Hypervolemia-Induced Immune Disturbances Do Not Involve IL-1ß but IL-6 and IL-10 Activation in Haemodialysis Patients
Abstract
1. Introduction
2. Results
2.1. Hypervolemia: Weight Gain and Clinical Signs of Inflammation
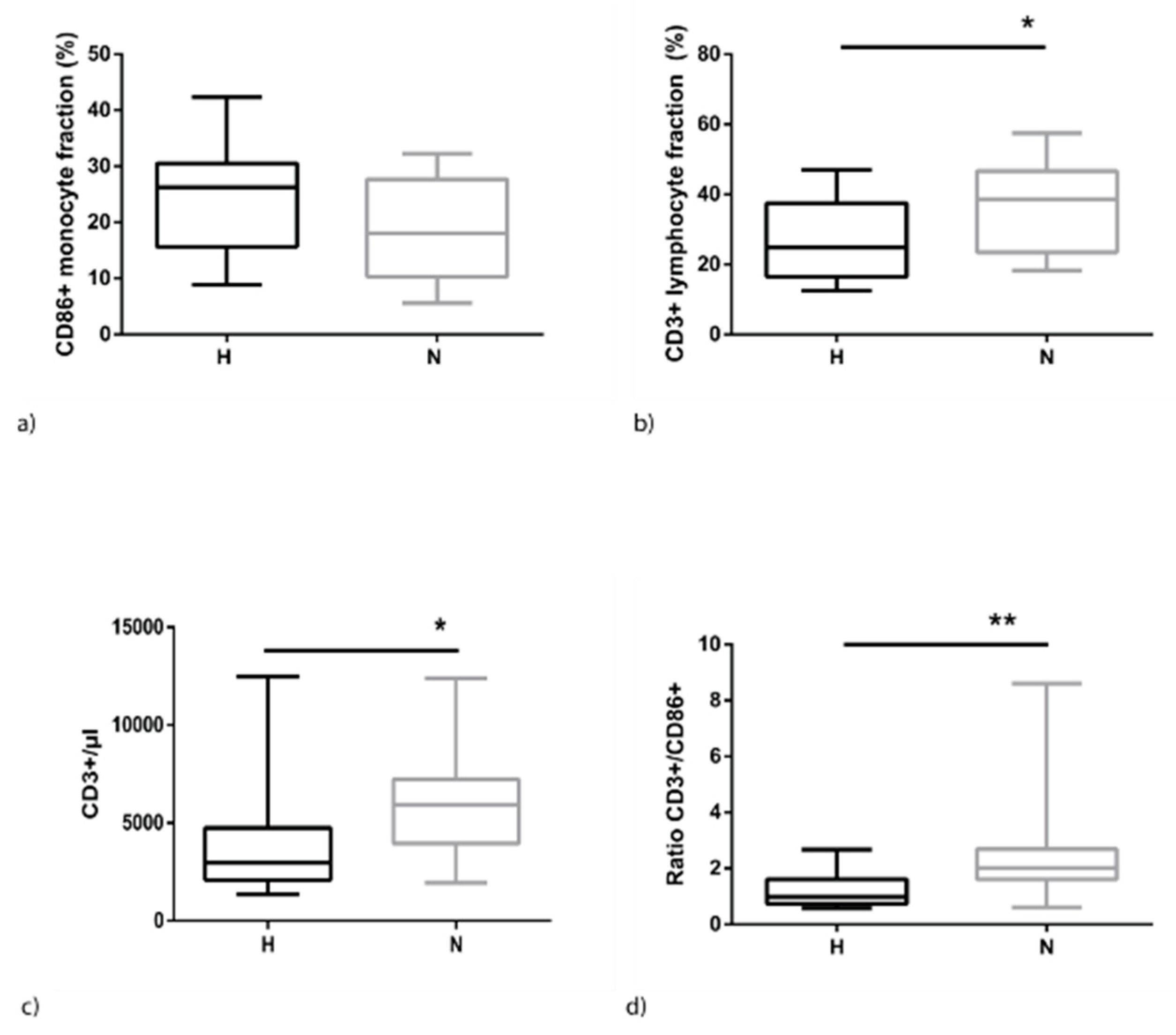
2.2. Hypervolemia is Associated with Higher Monocyte and Lower Lymphocyte Levels
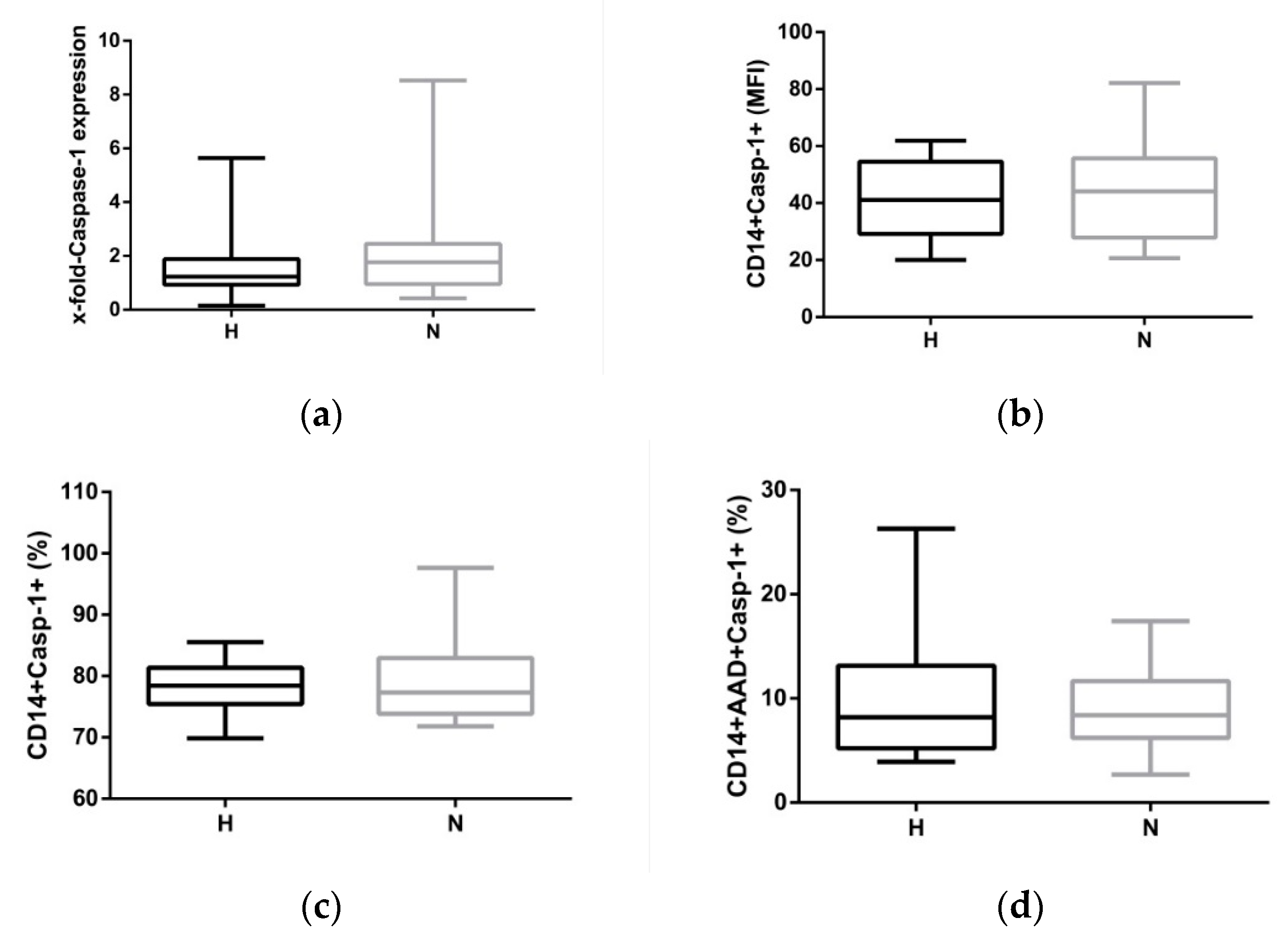
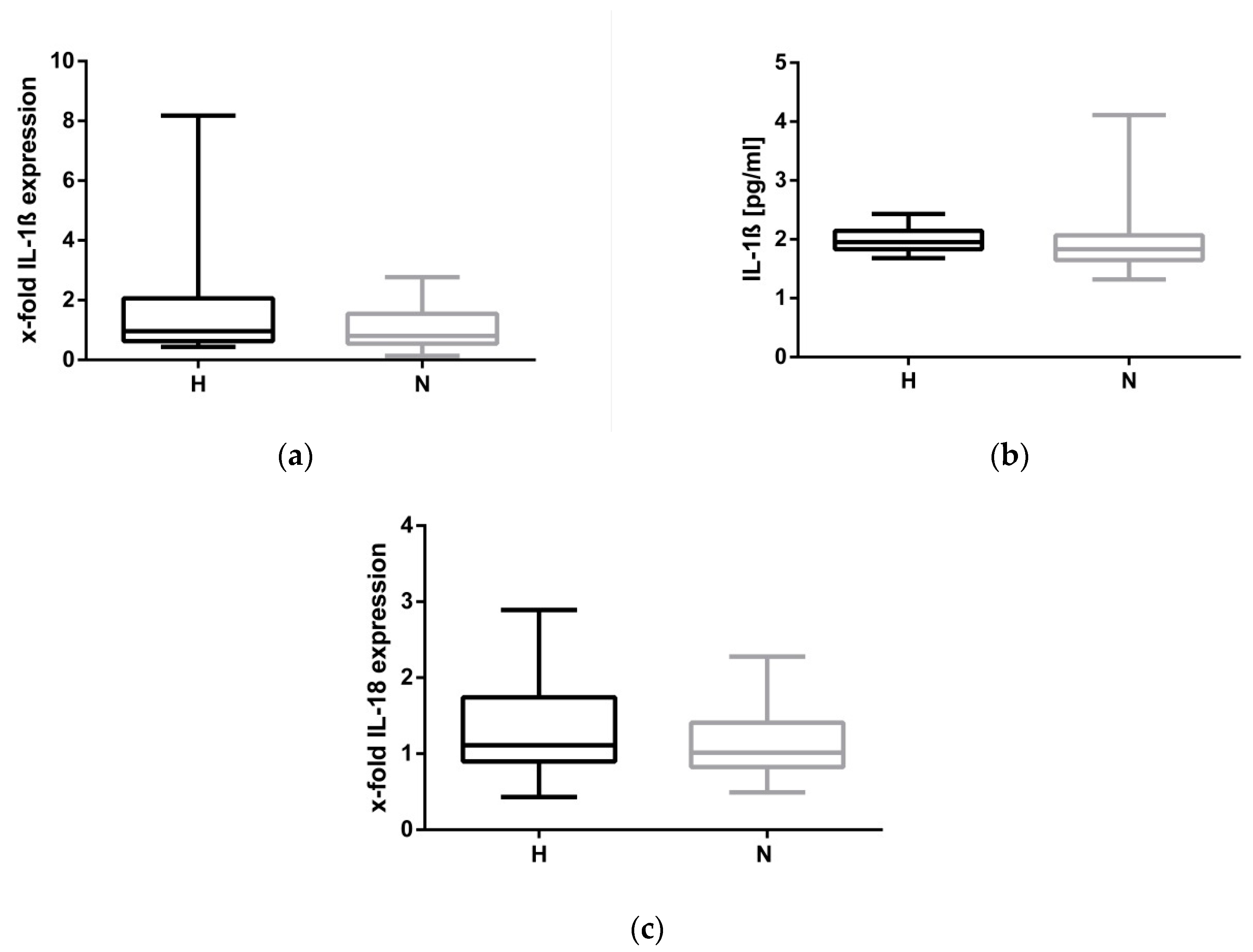
2.3. Hypervolemia: Low Impact on the NLRP3 Inflammasome
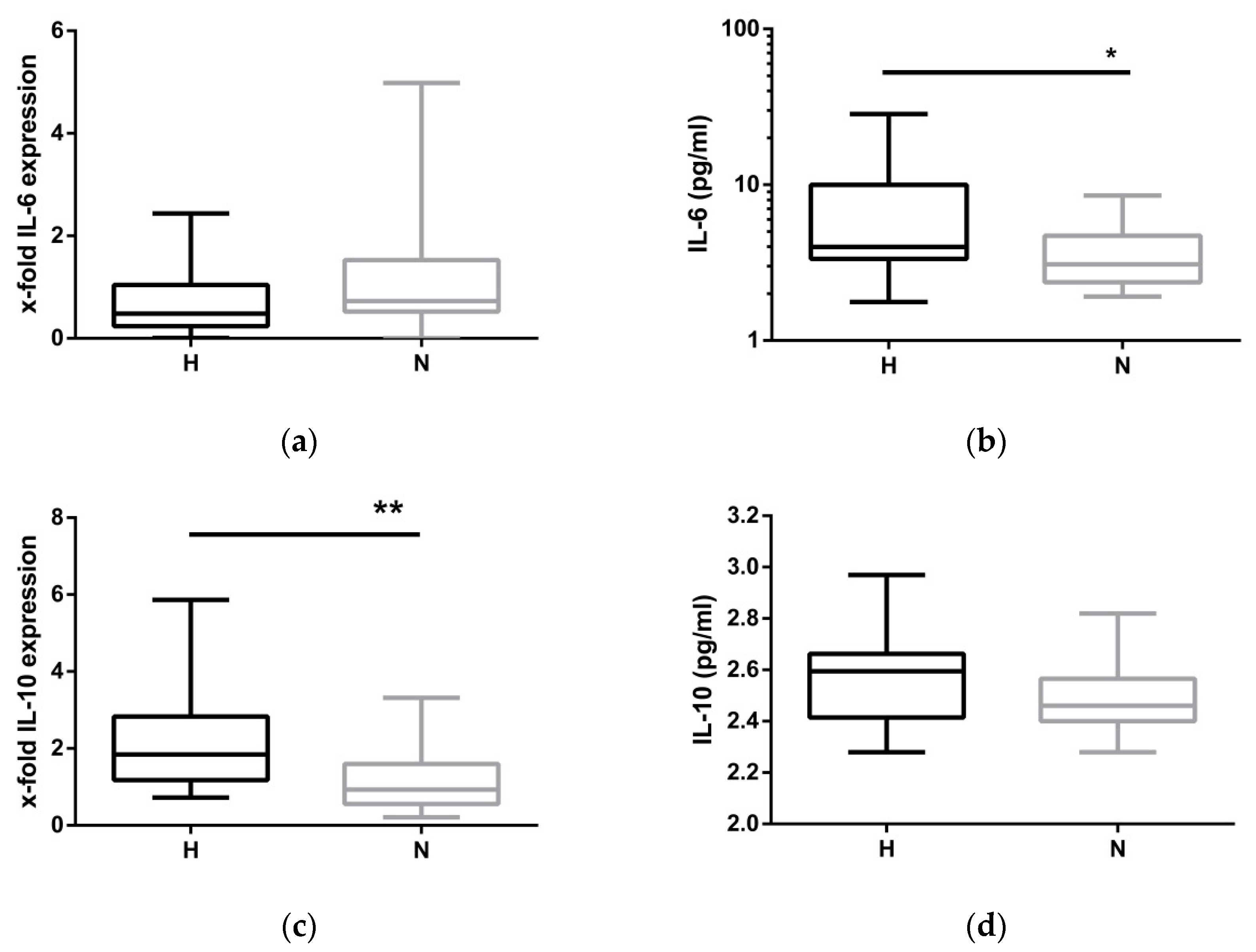
2.4. Compensatory Mechanisms Evoked by Hypervolemia
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. PBMC isolation
5.3. NLRP3 Inflammasome Stimulation Model
5.4. Antibodies for Flow Cytometry
5.5. Caspase-1-Assay
5.6. Mitochondrial Oxidative Stress
5.7. RNA/cDNA/qPCR
5.8. Cytokine Analysis
5.9. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agarwal, R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension 2010, 56, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, R.; Jehle, P.M.; Osten, B.; Dorligschaw, O.; Girndt, M. Clinical nutrition scores are superior for the prognosis of haemodialysis patients compared to lab markers and bioelectrical impedance. Nephrol. Dial. Transplant 2009, 24, 3812–3817. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A. Estimation of fluid volumes in hemodialysis patients: Comparing bioimpedance with isotopic and dilution methods. Kidney Int. 2014, 85, 738–741. [Google Scholar] [CrossRef]
