αM-Conotoxin MIIIJ Blocks Nicotinic Acetylcholine Receptors at Neuromuscular Junctions of Frog and Fish
Abstract
1. Introduction
2. Results
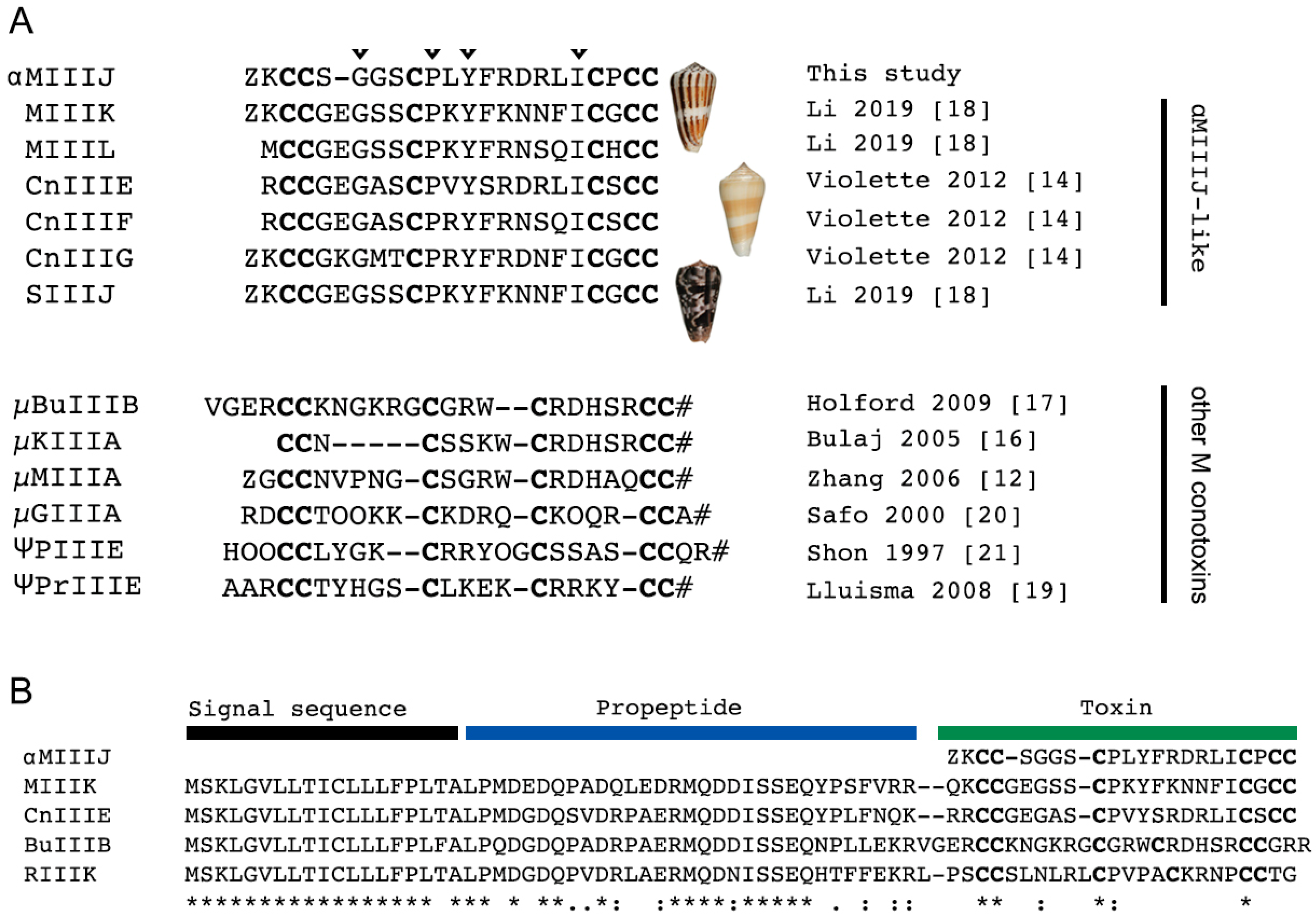
2.1. αM-MIIIJ Discovery
2.2. αM-MIIIJ Synthesis
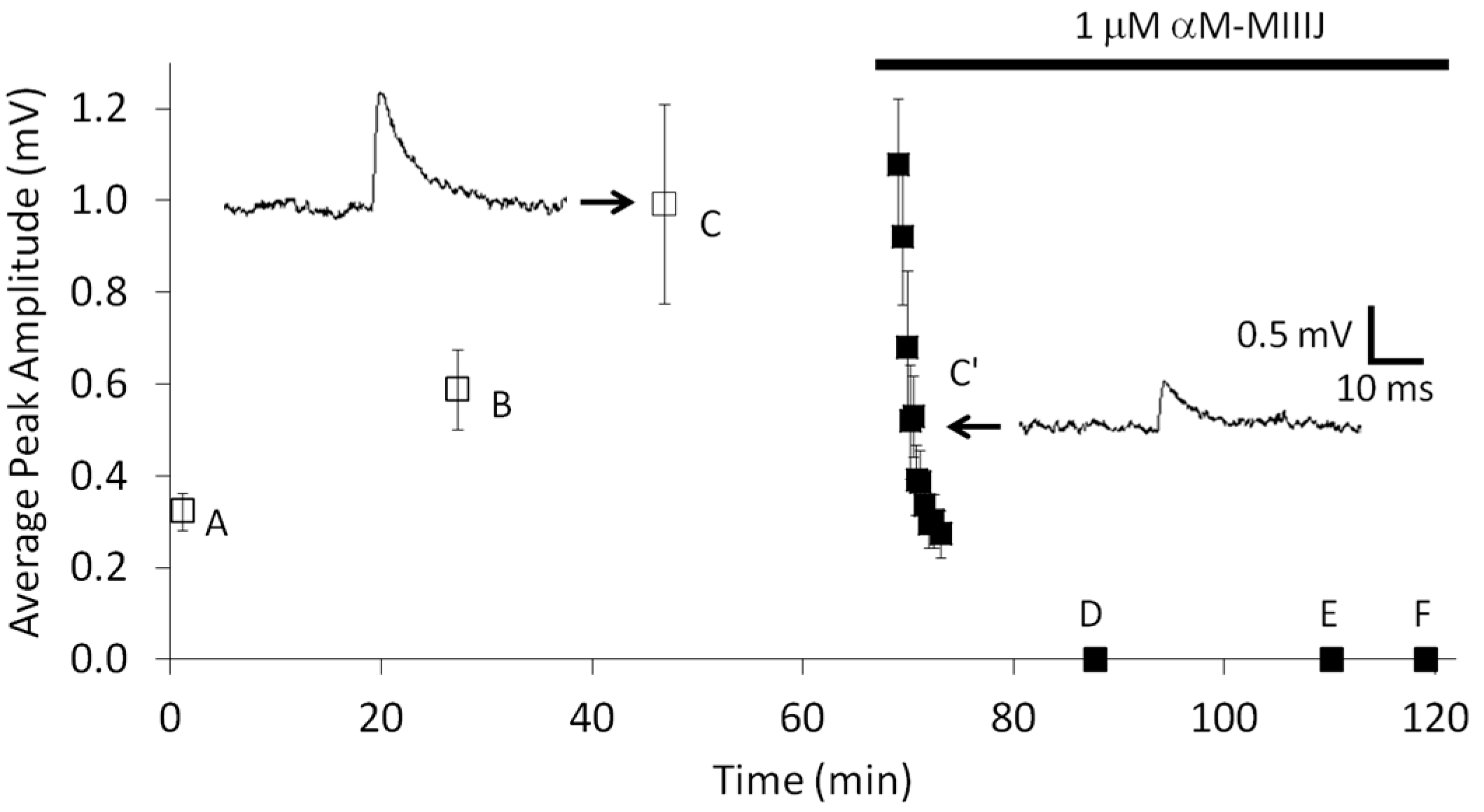
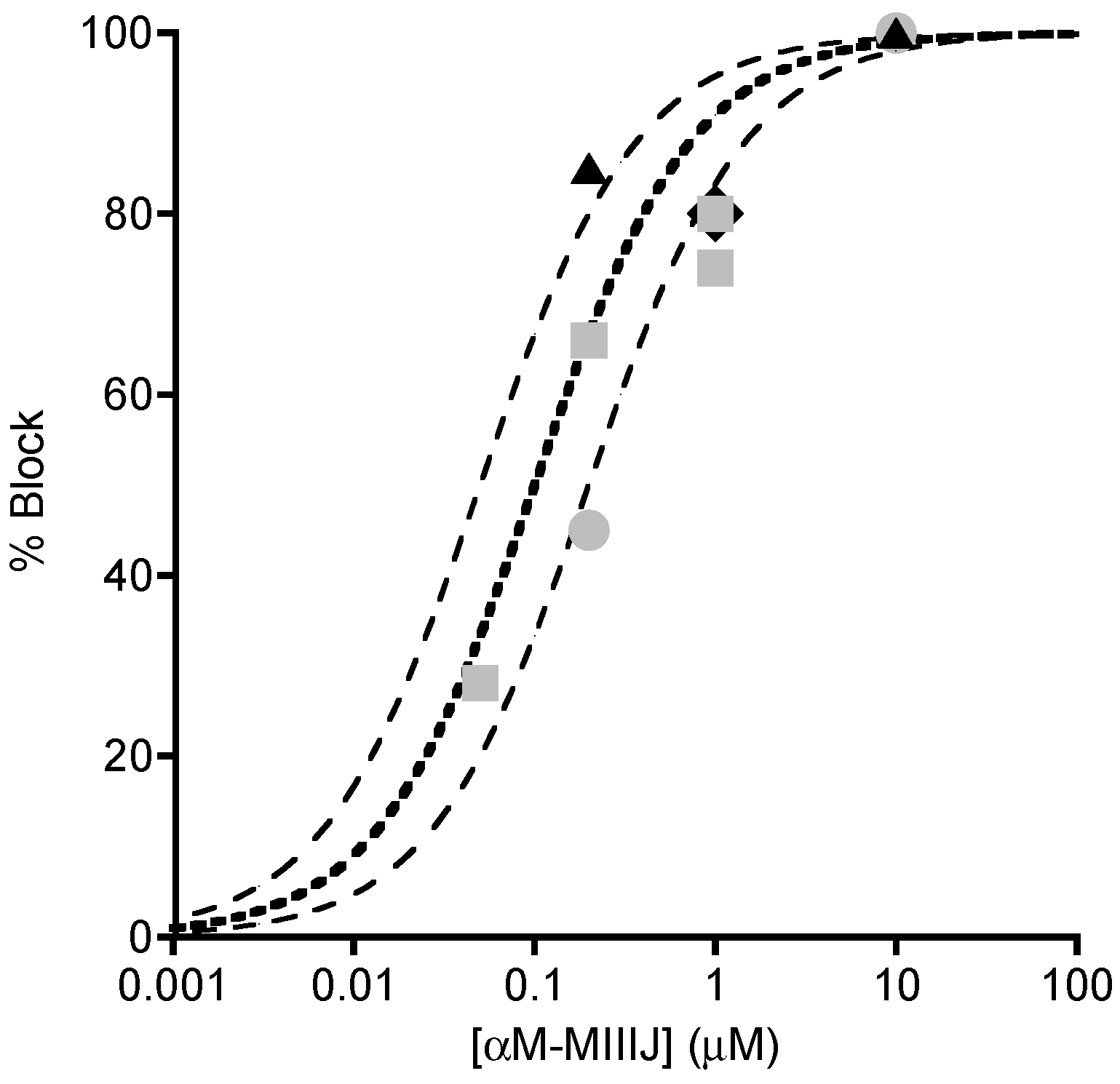
2.3. Paralytic Effects of αM-MIIIJ
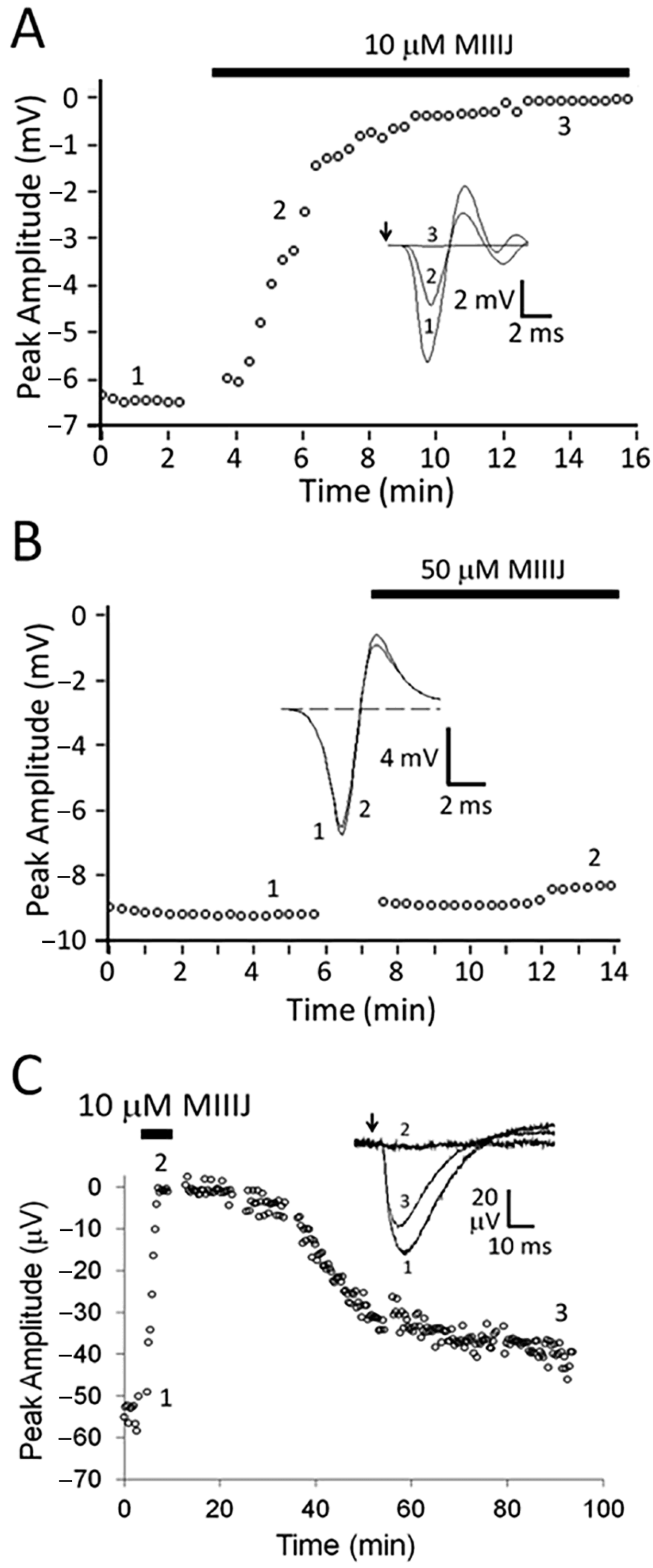
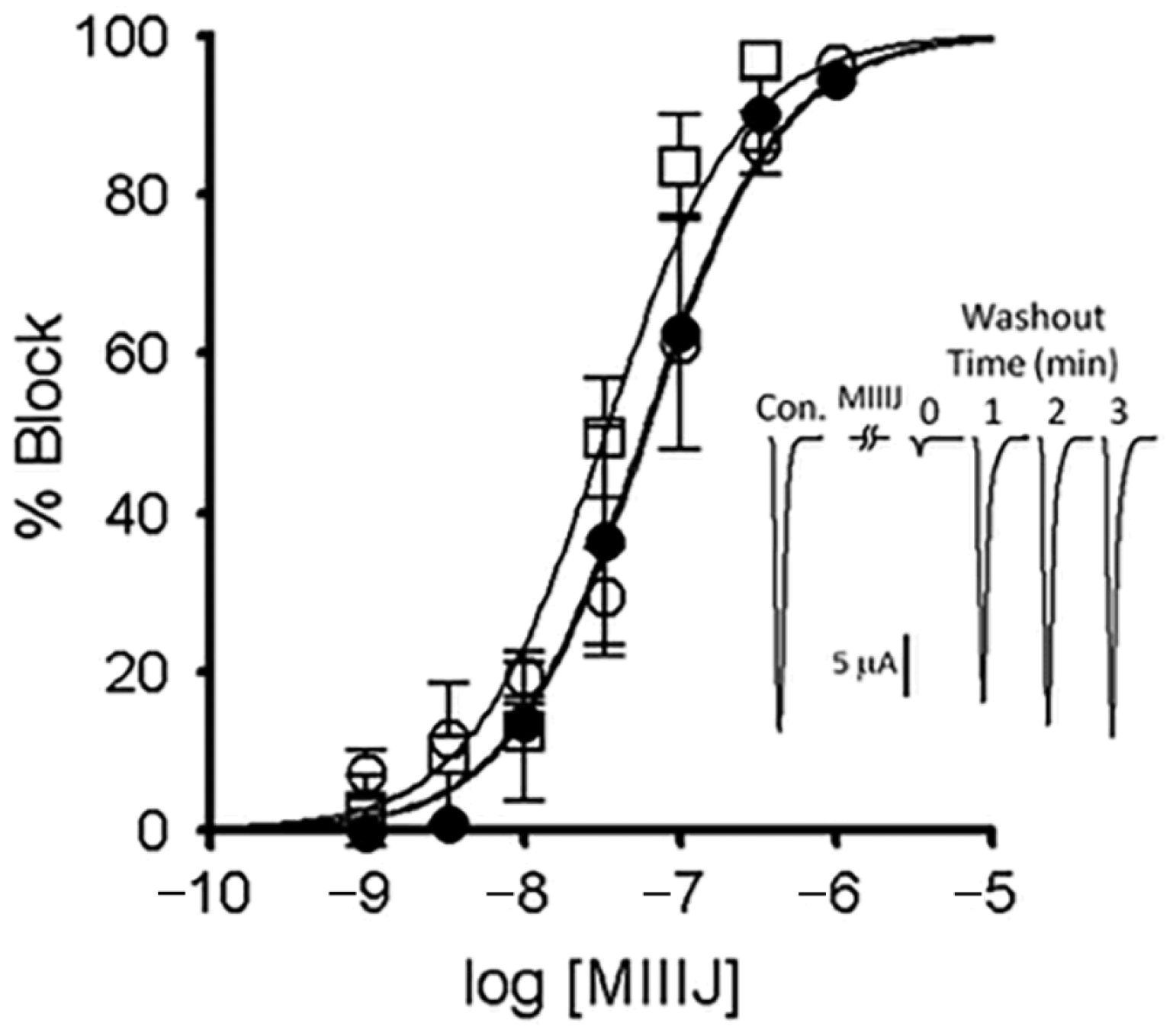
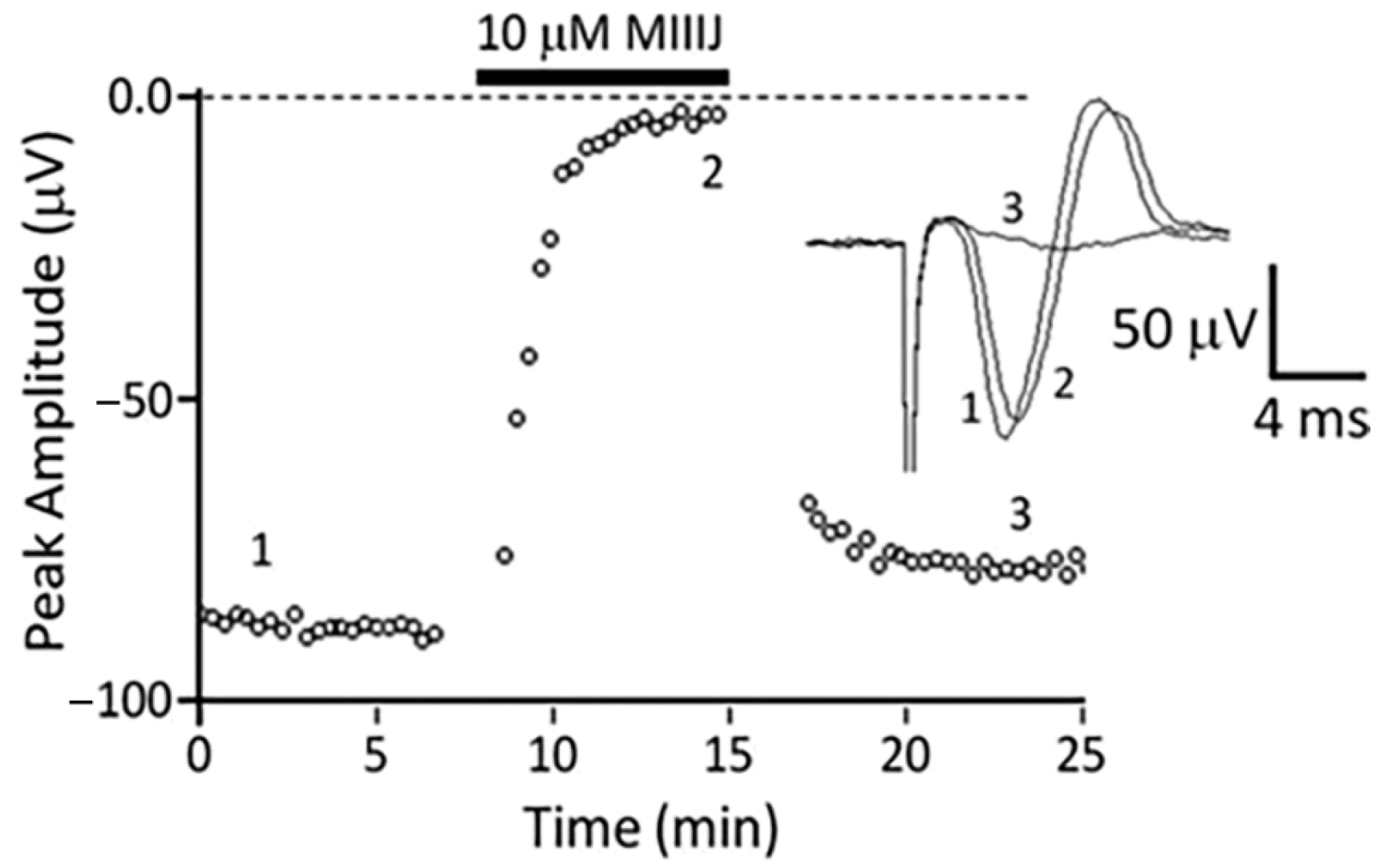
2.4. Extracellular Electrophysiology of Frog Muscle Preparations
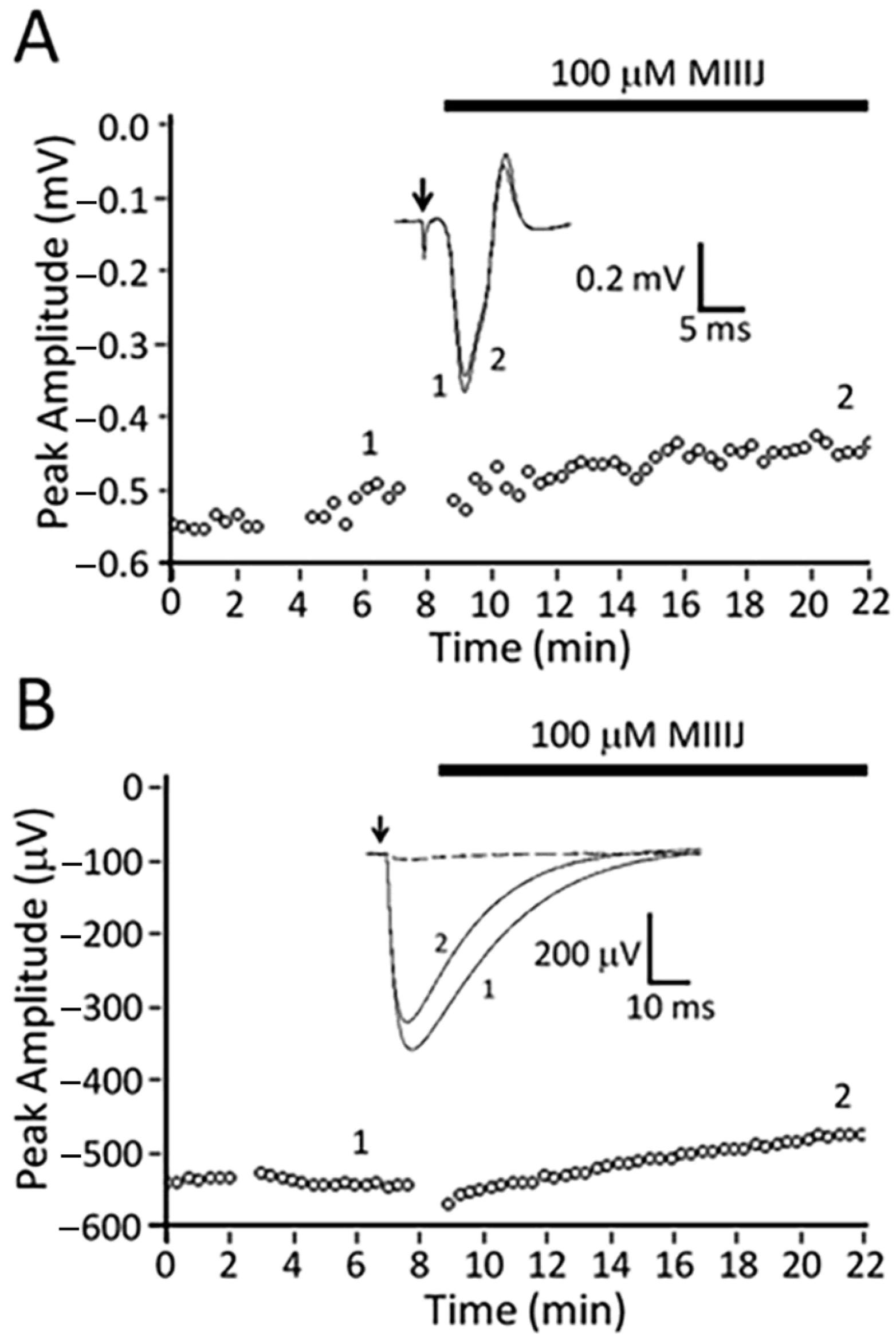
2.5. Intracellular Recording of Synaptic Activity in Goldfish Intercostal (IC) Muscle
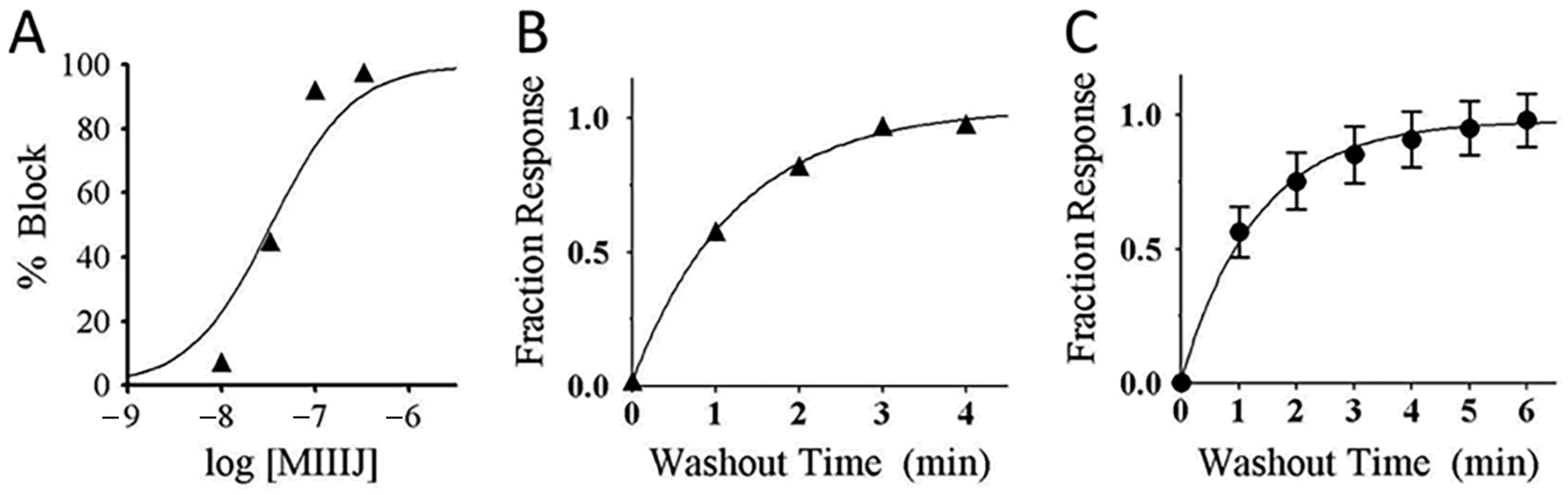
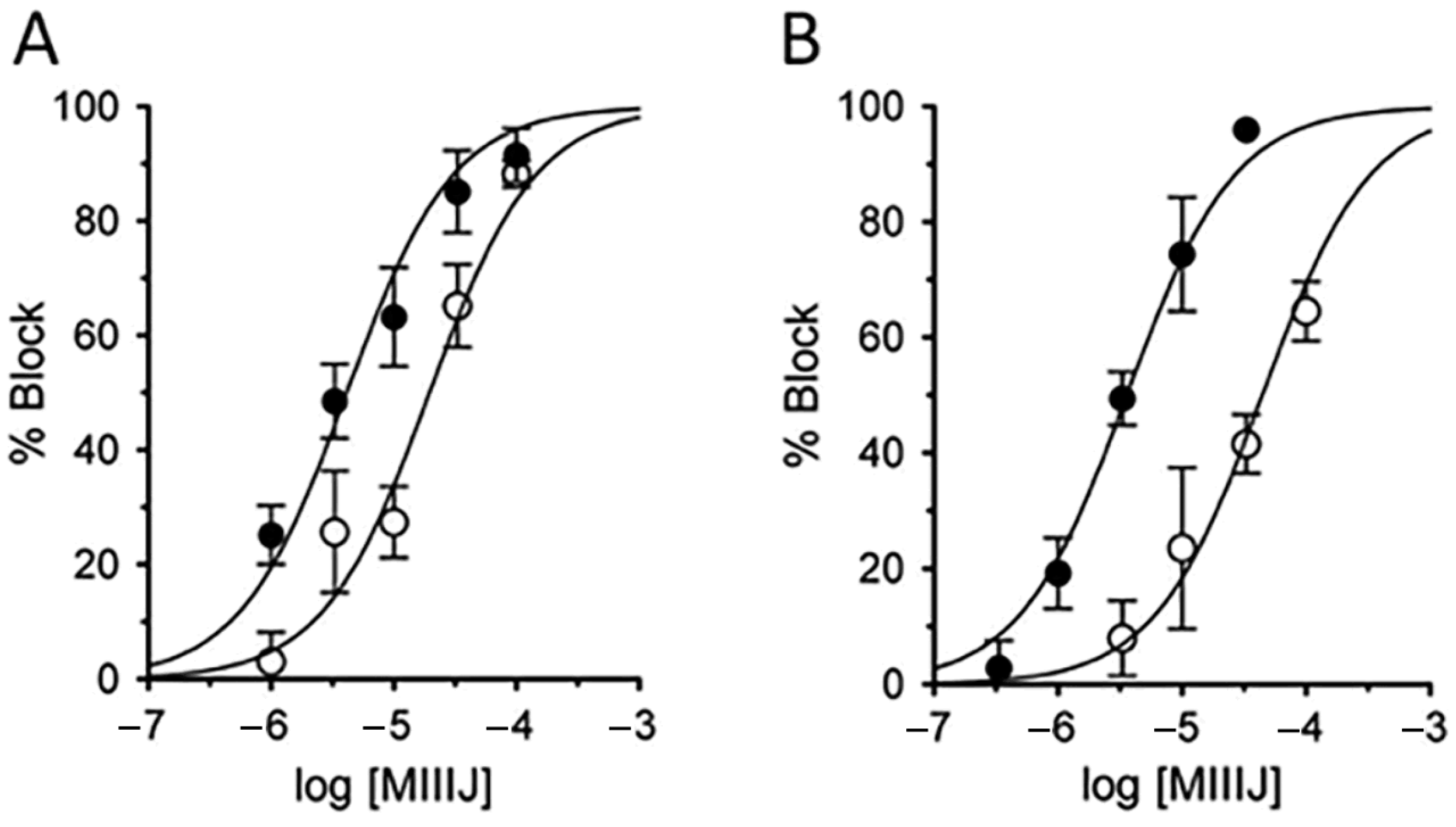
2.6. αM-MIIIJ Reversibly Blocks ACh-Gated Currents (IACh) of Zebrafish-Muscle nAChRs Exogenously Expressed in X. laevis Oocytes
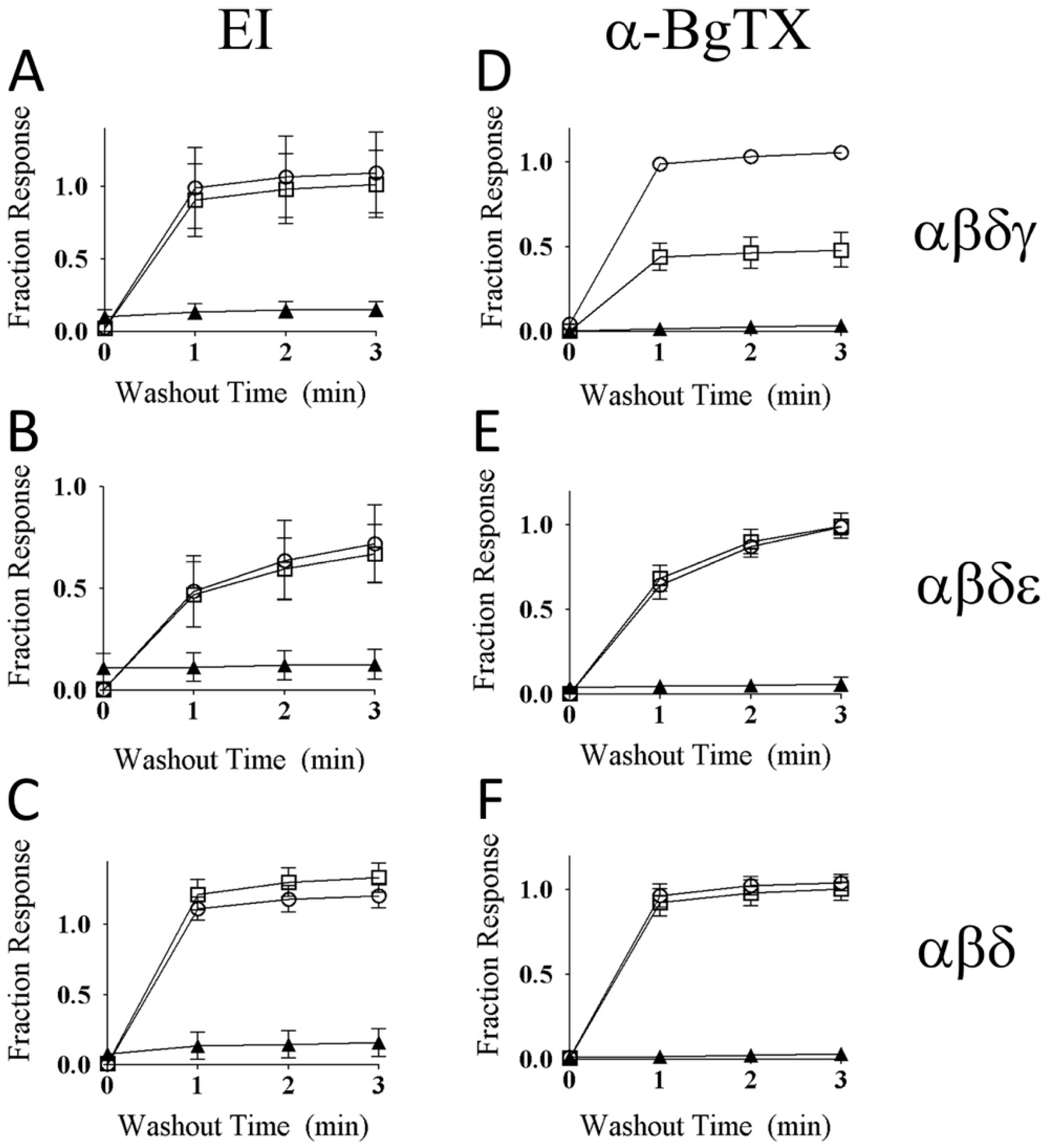
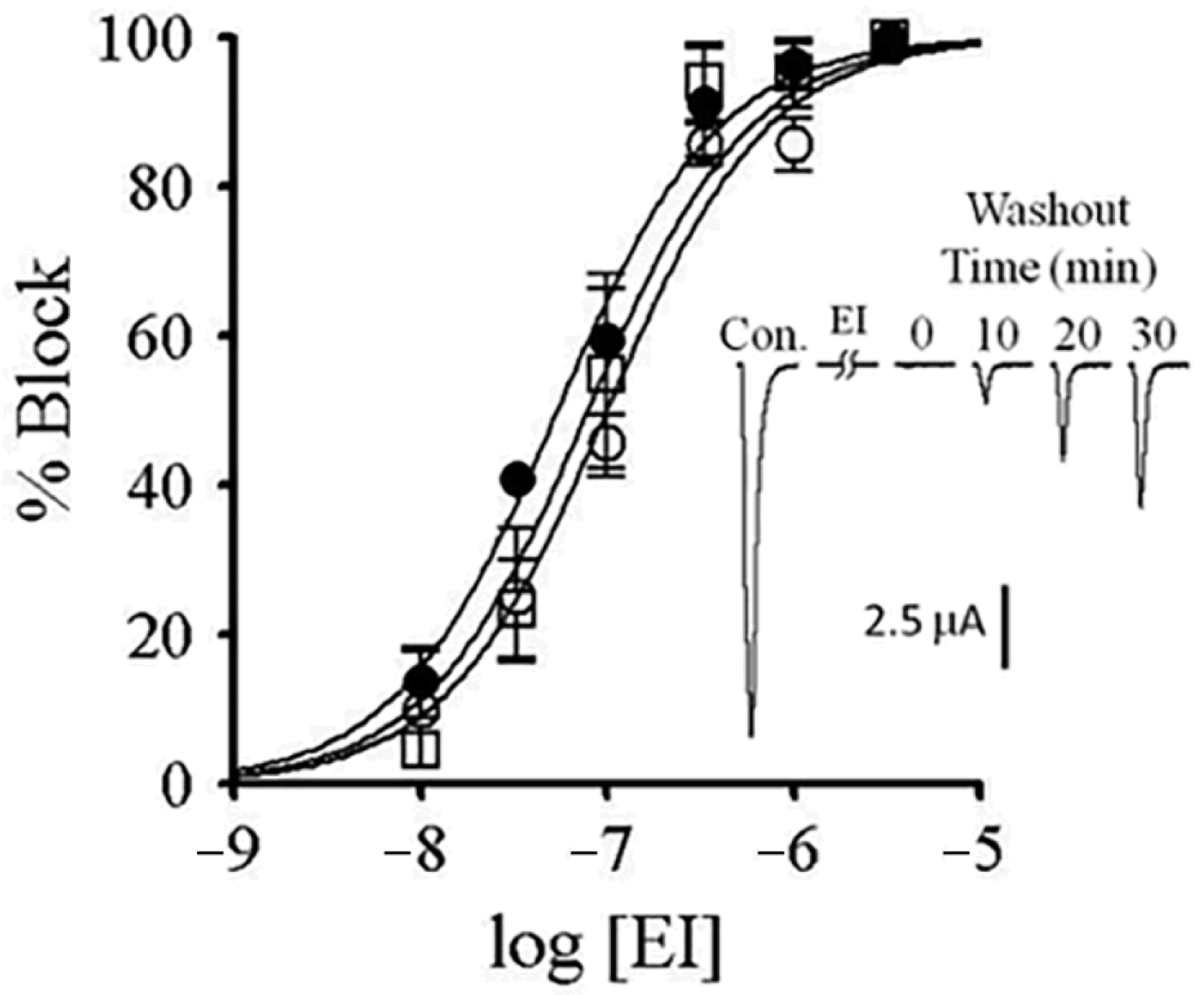
2.7. αM-MIIIJ Protects Zebrafish nAChRs Against A-Bungarotoxin (α-BgTX) and A-Conotoxin EI (α-EI)
2.8. αM-MIIIJ Blocks Indirectly-Evoked Mouse Muscle Action Potentials and Exogenously-Expressed Mouse and Human Muscle nAChRs
2.9. Lack of Effects of αM-MIIIJ on Neuronal nAChRs
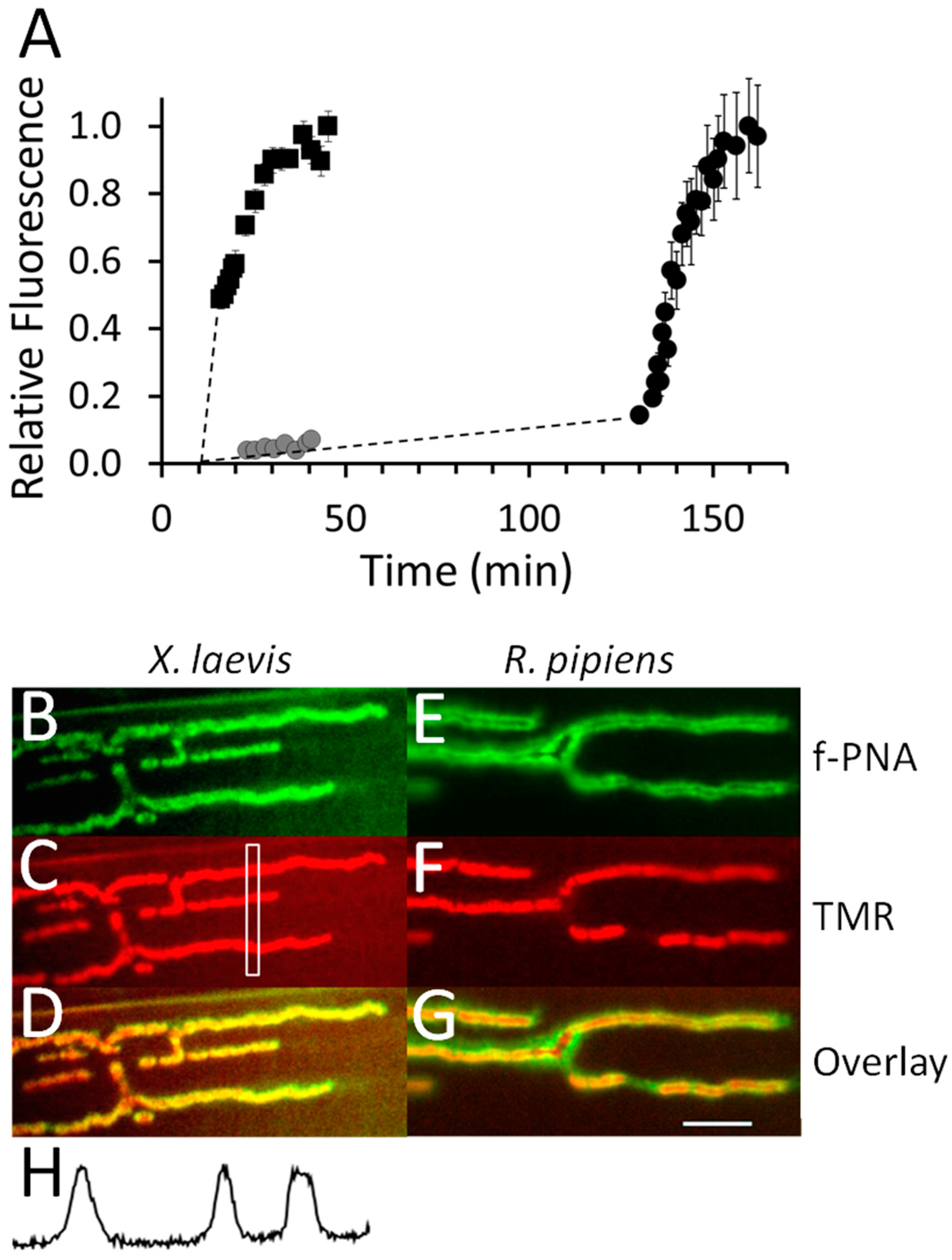
2.10. Imaging the Binding of Fluorescently-Labeled α-BgTX at Frog NMJs
2.11. Imaging the Binding of Fluorescently-Labeled α-BgTX at NMJs of Goldfish IC Muscle
2.12. Binding of Fluorescently-Labeled α-BgTX at Zebrafish NMJs
3. Discussion
3.1. αM-MIIIJ Uncovers a New Family of Muscle nAChR-Targeting Conotoxins
3.2. αM-MIIIJ Blocks Muscle nAChRs of X. laevis Frogs
3.3. αM-MIIIJ Blocks nAChRs of Goldfish Muscle
3.4. αM-MIIIJ Blocks IACh of Zebrafish-Muscle nAChRs Exogenously Expressed in X. laevis Oocytes
3.5. αM-MIIIJ Inhibits the Binding of α-BgTX to Frog and Fish NMJs
3.6. Possible Rationale for the Presence of both a-MI and αM-MIIIJ in C. magus Venom
4. Conclusions
5. Materials and Methods
5.1. αM-MIIIJ Purification and Sequencing
5.2. Identification of Homologous Sequences
5.3. αM-MIIIJ Synthesis
5.4. Animal Use
5.5. In Vivo Bioassays of αM-MIIIJ on Goldfish and Mice
5.6. Isolated Muscle Preparations
5.7. Muscle Electrophysiology
5.8. Voltage-Clamp of X. laevis Oocytes Exogenously Expressing Muscle nAChRs from Zebrafish, Mouse, and Human
5.9. Fluorescence Imaging
5.9.1. Imaging of Fluorescently-Tagged α-bungarotoxin (α-BgTX) and Fluorescein Peanut Agglutinin (f-PNA) Bound at NMJs
5.9.2. Fluorescence Intensity Measurements
5.10. Toxins and Their Application
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olivera, B.M.; Raghuraman, S.; Schmidt, E.W.; Safavi-Hemami, H. Linking neuroethology to the chemical biology of natural products: Interactions between cone snails and their fish prey, a case study. J. Comp. Physiol. A 2017, 203, 717–735. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.D.; Norton, R.S. Conotoxin gene superfamilies. Mar. Drugs 2014, 12, 6058–6101. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Olivera, B.M. Conus Venoms: A Rich Source of Novel Ion Channel-Targeted Peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [PubMed]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Boil. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Hemami, H.; Brogan, S.E.; Olivera, B.M. Pain therapeutics from cone snail venoms: From Ziconotide to novel non-opioid pathways. J. Proteom. 2019, 190, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Seger, J.; Horvath, M.P.; Fedosov, A.E. Prey-Capture Strategies of Fish-Hunting Cone Snails: Behavior, Neurobiology and Evolution. Brain Behav. Evol. 2015, 86, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M. Conus Venom Peptides, Receptor and Ion Channel Targets, and Drug Design: 50 Million Years of Neuropharmacology. Mol. Biol. Cell 1997, 8, 2101–2109. [Google Scholar] [CrossRef]
