Oral Charcoal Adsorbents Attenuate Neointima Formation of Arteriovenous Fistulas
Abstract
1. Introduction
2. Results
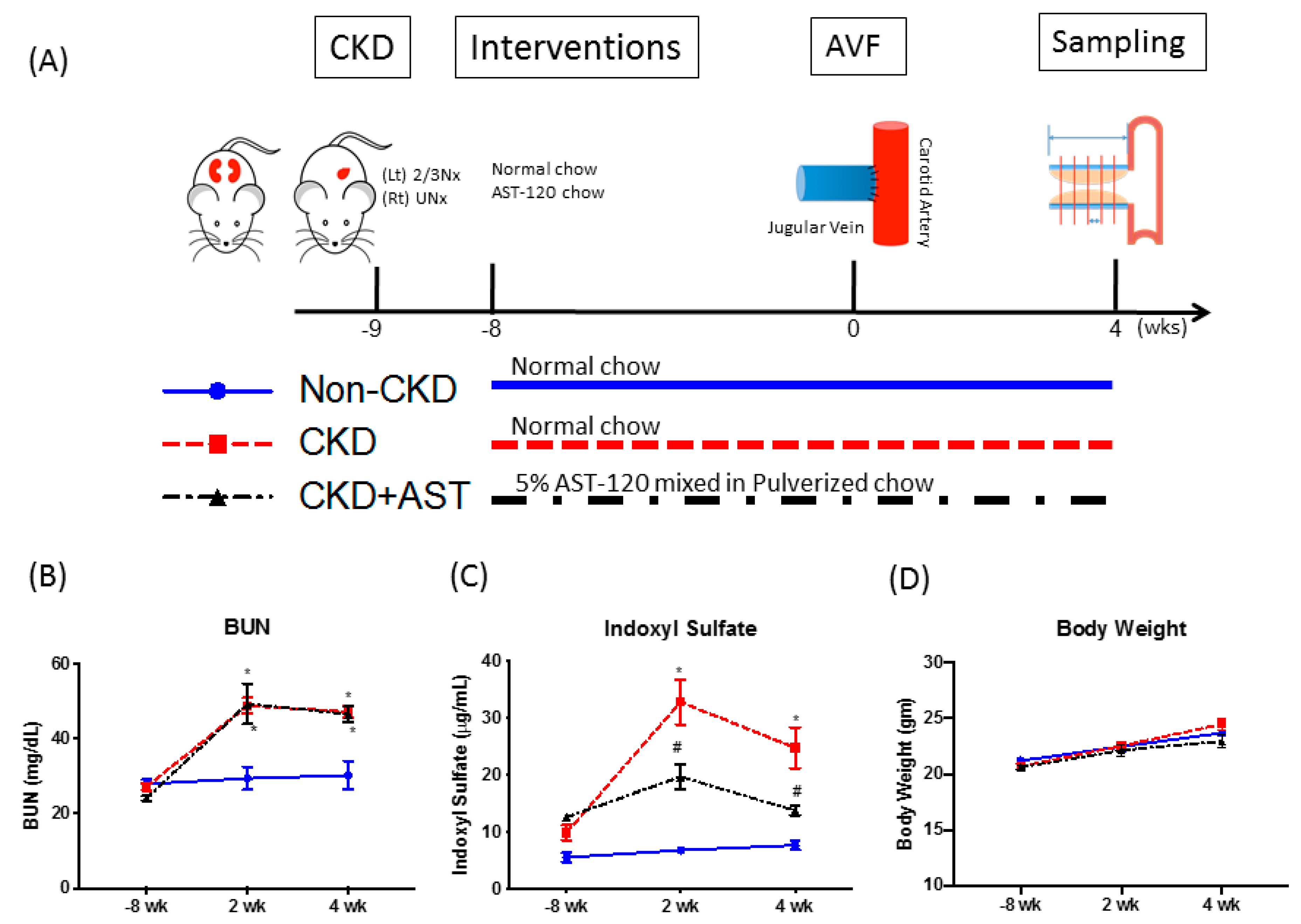
2.1. CKD Mice Had Elevated Serum IS Levels Which Was Reversed Upon AST-120 Administration
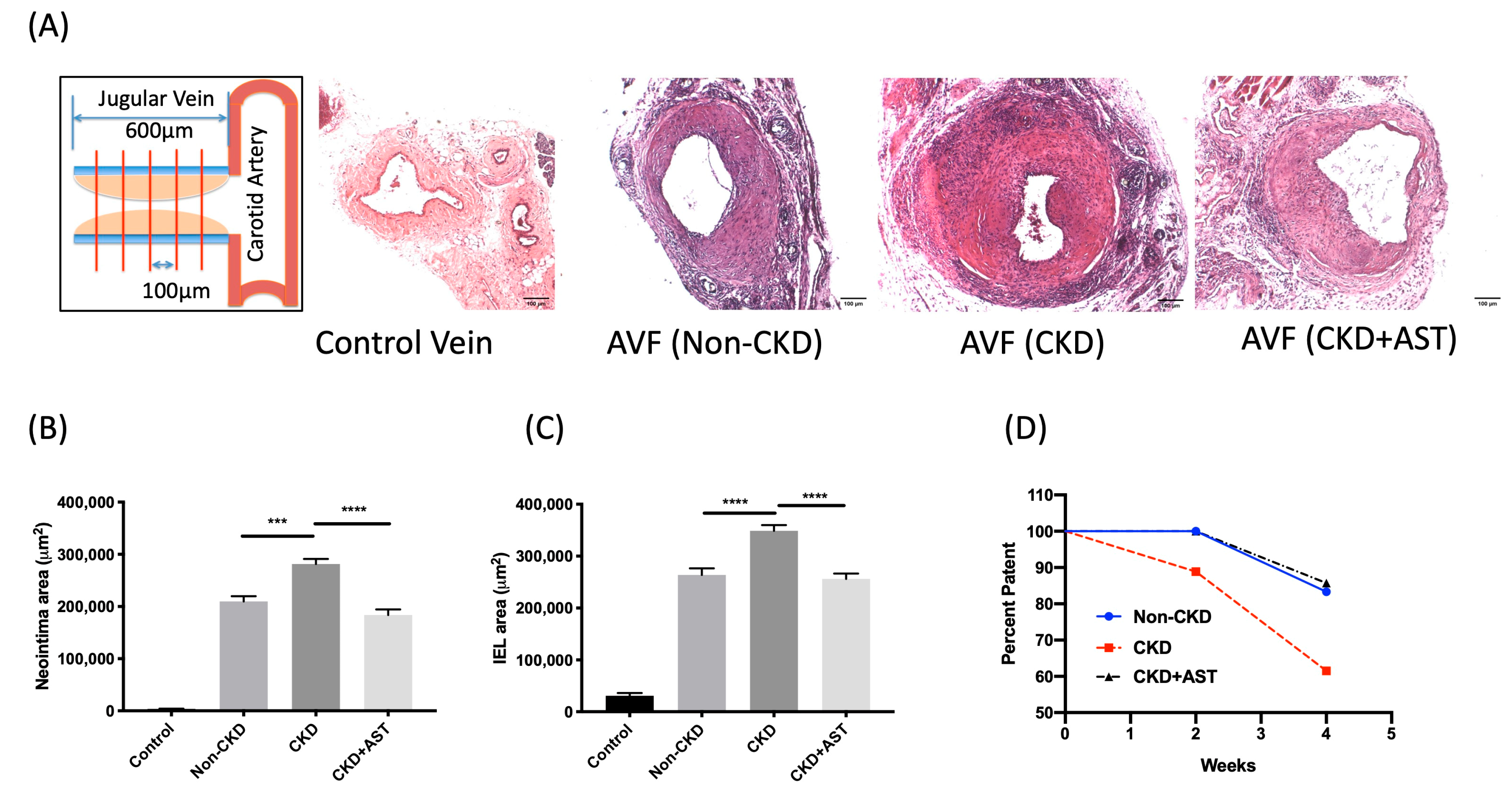
2.2. Morphometric Analysis of the Outflow Vein Segment of AV Fistulas
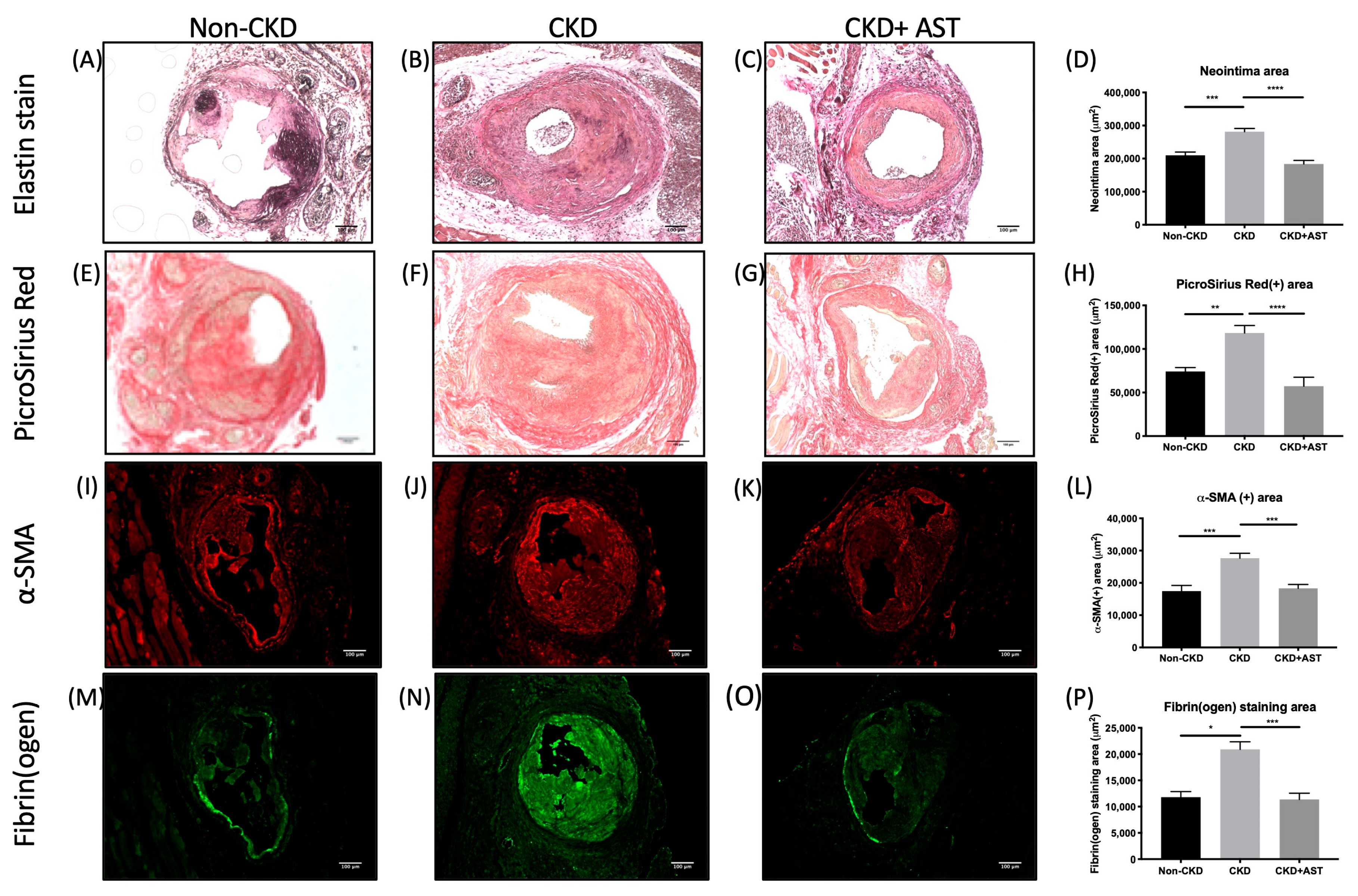
2.3. AST-120 Reduced the Smooth Muscle Cell, Collagen, and Thrombus Contents in the Neointima
2.4. The Effect of AST-120 on Gene Expression in AV Fistula of CKD Mice
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Experiments
5.2. CKD Mice, Interventions, AVF Creation, and Sampling (Figure 1A)
5.3. Determination of Serum Blood Urea Nitrogen and IS Levels
5.4. Quantitative Morphometric Analysis
5.5. Immunohistochemistry and Immunofluorescence Stain
5.6. RNA Isolation and Quantitative PCR Analysis
5.7. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheung, A.K.; Imrey, P.B.; Alpers, C.E.; Robbin, M.L.; Radeva, M.; Larive, B.; Shiu, Y.-T.; Allon, M.; Dember, L.M.; Greene, T.; et al. Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the hemodialysis fistula maturation study. J. Am. Soc. Nephrol. 2017, 28, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Rena Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; U.S. Renal Data System: Bethesda, MD, USA, 2010.