- Seibert, E.; Müller, S.G.; Pattmöller, J.; Kotanko, P.; Zhu, F.; Levin, N.W.; Fiedler, R.; Kuhlmann, M.K.; Girndt, M. Calf Resistivity Values in Chronic Kidney Disease in a Caucasian Population. Nephron 2017, 135, 196–200. [Google Scholar] [CrossRef]
- Hung, S.-C.; Kuo, K.-L.; Peng, C.-H.; Wu, C.-H.; Lien, Y.-C.; Wang, Y.-C.; Tarng, D.-C. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014, 85, 703–709. [Google Scholar] [CrossRef]
- Stegmayr, B.G. Ultrafiltration and dry weight-what are the cardiovascular effects? Artif. Organs 2003, 27, 227–229. [Google Scholar] [CrossRef]
- Mitsides, N.; Cornelis, T.; Broers, N.J.H.; Diederen, N.M.P.; Brenchley, P.; van der Sande, F.M.; Schalkwijk, C.G.; Kooman, J.P.; Mitra, S. Extracellular overhydration linked with endothelial dysfunction in the context of inflammation in haemodialysis dependent chronic kidney disease. PLoS ONE 2017, 12, e0183281. [Google Scholar] [CrossRef]
- Heine, G.H.; Ulrich, C.; Seibert, E.; Seiler, S.; Marell, J.; Reichart, B.; Krause, M.; Schlitt, A.; Köhler, H.; Girndt, M. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008, 73, 622–629. [Google Scholar] [CrossRef]
- Ulrich, C.; Seibert, E.; Heine, G.H.; Fliser, D.; Girndt, M. Monocyte angiotensin converting enzyme expression may be associated with atherosclerosis rather than arteriosclerosis in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 505–511. [Google Scholar] [CrossRef]