- McIntosh, M.; Cruz, L.J.; Hunkapiller, M.W.; Gray, W.R.; Olivera, B.M. Isolation and structure of a peptide toxin from the marine snail Conus magus. Arch. Bioch. Biophys. 1982, 218, 329–334. [Google Scholar] [CrossRef]
- Cartier, G.E.; Yoshikami, D.; Gray, W.R.; Luo, S.; Olivera, B.M.; McIntosh, J.M. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J. Biol. Chem. 1996, 271, 7522–7528. [Google Scholar] [CrossRef]
- Olivera, B.M.; Cruz, L.J.; de Santos, V.; LeCheminant, G.W.; Griffin, D.; Zeikus, R.; McIntosh, J.M.; Galyean, R.; Varga, J.; Gray, W.R. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry 1987, 26, 2086–2090. [Google Scholar] [CrossRef]
- Wilson, M.J.; Yoshikami, D.; Azam, L.; Gajewiak, J.; Olivera, B.M.; Bulaj, G.; Zhang, M.M. mu-Conotoxins that differentially block sodium channels NaV1.1 through 1.8 identify those responsible for action potentials in sciatic nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 10302–10307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-M.; Fiedler, B.; Green, B.R.; Catlin, P.; Watkins, M.; Garrett, J.E.; Smith, B.J.; Yoshikami, J.; Olivera, B.M.; Bulaj, G.; et al. Structural and Functional Diversities among μ-Conotoxins Targeting TTX-resistant Sodium Channels. Biochemistry 2006, 45, 3723–3732. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nakamura, H.; Ohizumi, Y.; Kobayashi, J.; Hirata, Y. The amino acid sequences of homologous hydroxyproline-containing myotoxins from the marine snail Conus geographus venom. FEBS Lett. 1983, 155, 277–280. [Google Scholar] [CrossRef]
- Violette, A.; Biass, D.; Dutertre, S.; Koua, D.; Piquemal, D.; Pierrat, F.; Stöcklin, R.; Favreau, P. Large-scale discovery of conopeptides and conoproteins in the injectable venom of a fish-hunting cone snail using a combined proteomic and transcriptomic approach. J. Proteom. 2012, 75, 5215–5225. [Google Scholar] [CrossRef]
- Bulaj, G.; West, P.J.; Garrett, J.E.; Watkins, M.; Zhang, M.-M.; Norton, R.S.; Smith, B.J.; Yoshikami, J.; Olivera, B.M. Novel Conotoxins from Conus striatusandConus kinoshitaiSelectively Block TTX-Resistant Sodium Channels. Biochemistry 2005, 44, 7259–7265. [Google Scholar] [CrossRef]
- Holford, M.; Zhang, M.-M.; Gowd, K.H.; Azam, L.; Green, B.R.; Watkins, M.; Ownby, J.-P.; Yoshikami, J.; Bulaj, G.; Olivera, B.M.; et al. Pruning nature: Biodiversity-derived discovery of novel sodium channel blocking conotoxins from Conus bullatus. Toxicon 2008, 53, 90–98. [Google Scholar] [CrossRef]
- Li, Q.; Watkins, M.; Robinson, S.D.; Safavi-Hemami, H.; Yandell, M. Discovery of Novel Conotoxin Candidates Using Machine Learning. Toxins 2018, 10, 503. [Google Scholar] [CrossRef]
- Lluisma, A.O.; Vera, E.L.; Bulaj, G.; Watkins, M.; Olivera, B.M. Characterization of a novel ψ-conotoxin from Conus parius Reeve. Toxicon 2007, 51, 174–180. [Google Scholar] [CrossRef]