- Kokubo, T.; Ishikawa, N.; Uchida, H.; Chasnoff, S.E.; Xie, X.; Mathew, S.; Hruska, K.A.; Choi, E.T. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J. Am. Soc. Nephrol. 2009, 20, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.; Kokozidou, M.; Heiss, C.; Kranz, J.; Kessler, T.; Paulus, N.; Kruger, T.; Jacobs, M.J.; Lente, C.; Koeppel, T.A. Chronic kidney disease aggravates arteriovenous fistula damage in rats. Kidney Int. 2010, 78, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Mair, R.D.; Sirich, T.L.; Plummer, N.S.; Meyer, T.W. Characteristics of colon-derived uremic solutes. Clin. J. Am. Soc. Nephrol. 2018, 13, 1398–1404. [Google Scholar] [CrossRef]
- Hung, S.C.; Kuo, K.L.; Wu, C.C.; Tarng, D.C. Indoxyl sulfate: A novel cardiovascular risk factor in chronic kidney disease. J. Am. Heart Assoc. 2017, 6, e005022. [Google Scholar] [CrossRef]
- Akiyama, Y.; Takeuchi, Y.; Kikuchi, K.; Mishima, E.; Yamamoto, Y.; Suzuki, C.; Toyohara, T.; Suzuki, T.; Hozawa, A.; Ito, S.; et al. A metabolomic approach to clarifying the effect of ast-120 on 5/6 nephrectomized rats by capillary electrophoresis with mass spectrometry (ce-ms). Toxins 2012, 4, 1309–1322. [Google Scholar] [CrossRef]
- Yamamoto, S.; Zuo, Y.; Ma, J.; Yancey, P.G.; Hunley, T.E.; Motojima, M.; Fogo, A.B.; Linton, M.F.; Fazio, S.; Ichikawa, I.; et al. Oral activated charcoal adsorbent (ast-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein e-deficient mice. Nephrol. Dial. Transpl. 2011, 26, 2491–2497. [Google Scholar] [CrossRef]
- Six, I.; Gross, P.; Rémond, M.C.; Chillon, J.M.; Poirot, S.; Drueke, T.B.; Massy, Z.A. Deleterious vascular effects of indoxyl sulfate and reversal by oral adsorbent ast-120. Atherosclerosis 2015, 243, 248–256. [Google Scholar] [CrossRef]
- Ito, S.; Higuchi, Y.; Yagi, Y.; Nishijima, F.; Yamato, H.; Ishii, H.; Osaka, M.; Yoshida, M. Reduction of indoxyl sulfate by ast-120 attenuates monocyte inflammation related to chronic kidney disease. J. Leukoc. Biol. 2013, 93, 837–845. [Google Scholar] [CrossRef]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Vesey, D.A.; Coombes, J.S.; Weston, K.S.; Hawley, C.M.; McWhinney, B.C.; Ungerer, J.P.; et al. Protein-bound uremic toxins, inflammation and oxidative stress: A cross-sectional study in stage 3-4 chronic kidney disease. Arch. Med. Res. 2014, 45, 309–317. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Singh, G.; Jonah, G.; Sikalas, N.; Labropoulos, N. Mechanical and biochemical role of fibrin within a venous thrombus. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Bouzeghrane, F.; Zhang, X.; Gevry, G.; Raymond, J. Deep vein thrombosis resolution is impaired in diet-induced type 2 diabetic mice. J. Vasc. Surg. 2008, 48, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, A.; Remuzzi, A.; Franzoni, M.; Misra, S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. 2016, 89, 303–316. [Google Scholar] [CrossRef]
- Wasse, H.; Huang, R.; Naqvi, N.; Smith, E.; Wang, D.; Husain, A. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from avf creation in ckd patients. J. Vasc. Access 2012, 13, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Chen, T.-Y.; Hsieh, M.-Y.; Lin, L.; Yang, C.-W.; Chuang, S.-Y.; Tarng, D.-C. Monocyte chemoattractant protein-1 levels and postangioplasty restenosis of arteriovenous fistulas. Clin. J. Am. Soc. Nephrol. 2017, 12, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Shang, F.; Wang, S.-C.; Hsu, C.-Y.; Miao, Y.; Martin, M.; Yin, Y.; Wu, C.-C.; Wang, Y.-T.; Wu, G.; Chien, S.; et al. Microrna-92a mediates endothelial dysfunction in ckd. J. Am. Soc. Nephrol. 2017, 28, 3251–3261. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, P.H.; Lai, C.L.; Leu, H.B.; Chen, J.W.; Lin, S.J. The impact of endothelial progenitor cells on restenosis after percutaneous angioplasty of hemodialysis vascular access. PLoS ONE 2014, 9, e101058. [Google Scholar] [CrossRef]