- Girndt, M.; Kaul, H.; Leitnaker, C.K.; Sester, M.; Sester, U.; Köhler, H. Selective sequestration of cytokine-producing monocytes during hemodialysis treatment. Am. J. Kidney Dis. 2001, 37, 954–963. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Schmidt, T.; Schmid-Burgk, J.L.; Rapino, F.; Robertson, A.A.B.; Cooper, M.A.; Graf, T.; Hornung, V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 2016, 44, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.E.; Marcelli, D.; Canaud, B.; Konings, C.J.A.M.; Leunissen, K.M.; Levin, N.W.; Carioni, P.; Maheshwari, V.; Raimann, J.G.; van der Sande, F.M.; et al. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: Results from the international MONDO initiative. Eur. J. Clin. Nutr. 2016, 70, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.E.; Marcelli, D.; Canaud, B.J.; Carioni, P.; Wang, Y.; Grassmann, A.; Konings, C.J.A.M.; Kotanko, P.; Leunissen, K.M.; Levin, N.W.; et al. Impact of fluid status and inflammation and their interaction on survival: A study in an international hemodialysis patient cohort. Kidney Int. 2017, 91, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Antlanger, M.; Hecking, M.; Haidinger, M.; Werzowa, J.; Kovarik, J.J.; Paul, G.; Eigner, M.; Bonderman, D.; Hörl, W.H.; Säemann, M.D. Fluid overload in hemodialysis patients: A cross-sectional study to determine its association with cardiac biomarkers and nutritional status. BMC Nephrol. 2013, 14, 266. [Google Scholar] [CrossRef]