- Safo, P.; Rosenbaum, T.; Shcherbatko, A.; Choi, D.Y.; Han, E.; Toledo-Aral, J.J.; Olivera, B.M.; Brehm, P.; Mandel, G. Distinction among neuronal subtypes of voltage-activated sodium channels by mu-conotoxin PIIIA. J. Neurosci. 2000, 20, 76–80. [Google Scholar] [CrossRef]
- Shon, K.J.; Grilley, M.; Jacobsen, R.; Cartier, G.E.; Hopkins, C.; Gray, W.R.; Watkins, M.; Hillyard, D.R.; Rivier, J.; Torres, J.; et al. A noncompetitive peptide inhibitor of the nicotinic acetylcholine receptor from Conus purpurascens venom. Biochemistry 1997, 36, 9581–9587. [Google Scholar] [CrossRef]
- Jacob, R.B.; McDougal, O.M. The M-superfamily of conotoxins: A review. Cell. Mol. Life Sci. 2009, 67, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.S.; Olivera, B.M.; Gray, W.R.; Craig, A.G.; Groebe, D.R.; Abramson, S.N.; McIntosh, J.M. alpha-Conotoxin EI, a new nicotinic acetylcholine receptor antagonist with novel selectivity. Biochemistry 1995, 34, 14519–14526. [Google Scholar] [CrossRef] [PubMed]
- Shon, K.-J.; Olivera, B.M.; Watkins, M.; Jacobsen, R.B.; Gray, W.R.; Floresca, C.Z.; Cruz, L.J.; Hillyard, D.R.; Brink, A.; Terlau, H.; et al. μ-Conotoxin PIIIA, a New Peptide for Discriminating among Tetrodotoxin-Sensitive Na Channel Subtypes. J. Neurosci. 1998, 18, 4473–4481. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, E.M.; Martin, A.R. Non-linear summation of end-plate potentials in the frog and mouse. J. Physiol. 1981, 311, 307–324. [Google Scholar] [CrossRef]
- Mongeon, R.; Walogorsky, M.; Urban, J.; Mandel, G.; Ono, F.; Brehm, P. An acetylcholine receptor lacking both γ and ε subunits mediates transmission in zebrafish slow muscle synapses. J. Gen. Physiol. 2011, 138, 353–366. [Google Scholar] [CrossRef]
- Drapeau, P.; Buss, R.R.; Ali, D.W.; Legendre, P.; Rotundo, R.L. Limits to the development of fast neuromuscular transmission in zebrafish. J. Neurophysiol. 2001, 86, 2951–2956. [Google Scholar] [CrossRef][Green Version]
- Nguyen, P.V.; Aniksztejn, L.; Catarsi, S.; Drapeau, P. Maturation of neuromuscular transmission during early development in zebrafish. J. Neurophysiol. 1999, 81, 2852–2861. [Google Scholar] [CrossRef][Green Version]
- López-Vera, E.; Aguilar, M.B.; Schiavon, E.; Marinzi, C.; Ortiz, E.; Restano Cassulini, R.; Batista, C.V.; Possani, L.D.; Heimer de la Cotera, E.P.; Peri, F.; et al. Novel alpha-conotoxins from Conus spurius and the alpha-conotoxin EI share high-affinity potentiation and low-affinity inhibition of nicotinic acetylcholine receptors. FEBS J. 2007, 274, 3972–3985. [Google Scholar] [CrossRef]
- Jimenez, E.C.; Olivera, B.M.; Teichert, R.W. AlphaC-conotoxin PrXA: A new family of nicotinic acetylcholine receptor antagonists. Biochemistry 2007, 46, 8717–8724. [Google Scholar] [CrossRef]
- Luna, V.M.; Daikoku, E.; Ono, F. “Slow” skeletal muscles across vertebrate species. Cell Biosci. 2015, 5, 62. [Google Scholar] [CrossRef]
- Green, B.R.; Olivera, B.M. Venom Peptides From Cone Snails: Pharmacological Probes for Voltage-Gated Sodium Channels. Curr. Top. Membr. 2016, 78, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Kerr, L.M.; Yoshikami, D.A. Venom peptide with a novel presynaptic blocking action. Nature 1984, 308, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, R.; Yoshikami, D.; Ellison, M.; Martinez, J.; Gray, W.R.; Cartier, G.E.; Shon, K.J.; Groebe, D.R.; Abramson, S.N.; Olivera, B.M.; et al. Differential targeting of nicotinic acetylcholine receptors by novel alphaA-conotoxins. J. Biol. Chem. 1997, 272, 22531–22537. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Cohen, M.W. Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle. J. Physiol. 1974, 237, 385–400. [Google Scholar] [CrossRef]