- Hsieh, M.-Y.; Lee, C.-K.; Lo, C.-M.; Chen, C.-H.; Chuang, S.-Y.; Wu, C.-C. Temporal distribution and biological determinants of thrombotic events after interventions for dialysis vascular access. Sci. Rep. 2019, 9, 10720. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Dou, L.; Sabatier, F.; Calaf, R.; Cerini, C.; Robert, S.; Camoin-Jau, L.; Charpiot, P.; Argiles, A.; Dignat-George, F.; et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J. Thromb. Haemost. 2009, 7, 1576–1584. [Google Scholar] [CrossRef]
- Madsen, M.; Aarup, A.; Albinsson, S.; Hartvigsen, K.; Sørensen, C.M.; Turczynska, K.; Nielsen, L.B.; Pedersen, T.X. Uremia modulates the phenotype of aortic smooth muscle cells. Atherosclerosis 2017, 257, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Monroy, M.A.; Fang, J.; Li, S.; Ferrer, L.; Birkenbach, M.P.; Lee, I.J.; Wang, H.; Yang, X.-F.; Choi, E.T. Chronic kidney disease alters vascular smooth muscle cell phenotype. Front. Biosci. 2015, 20, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Aarup, A.; Nielsen, C.H.; Bisgaard, L.S.; Bot, I.; El-Ali, H.H.; Kjaer, A.; Nielsen, L.B.; Pedersen, T.X. Uremia does not affect neointima formation in mice. Sci. Rep. 2017, 7, 6496. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Kompa, A.R.; Manabe, M.; Wang, B.H.; Langham, R.G.; Nishijima, F.; Kelly, D.J.; Krum, H. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS ONE 2012, 7, e41281. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Du, C.; Wang, X.; Li, F.; Xu, Y.; Wang, S.; Chen, S.; Chen, F.; Shen, M.; Chen, M.; et al. Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease–associated thrombosis in mice. Blood 2017, 129, 2667–2679. [Google Scholar] [CrossRef]
- Shivanna, S.; Kolandaivelu, K.; Shashar, M.; Belghasim, M.; Al-Rabadi, L.; Balcells, M.; Zhang, A.; Weinberg, J.; Francis, J.; Pollastri, M.P.; et al. The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J. Am. Soc. Nephrol. 2016, 27, 189–201. [Google Scholar] [CrossRef]
- Chitalia, V.C.; Shivanna, S.; Martorell, J.; Balcells, M.; Bosch, I.; Kolandaivelu, K.; Edelman, E.R. Uremic serum and solutes increase post–vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation 2013, 127, 365–376. [Google Scholar] [CrossRef]
- Wu, C.-C.; Hsieh, M.-Y.; Hung, S.-C.; Kuo, K.-L.; Tsai, T.-H.; Lai, C.-L.; Chen, J.-W.; Lin, S.-J.; Huang, P.-H.; Tarng, D.-C. Serum indoxyl sulfate associates with postangioplasty thrombosis of dialysis grafts. J. Am. Soc. Nephrol. 2016, 27, 1254–1264. [Google Scholar] [CrossRef]
- Kolachalama, V.B.; Shashar, M.; Alousi, F.; Shivanna, S.; Rijal, K.; Belghasem, M.E.; Walker, J.; Matsuura, S.; Chang, G.H.; Gibson, C.M.; et al. Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J. Am. Soc. Nephrol. 2018, 29, 1063–1072. [Google Scholar] [CrossRef]
- Chan, C.-Y.; Chen, Y.-S.; Ma, M.-C.; Chen, C.-F. Remodeling of experimental arteriovenous fistula with increased matrix metalloproteinase expression in rats. J. Vasc. Surg. 2007, 45, 804–811. [Google Scholar] [CrossRef]