- Braam, B.; Lai, C.F.; Abinader, J.; Bello, A.K. Extracellular fluid volume expansion, arterial stiffness and uncontrolled hypertension in patients with chronic kidney disease. Nephrol. Dial. Transplant 2019. [Google Scholar] [CrossRef]
- Dekker, M.J.E.; van der Sande, F.M.; van den Berghe, F.; Leunissen, K.M.L.; Kooman, J.P. Fluid Overload and Inflammation Axis. Blood Purif. 2018, 45, 159–165. [Google Scholar] [CrossRef]
- Gonçalves, S.; Pecoits-Filho, R.; Perreto, S.; Barberato, S.H.; Stinghen, A.E.M.; Lima, E.G.A.; Fuerbringer, R.; Sauthier, S.M.; Riella, M.C. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol. Dial. Transplant 2006, 21, 2788–2794. [Google Scholar] [CrossRef]
- McIntyre, C.W.; Harrison, L.E.A.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.-C.; John, S.G.; Sigrist, M.K.; Burton, J.O.; Hothi, D.; Korsheed, S.; et al. Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 133–141. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Hornung, V. Alternative inflammasome activation enables IL-1β release from living cells. Curr. Opin. Immunol. 2017, 44, 7–13. [Google Scholar] [CrossRef]