- Tarvin, R.D.; Borghese, C.M.; Sachs, W.; Santos, J.C.; Lu, Y.; Connell, L.A.; Cannatella, D.C.; Harris, R.A.; Zakon, H.H. Interacting amino acid replacements allow poison frogs to evolve epibatidine resistance. Science 2017, 357, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, S.F.; Tavazoie, M.F.; McIntosh, J.M.; Olivera, B.M.; Yoshikami, D. Differential block of nicotinic synapses on B versus C neurones in sympathetic ganglia of frog by alpha-conotoxins MII and ImI. Br. J. Pharmacol. 1997, 120, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.R.; Luque, A.; Olivera, B.M. Peptide Toxins from Conus geographus Venom. J. Biol. Chem. 1981, 256, 4734–4740. [Google Scholar]
- Groebe, D.R.; Dumm, J.M.; Levitan, E.S.; Abramson, S.N. alpha-Conotoxins selectively inhibit one of the two acetylcholine binding sites of nicotinic receptors. Mol. Pharmacol. 1995, 48, 105–111. [Google Scholar]
- Kapono, C.A.; Thapa, P.; Cabalteja, C.C.; Guendisch, D.; Collier, A.C.; Bingham, J.-P. Conotoxin truncation as a post-translational modification to increase the pharmacological diversity within the milked venom of Conus magus. Tox. Off. J. Int. Soc. Toxinol. 2013, 70, 170–178. [Google Scholar] [CrossRef]
- McIntosh, J.M.; Dowell, C.; Watkins, M.; Garrett, J.E.; Yoshikami, D.; Olivera, B.M. a-Conotoxin GIC from Conus geographus, a Novel Peptide Antagonist of Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2002, 277, 33610–33615. [Google Scholar] [CrossRef]
- Zafaralla, G.C.; Ramilo, C.; Gray, W.R.; Karlstrom, R.; Olivera, B.M.; Cruz, L.J. Phylogenetic specificity of cholinergic ligands: Alpha-conotoxin SI. Biochemistry 1988, 27, 7102–7105. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.A.; Zafaralla, G.C.; Gray, W.R.; Abbott, J.; Cruz, L.J.; Olivera, B.M. alpha-Conotoxins, small peptide probes of nicotinic acetylcholine receptors. Biochemistry 1991, 30, 9370–9377. [Google Scholar] [CrossRef] [PubMed]
- Ramilo, C.A.; Zafaralla, G.C.; Nadasdi, L.; Hammerland, L.G.; Yoshikami, D.; Gray, W.R.; Kristipati, R.; Ramachandran, J.; Miljanich, G.; Olivera, B.M. Novel alpha- and omega-conotoxins from Conus striatus venom. Biochemistry 1992, 31, 9919–9926. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chew, G.; Hawrot, E.; Chi, C.; Wang, C. Two potent alpha3/5 conotoxins from piscivorous Conus achatinus. Acta Biochim. Biophys. Sinica 2007, 39, 438–444. [Google Scholar] [CrossRef]
- Favreau, P.; Krimm, I.; Le Gall, F.; Bobenrieth, M.J.; Lamthanh, H.; Bouet, F.; Servent, D.; Molgo, J.; Ménez, A.; Letourneux, Y.; et al. Biochemical characterization and nuclear magnetic resonance structure of novel alpha-conotoxins isolated from the venom of Conus consors. Biochemistry 1999, 38, 6317–6326. [Google Scholar] [CrossRef]
- Nicke, A.; Loughnan, M.L.; Millard, E.L.; Alewood, P.F.; Adams, D.J.; Daly, N.L.; Craik, D.J.; Lewis, R.J. Isolation, structure, and activity of GID, a novel alpha 4/7-conotoxin with an extended N-terminal sequence. J. Biol. Chem. 2003, 278, 3137–3144. [Google Scholar] [CrossRef]
- Dowell, C.; Olivera, B.M.; Garrett, J.E.; Staheli, S.T.; Watkins, M.; Kuryatov, A.; Yoshikami, D.; Lindstrom, J.M.; McIntosh, J.M. a-Conotoxin PIA Is Selective for a6 Subunit-Containing Nicotinic Acetylcholine Receptors. J. Neurosci. 2003, 23, 8445–8452. [Google Scholar] [CrossRef]
