 5 | 3 | SPL | 14 | 10 |
| 13 | M | 56 | 4 | 5 | SPL | 13 | 9 |
| 14 | F | 57 | 3 | 1 | SCM, SPL, TPZ | 15 | 12 |
| Avg | F/M = 12/2 | 54 ± 9 | 11 ± 8 | 8 ± 6 | 12 ± 3 | 9 ± 2 |
| Pz | Gender | Age (yrs) | Disease Duration (yrs) | BT-A Treatment Duration (yrs) | Muscles Injected with BT-A | BSRS Off | BSRS On |
|---|---|---|---|---|---|---|---|
| 1 | F | 71 | 8 | 8 | OO | 13 | 12 |
| 2 | F | 68 | 10 | 3 | OO | 8 | 6 |
| 3 | F | 65 | 8 | 7 | OO, Pretarsal | 6 | 2 |
| 4 | F | 54 | 4 | 3 | OO, Pretarsal | 13 | 12 |
| 5 | F | 47 | 8 | 4 | OO | 8 | 6 |
| 6 | F | 50 | 9 | 9 | OO | 6 | 2 |
| 7 | M | 66 | 9 | 4 | OO, Pretarsal | 14 | 12 |
| 8 | F | 55 | 8 | 3 | OO, Pretarsal | 6 | 6 |
| 9 | M | 54 | 5 | 3 | OO | 8 | 6 |
| 10 | M | 64 | 13 | 10 | OO, Pretarsal | 8 | 8 |
| 11 | M | 60 | 9 | 6 | OO, Pretarsal | 11 | 9 |
| Avg | F/M = 7/4 | 59 ± 8 | 8 ± 2 | 5 ± 3 | 9 ± 3 | 7 ± 3 |
| Pz | Gender | Age (ys) | Side Affected | Duration Disease (yrs) | BT-A Treatment Duration (yrs) | Muscles Injected with BT-A | BFMOff | BFM On |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 52 | R | 2 | 1 | FCR | 4 | 3 |
| 2 | M | 49 | L | 25 | 5 | FDS | 2 | 2 |
| 3 | M | 58 | R | 22 | 18 | FCR, FCU | 6 | 4 |
| 4 | M | 59 | R | 23 | 9 | FDP, FDS | 4 | 2 |
| 5 | F | 66 | R | 25 | 23 | FCR, FCU | 5 | 4 |
| 6 | F | 40 | L | 1 | <1 | FCR, FCU | 2 | 2 |
| 7 | F | 50 | L | 8 | 5 | FDP, FDS | 5 | 4 |
| 8 | F | 18 | R | 1 | <1 | FCU | 2 | 2 |
| 9 | F | 22 | R | 1 | <1 | FCR, FCU | 12 | 10 |
| 10 | F | 52 | R | 8 | 8 | ECR | 6 | 4 |
| Avg | F/M = 6/4 | 47 ± 16 | R/L = 7/10 | 12 ± 11 | 5 ± 3 | 4 ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bartolo, M.I.; Manzo, N.; Ferrazzano, G.; Baione, V.; Belvisi, D.; Fabbrini, G.; Berardelli, A.; Conte, A. Botulinum Toxin Effects on Sensorimotor Integration in Focal Dystonias. Toxins 2020, 12, 277. https://doi.org/10.3390/toxins12050277
De Bartolo MI, Manzo N, Ferrazzano G, Baione V, Belvisi D, Fabbrini G, Berardelli A, Conte A. Botulinum Toxin Effects on Sensorimotor Integration in Focal Dystonias. Toxins. 2020; 12(5):277. https://doi.org/10.3390/toxins12050277
Chicago/Turabian StyleDe Bartolo, Maria Ilenia, Nicoletta Manzo, Gina Ferrazzano, Viola Baione, Daniele Belvisi, Giovanni Fabbrini, Alfredo Berardelli, and Antonella Conte. 2020. "Botulinum Toxin Effects on Sensorimotor Integration in Focal Dystonias" Toxins 12, no. 5: 277. https://doi.org/10.3390/toxins12050277
APA StyleDe Bartolo, M. I., Manzo, N., Ferrazzano, G., Baione, V., Belvisi, D., Fabbrini, G., Berardelli, A., & Conte, A. (2020). Botulinum Toxin Effects on Sensorimotor Integration in Focal Dystonias. Toxins, 12(5), 277. https://doi.org/10.3390/toxins12050277