- Ding, S.; Liang, Y.; Zhao, M.; Liang, G.; Long, H.; Zhao, S.; Wang, Y.; Yin, H.; Zhang, P.; Zhang, Q.; et al. Decreased microRNA-142-3p/5p expression causes CD4+ T cell activation and B cell hyperstimulation in systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, M.; Aich, J.; Hariharan, M.; Brahmachari, S.K.; Agrawal, A.; Ghosh, B. Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proc. Natl. Acad. Sci. USA 2009, 106, 5761–5766. [Google Scholar] [CrossRef] [PubMed]
- Girndt, M.; Pietsch, M.; Köhler, H. Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. Am. J. Kidney Dis. 1995, 26, 454–460. [Google Scholar] [CrossRef]
- Girndt, M.; Ulrich, C.; Kaul, H.; Sester, U.; Sester, M.; Köhler, H. Uremia-associated immune defect: The IL-10-CRP axis. Kidney Int. Suppl. 2003, 63, S76–S79. [Google Scholar] [CrossRef] [PubMed]
- Alscher, D.M.; Pauli-Magnus, C.; Kuhlmann, U.; Mettang, T. Stimulated IFNgamma and IL-10 secretion of blood mononuclear cells in patients on renal replacement therapies show different secretion patterns. Artif. Organs 2000, 24, 767–772. [Google Scholar] [CrossRef]
- Perianayagam, M.C.; Jaber, B.L.; Guo, D.; King, A.J.; Pereira, B.J.G.; Balakrishnan, V.S. Defective interleukin-10 synthesis by peripheral blood mononuclear cells among hemodialysis patients. Blood Purif. 2002, 20, 543–550. [Google Scholar] [CrossRef]
- Wong, J.; Vilar, E.; Farrington, K. Endotoxemia in end-stage kidney disease. Semin. Dial. 2015, 28, 59–67. [Google Scholar] [CrossRef]
| Patient’s Characteristics | H (n = 17) | N (n = 19) | Statistics |
|---|---|---|---|