- McIntosh, J.M.; Plazas, P.V.; Watkins, M.; Gomez-Casati, M.E.; Olivera, B.M.; Elgoyhen, A.B. A Novel α-Conotoxin, PeIA, Cloned from Conus pegrandis, Discriminates between Rat α9α10 and α7 Nicotinic Cholinergic Receptors. J. Biol. Chem. 2005, 34, 30107–30112. [Google Scholar] [CrossRef]
- Azam, L.; Dowell, C.; Watkins, M.; Stitzel, J.A.; Olivera, B.M.; McIntosh, J.M. α-Conotoxin BuIA, a Novel Peptide from Conus bullatus, distinguishes among Neuronal Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2005, 280, 80–87. [Google Scholar] [CrossRef]
- Lopez-Vera, E.; Jacobsen, R.B.; Ellison, M.; Olivera, B.M.; Teichert, R.W. A novel alpha conotoxin (a-PIB) isolated from C. purpurascens is selective for skeletal muscle nicotinic acetylcholine receptors. Toxicon 2007, 49, 1193–1199. [Google Scholar] [CrossRef]
- Quinton, L.; Servent, D.; Girard, E.; Molgó, J.; Caer, J.-P.L.; Malosse, C.; Haidar, E.A.; Lecoq, A.; Gilles, N.; Chamot-Rooke, J.; et al. Identification and functional characterization of a novel α-conotoxin (EIIA) from Conus ermineus. Anal. Bioanal. Chem. 2013, 405, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Grilley, M.; Miller, C.; Shon, K.J.; Cruz, L.J.; Gray, W.R.; Dykert, J.; Rivier, J.; Yoshikami, D.; Olivera, B.M.; et al. A New Family of Conus Peptides Targeted to the Nicotinic Acetylcholine-Receptor. J. Biol. Chem. 1995, 270, 22361–22367. [Google Scholar] [CrossRef] [PubMed]
- Teichert, R.W.; Rivier, J.; Dykert, J.; Cervini, L.; Gulyas, J.; Bulaj, G.; Ellison, M.; Olivera, B.M. AlphaA-Conotoxin OIVA defines a new alphaA-conotoxin subfamily of nicotinic acetylcholine receptor inhibitors. Toxicon 2004, 44, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Teichert, R.W.; Jimenez, E.C.; Olivera, B.M. Alpha S-contoxin RVIIIA: A structurally unique conotoxin that broadly targets nicotinic acetylcholine receptors. Biochemistry 2005, 44, 7897–7902. [Google Scholar] [CrossRef] [PubMed]
- Teichert, R.W.; Lopez-Vera, E.; Gulyas, J.; Watkins, M.; Rivier, J.; Olivera, B.M. Definition and characterization of the short alphaA-conotoxins: A single residue determines dissociation kinetics from the fetal muscle nicotinic acetylcholine receptor. Biochemistry 2006, 45, 1304–1312. [Google Scholar] [CrossRef]
- Christensen, S.B.; Bandyopadhyay, P.K.; Olivera, B.M.; McIntosh, J.M. alphaS-conotoxin GVIIIB potently and selectively blocks alpha9alpha10 nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015, 96, 349–356. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Jacobsen, R.B.; Olivera, B.M.; Ireland, C.M. Characterization and three-dimensional structure determination of psi-conotoxin Piiif, a novel noncompetitive antagonist of nicotinic acetylcholine receptors. Biochemistry 2003, 42, 6353–6362. [Google Scholar] [CrossRef]
- Ellison, M.; McIntosh, J.M.; Olivera, B.M. Alpha-conotoxins ImI and ImII. Similar alpha 7 nicotinic receptor antagonists act at different sites. J. Biol. Chem. 2003, 278, 757–764. [Google Scholar] [CrossRef]
- Johnson, D.S.; Martinez, J.; Elgoyhen, A.B.; Heinemann, S.F.; McIntosh, J.M. Alpha-Conotoxin ImI exhibits subtype-specific nicotinic acetylcholine receptor blockade: Preferential inhibition of homomeric alpha 7 and alpha 9 receptors. Mol. Pharmacol. 1995, 48, 194–199. [Google Scholar]