- Tronc, F.; Mallat, Z.; Lehoux, S.; Wassef, M.; Esposito, B.; Tedgui, A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: Interaction with no. Arterioscler. Thrombosis Vasc. Biol. 2000, 20, E120–E126. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Young, G.H.; Hsieh, Y.T.; Chen, Y.H.; Wu, M.S.; Wu, V.C.; Lee, J.H.; Lee, C.C. Protein-bound uremic toxins induce tissue remodeling by targeting the egf receptor. J. Am. Soc. Nephrol. 2015, 26, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.C.; Da Silva, A. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database Syst. Rev. 2015, 7, Cd002786. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.C.; da Silva, A.F. Medical adjuvant treatment to improve the patency of arteriovenous fistulae and grafts: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 243–252. [Google Scholar] [CrossRef]
- Gallieni, M.; Hollenbeck, M.; Inston, N.; Kumwenda, M.; Powell, S.; Tordoir, J.; Al Shakarchi, J.; Berger, P.; Bolignano, D.; Cassidy, D.; et al. Clinical practice guideline on peri- and postoperative care of arteriovenous fistulas and grafts for haemodialysis in adults. Nephrol. Dial. Transpl. 2019, 34, ii1–ii42. [Google Scholar] [CrossRef]
- Schmidli, J.; Widmer, M.K.; Basile, C.; de Donato, G.; Gallieni, M.; Gibbons, C.P.; Haage, P.; Hamilton, G.; Hedin, U.; Kamper, L.; et al. Editor’s choice—Vascular access: 2018 clinical practice guidelines of the european society for vascular surgery (esvs). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 757–818. [Google Scholar] [CrossRef]
- Liu, W.-C.; Tomino, Y.; Lu, K.-C. Impacts of indoxyl sulfate and p-cresol sulfate on chronic kidney disease and mitigating effects of ast-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef]
- Schulman, G.; Agarwal, R.; Acharya, M.; Berl, T.; Blumenthal, S.; Kopyt, N. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of ast-120 (kremezin) in patients with moderate to severe ckd. Am. J. Kidney Dis. 2006, 47, 565–577. [Google Scholar] [CrossRef]
- Akizawa, T.; Asano, Y.; Morita, S.; Wakita, T.; Onishi, Y.; Fukuhara, S.; Gejyo, F.; Matsuo, S.; Yorioka, N.; Kurokawa, K. Effect of a carbonaceous oral adsorbent on the progression of ckd: A multicenter, randomized, controlled trial. Am. J. Kidney Dis. 2009, 54, 459–467. [Google Scholar] [CrossRef]
- Kuo, K.L.; Hung, S.C.; Lee, T.S.; Tarng, D.C. Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in ckd. J. Am. Soc. Nephrol. 2014, 25, 2596–2606. [Google Scholar] [CrossRef]
- Kang, L.; Grande, J.P.; Hillestad, M.L.; Croatt, A.J.; Barry, M.A.; Katusic, Z.S.; Nath, K.A. A new model of an arteriovenous fistula in chronic kidney disease in the mouse: Beneficial effects of upregulated heme oxygenase-1. Am. J. Physiol. Renal Physiol. 2016, 310, F466–F476. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; de Vries, M.R.; Wang, Y.; van der Vorst, J.R.; Vahrmeijer, A.L.; van Zonneveld, A.J.; Roy-Chaudhury, P.; Rabelink, T.J.; Quax, P.H.; Rotmans, J.I. Vascular remodeling and intimal hyperplasia in a novel murine model of arteriovenous fistula failure. J. Vasc. Surg. 2014, 59, 192–201. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, Y.-C.; Wu, C.-C.; Wang, S.-C.; Liou, J.-Y.; Huang, P.-H.; Tarng, D.-C. Oral Charcoal Adsorbents Attenuate Neointima Formation of Arteriovenous Fistulas. Toxins 2020, 12, 237. https://doi.org/10.3390/toxins12040237
Shih Y-C, Wu C-C, Wang S-C, Liou J-Y, Huang P-H, Tarng D-C. Oral Charcoal Adsorbents Attenuate Neointima Formation of Arteriovenous Fistulas. Toxins. 2020; 12(4):237. https://doi.org/10.3390/toxins12040237
Chicago/Turabian StyleShih, Yu-Chung, Chih-Cheng Wu, Shen-Chih Wang, Jun-Yang Liou, Po-Hsun Huang, and Der-Cherng Tarng. 2020. "Oral Charcoal Adsorbents Attenuate Neointima Formation of Arteriovenous Fistulas" Toxins 12, no. 4: 237. https://doi.org/10.3390/toxins12040237
APA StyleShih, Y.-C., Wu, C.-C., Wang, S.-C., Liou, J.-Y., Huang, P.-H., & Tarng, D.-C. (2020). Oral Charcoal Adsorbents Attenuate Neointima Formation of Arteriovenous Fistulas. Toxins, 12(4), 237. https://doi.org/10.3390/toxins12040237