| Age (years) | 62.2 ± 17.1 | 56.2 ± 15.8 | 0.286 |
| Sex (f, %) | 35.3 | 26.3 | 0.721 |
| Diabetes (%) | 47.1 | 15.8 | 0.070 |
| BMI (Kg/m2) | 29.7 ± 6.7 | 26.2 ± 4.9 | 0.090 |
| Dialysis vintage (years) | 3.3 ± 3.0 | 5.1 ± 5.2 | 0.235 |
| Dialysis (hours/week) | 13.4 ± 1.3 | 12.7 ± 1.4 | 0.115 |
| Kt/V (BS1) | 1.3 ± 0.3 | 1.3 ± 0.2 | 0.267 |
| Kt/V (BS2) | 1.2 ± 0.2 | 1.4 ± 0.3 | 0.051 |
| Residual urine volume (mL) | 541.2 ± 529.5 | 581.1 ± 468.8 | 0.158 |
| CRP (mg/dL) | 12.9 ± 11.6 | 7.0 ± 8.9 | 0.056 |
| Albumin (g/L) | 38.5 ± 4.7 | 40.3 ± 2.5 | 0.252 |
| Haemoglobin (mmol/L) | 6.7 ± 0.8 | 6.8 ± 0.8 | 0.643 |
| Creatinine (µmol/L) | 778 ± 280 | 879 ± 320 | 0.327 |
| ECW (L) | 20.8 ± 5.0 | 15.5 ± 3.1 | 0.001 |
| ICW (L) | 26.4 ± 5.0 | 24.2 ± 3.5 | 0.144 |
| TBW (L) | 47.2 ± 9.6 | 39.8 ± 6.4 | 0.001 |
| ECW/TBW (%) | 44.0 ± 3.6 | 37.2 ± 1.8 | 0.001 |
| Interdialytic weight gain (kg) | 3.4 ± 0.9 | 2.7 ± 0.6 | 0.018 |
| Hypertensive drugs (n) | 2.8 ± 0.4 | 2.4 ± 0.4 | 0.535 |
| Diuretic drugs (n) | 0.8 ± 0.2 | 1.3 ± 0.2 | 0.054 |
| MAP (mm Hg, BS1) | 102 ± 20 | 100 ± 15 | 0.757 |
| MAP (mm Hg, BS2) | 108 ± 15 | 110 ± 21 | 0.781 |
| CCI | 6.1 ± 2.2 | 5.3 ± 2.6 | 0.323 |
| Caspase-1 Expression/Frequency | H | N | p-Value |
|---|---|---|---|
| Caspase-1 mRNA (x-fold) | 1.4 ± 1.3 | 2.1 ± 2.1 | 0.643 |
| CD14+Casp-1+ (MFI) | 46.0 ± 16.7 | 43.1 ± 29.9 | 0.243 |
| CD14+Casp-1+ (%) | 77.5 ± 4.7 | 79.2 ± 6.2 | 0.370 |