- Ellison, M.; Gao, F.; Wang, H.L.; Sine, S.M.; McIntosh, J.M.; Olivera, B.M. Alpha-conotoxins ImI and ImII target distinct regions of the human alpha7 nicotinic acetylcholine receptor and distinguish human nicotinic receptor subtypes. Biochemistry 2004, 43, 16019–16026. [Google Scholar] [CrossRef]
- Ellison, M.; Olivera, B.M. Alpha4/3 conotoxins: Phylogenetic distribution, functional properties, and structure-function insights. Chem. Rec. 2007, 7, 341–353. [Google Scholar] [CrossRef]
- McIntosh, J.M.; Hasson, A.; Spira, M.E.; Gray, W.R.; Li, W.; Marsh, M.; Hillyard, D.R.; Olivera, B.M. A new family of conotoxins that blocks voltage-gated sodium channels. J. Biol. Chem. 1995, 270, 16796–16802. [Google Scholar] [CrossRef] [PubMed]
- Fainzilber, M.; van der Schors, R.; Lodder, J.C.; Li, K.W.; Geraerts, W.P.; Kits, K.S. New sodium channel-blocking conotoxins also affect calcium currents in Lymnaea neurons. Biochemistry 1995, 34, 5364–5371. [Google Scholar] [CrossRef] [PubMed]
- Bulaj, G.; Zhang, M.-M.; Green, B.R.; Fiedler, B.; Layer, R.T.; Wei, S.; Nielsen, J.S.; Low, S.J.; Klein, B.D.; Wagstaff, J.D.; et al. Synthetic muO-conotoxin MrVIB blocks TTX-resistant sodium channel NaV1.8 and has a long-lasting analgesic activity. Biochemistry 2006, 45, 7404–7414. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, J.; Jayamanne, A.; Vaughan, C.W.; Aslan, S.; Thomas, L.; Mould, J.; Drinkwater, R.; Baker, M.D.; Abrahamsen, B.; Wood, J.N.; et al. muO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc. Natl Acad. Sci. USA 2006, 103, 17030–17035. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Stocker, M.; Shon, K.J.; McIntosh, J.M.; Olivera, B.M. mu O-conotoxin MrVIA inhibits mammalian sodium channels, but not through site I. J. Neurophys. 1996, 76, 1423–1429. [Google Scholar] [CrossRef]
- Favreau, P.; Benoit, E.; Hocking, H.G.; Carlier, L.; D’hoedt, D.; Leipold, E.; Markgraf, R.; Schlumberger, S.; Córdova, M.A.; Gaertner, H.; et al. A novel µ-conopeptide, CnIIIC, exerts potent and preferential inhibition of NaV1.2/1.4 channels and blocks neuronal nicotinic acetylcholine receptors. Br. J. Pharmacol. 2012, 166, 1654–1668. [Google Scholar] [CrossRef]
- Leipold, E.; Ullrich, F.; Thiele, M.; Tietze, A.A.; Terlau, H.; Imhof, D.; Heinemann, S.H. Subtype-specific block of voltage-gated K(+) channels by μ-conopeptides. Biochem. Biophys. Res. Commun. 2016. [Google Scholar] [CrossRef]
- Liu, Z.; Bartels, P.; Sadeghi, M.; Du, T.; Dai, Q.; Zhu, C.; Yu, S.; Wang, S.; Dong, M.; Sun, T.; et al. A novel α-conopeptide Eu1.6 inhibits N-type (CaV2.2) calcium channels and exhibits potent analgesic activity. Sci. Rep. 2018, 8, 1004. [Google Scholar] [CrossRef]
- Andreson, R.; Roosaare, M.; Kaplinski, L.; Laht, S.; Kõressaar, T.; Lepamets, M.; Brauer, A.; Kukuškina, V.; Remm, M. Gene content of the fish-hunting cone snail Conus consors. bioRxiv 2020. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Stewart, J.; Young, J. Solid Phase Peptide Synthesis; Pierce Chemical Company: Rockford, IL, USA, 1984. [Google Scholar]