| CD14+Casp-1+AAD+ (%) | 10.2 ± 4.7 | 7.9 ± 3.7 | 0.046 |
| Caspase-1 Expression/Frequency | H | N | p-Value |
|---|---|---|---|
| CD14 + Casp-1 + (MFI) | 89.9 ± 22.6 | 88.0 ± 23.3 | 0.900 |
| CD14 + Casp-1 + (%) | 89.65 ± 4.4 | 88.5 ± 5.7 | 0.749 |
| CD14 + Casp-1 + AAD + (%) | 54.8 ± 22.1 | 52.3 ± 20.9 | 0.720 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulrich, C.; Wilke, A.; Schleicher, N.; Girndt, M.; Fiedler, R. Hypervolemia-Induced Immune Disturbances Do Not Involve IL-1ß but IL-6 and IL-10 Activation in Haemodialysis Patients. Toxins 2020, 12, 159. https://doi.org/10.3390/toxins12030159
Ulrich C, Wilke A, Schleicher N, Girndt M, Fiedler R. Hypervolemia-Induced Immune Disturbances Do Not Involve IL-1ß but IL-6 and IL-10 Activation in Haemodialysis Patients. Toxins. 2020; 12(3):159. https://doi.org/10.3390/toxins12030159
Chicago/Turabian StyleUlrich, Christof, Annegret Wilke, Nadja Schleicher, Matthias Girndt, and Roman Fiedler. 2020. "Hypervolemia-Induced Immune Disturbances Do Not Involve IL-1ß but IL-6 and IL-10 Activation in Haemodialysis Patients" Toxins 12, no. 3: 159. https://doi.org/10.3390/toxins12030159
APA StyleUlrich, C., Wilke, A., Schleicher, N., Girndt, M., & Fiedler, R. (2020). Hypervolemia-Induced Immune Disturbances Do Not Involve IL-1ß but IL-6 and IL-10 Activation in Haemodialysis Patients. Toxins, 12(3), 159. https://doi.org/10.3390/toxins12030159