- Horiki, K.; Igano, K.; Inouye, K. Amino acids and peptides. Part 6. Synthesis of the Merrifield resin esters of N-protected amino acids with the aid of hydrogen bonding. Chem. Lett. 1978, 2, 165–168. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.; Bossinger, C.; Cook, P. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Ellman, G. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Betz, W.J.; Bewick, G.S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 1992, 255, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Peper, K.; McMahan, U.J. Distribution of acetylcholine receptors in the vincinity of nerve terminals on skeletal muscle of the frog. Proc. R. Soc. B. 1972, 181, 431–440. [Google Scholar]
- Jimenez, E.C.; Shetty, R.P.; Lirazan, M.; Rivier, J.; Walker, C.; Abogadie, F.C.; Yoshikami, D.; Cruz, L.J.; Olivera, B.M. Novel excitatory Conus peptides define a new conotoxin superfamily. J. Neurochem. 2003, 85, 610–621. [Google Scholar] [CrossRef]
- Gilchrist, J.D.F.; von Bonde, C. Dissection of the Platana and the Frog; Good Press: Glasgow, UK, 1919; p. 8. [Google Scholar] [CrossRef]
- Brady, J. A simple technique for making very fine, durable dissecting needles by sharpening tungsten wire electrolytically. Bull. WHO 1965, 32, 143–144. [Google Scholar]
- Li, W.; Ono, F.; Brehm, P. Optical measurements of presynaptic release in mutant zebrafish lacking postsynaptic receptors. J. Neurosci. 2003, 23, 10467–10474. [Google Scholar] [CrossRef]
- Ono, F.; Higashijima, S.; Shcherbatko, A.; Fetcho, J.R.; Brehm, P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J. Neurosci. 2001, 21, 5439–5448. [Google Scholar] [CrossRef]
- Angaut-Petit, D.; Molgó, J.; Connold, A.L.; Faille, L. The levator auris longus muscle of the mouse: A convenient preparation for studies of short-and long-term presynaptic effects of drugs or toxins. Neurosci. Lett. 1987 82, 83–88. [CrossRef]
- Kuffler, S.W.; Yoshikami, D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: Iontophoretic mapping in the micron range. J. Physiol. 1975, 244, 703–730. [Google Scholar] [CrossRef] [PubMed]
- Kamata, R.; Washio, H. Neuromuscular transmission of pectoral fin muscles of the goldfish Carassius auratus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 135, 435–442. [Google Scholar] [CrossRef]
- Filchakova, O.; McIntosh, J.M. Functional expression of human α9* nicotinic acetylcholine receptors in X. laevis oocytes is dependent on the α9 subunit 5′ UTR. PLoS ONE 2013, 8, e64655. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.P. A lectin, peanut agglutinin, as a probe for the extracellular matrix in living neuromuscular junctions. J. Neurocytol. 1987, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]

| Peptide | Sequence 1 | Ion-Channel Target | Reference |
|---|---|---|---|
| α-MI | GRCCHPACGKNYSC# | muscle nAChR | McIntosh 1982 [8] |
| α-MII 2 | GCCSNPVCHLEHSNLC# | neuronal nAChR | Cartier 1996 [9] |
| ω-MVIIA | CKGKGAKCSRLMYDCCTGSCRSGKC# | presynaptic Ca channel | Olivera 1987 [10] |
| μ-MIIIA | ZGCCNVPNGCSGRWCRDHAQCC# | muscle Na channel | Wilson 2011 [11] Zhang 2006 [12] |
| αM-MIIIJ | ZKCCSGGSCPLYFRDRLICPCC | muscle nAChR | Rybin 2020 [this report] |
| nAChR 2 | αM-MIIIJ | α-EI |
|---|---|---|
| zαβδ | 0.033 (0.023–0.048) 3 | 0.073 (0.045–0.118) 4 |
| zαβδγ | 0.061 (0.049–0.076) 3 | 0.103 (0.069–0.155) 4 |
| zαβδε | 0.058 (0.040–0.085) 3 | 0.058 (0.039–0.087) 4 |
| zαβε | 0.034 (0.022–0.053) 5 | N. A. |
| mαβδγ | 44.4 (25.4–77.5) 6 | N.A. |
| mαβδε | 3.6 (2.6–4.9) 6 | N. A. |
| hαβδγ | 19.6 (13.8–27.7) 6 | N. A. 7 |
| hαβδε | 4.1 (2.9–5.9) 6 | N. A. |
| hα9α10 | >>10 8 | N. A. |
| rα4β2 | >>10 8 | N. A. 9 |
| rα3β4 | >>10 8 | N. A. 10 |
| Peptide 2 | Sequence 3 | Reference |
|---|---|---|
| α(3/5) | ||
| α-GI | ECCNPACGRHYSC# | Gray 1981 [37] |
| α-GIA | ECCNPACGRHYSCGK# | Gray 1981 [37] |
| α-GIB | ECCNPACGRHYSCKG# | McIntosh 2002 [40] |
| α-GII | ECCHPACGKHFSC# | Gray 1981 [37] |
| α-MI | GRCCHPACGKNYSC# | McIntosh 1982 [8] |
| α-MIA | DGRCCHPACAKHFNC# | McIntosh 2002 [40] |
| α-MIB | NGRCCHPACGKNYSC# | McIntosh 2002 [40] |
| α-MIC | CCHPACGKNYSC# | Kapono 2013 [39] |
| α-SI | ICCNPACGPKYSC# | Zafaralla 1988 [41] |
| α-SIA | YCCHPACGKNFDC# | Myers 1991 [42] |
| α-SII | GCCCNPACGPNYGCGTSCS | Ramilo 1992 [43] |
| α-Ac1.1a | NGRCCHPACGKHFNC# | Liu 2007 [44] |
| α-Ac1.1b | NGRCCHPACGKHFSC# | Liu 2007 [44] |
| α-CnIA | GRCCHPACGKYYSC# | Favreau 1999 [45] |
| α-CnIB | CCHPACGKYYSC# | Favreau 1999 [45] |
| α(4/7) | ||
| α-EI | RDOCCYHPTCNMSNPQIC# | Martinez 1995 [22] |
| α-MII† | GCCSNPVCHLEHSNLC# | Cartier 1996 [9] |
| α-GIC† | GCCSHPACAGNNQHIC# | McIntosh 2002 [40] |
| α-GID† | IRDγCCSNPACRVNNOHVC | Nicke 2003 [46] |
| α-PIA† | RDPCCSNPVCTVHNPQIC# | Dowell 2003 [47] |
| α-PeIA† | GCCSHPACSVNHPELC# | McIntosh 2005 [48] |
| α(4/4) | ||
| α-BuIA† | GCCSTPPCAVLYC# | Azam 2005 [49] |
| α-PIB | ZSOGCCWNPACVKNRC# | Lopez-Vera 2007 [50] |
| α-EIIA | ZTOGCCWNPACVKNRC# | Quinton 2013 [51] |
| αALong | ||
| αA-PIVA | GCCGSYONAACHOCSCKDROSYCGQ# | Hopkins 1995 [52] |
| αA-EIVA | GCCGPYONAACHOCGCKVGROOYCDROSGG# | Jacobsen 1997 [33] |
| αA-EIVB | GCCGKYONAACHOCGCTVGROOYCDROSGG# | Jacobsen 1997 [33] |
| αAShort | ||
| αA-OIVA | CCGVONAACHOCVCKNTC# | Teichert 2004 [53] |
| αA-OIVB | CCGVONAACPOCVCNKTCG# | Teichert 2005b [54] |
| αA-PeIVA | CCGVONAACHOCVCTGKC | Teichert 2006 [55] |
| αS | ||
| αS-RVIIA | KCNFDKCKGTGVYNCGγSCSCγGLHSCRCTY | |
| NIGSMKSGCACICTYY | Teichert 2005a [54] | |
| αS-GVIIIB† | SGSTCTCFTSTNCQGSCECLSPPGCYCSNN | |
| GIRQRGCSCTCPGT# | Christensen 2015 [56] | |
| αC | ||
| αC-PrXA | TYGIYDAKPOFSCAGLRGGCVLPONLROKFKE# | Jimenez 2007 [29] |
| αM | ||
| ψ-PIIIE | HOOCCLYGKCRRYOGCSSASCCQR# | Shon 1997 [20] |
| ψ-PIIIF | GOOCCLYGSCROFOGCYNALCCRK# | Van Wagoner 2003 [57] |
| ψ-Pr3e | AARCCTYHGSCLKEKCRRKYCC# | Lluisma 2008 [18] |
| αM-MIIIJ | ZKCCSGGSCPLYFRDRLICPCC | This report |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybin, M.J.; O’Brien, H.; Ramiro, I.B.L.; Azam, L.; McIntosh, J.M.; Olivera, B.M.; Safavi-Hemami, H.; Yoshikami, D. αM-Conotoxin MIIIJ Blocks Nicotinic Acetylcholine Receptors at Neuromuscular Junctions of Frog and Fish. Toxins 2020, 12, 197. https://doi.org/10.3390/toxins12030197
Rybin MJ, O’Brien H, Ramiro IBL, Azam L, McIntosh JM, Olivera BM, Safavi-Hemami H, Yoshikami D. αM-Conotoxin MIIIJ Blocks Nicotinic Acetylcholine Receptors at Neuromuscular Junctions of Frog and Fish. Toxins. 2020; 12(3):197. https://doi.org/10.3390/toxins12030197
Chicago/Turabian StyleRybin, Matthew J., Henrik O’Brien, Iris Bea L. Ramiro, Layla Azam, J. Michael McIntosh, Baldomero M. Olivera, Helena Safavi-Hemami, and Doju Yoshikami. 2020. "αM-Conotoxin MIIIJ Blocks Nicotinic Acetylcholine Receptors at Neuromuscular Junctions of Frog and Fish" Toxins 12, no. 3: 197. https://doi.org/10.3390/toxins12030197
APA StyleRybin, M. J., O’Brien, H., Ramiro, I. B. L., Azam, L., McIntosh, J. M., Olivera, B. M., Safavi-Hemami, H., & Yoshikami, D. (2020). αM-Conotoxin MIIIJ Blocks Nicotinic Acetylcholine Receptors at Neuromuscular Junctions of Frog and Fish. Toxins, 12(3), 197. https://doi.org/10.3390/toxins12030197





