Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins
Abstract
1. Introduction
2. The Development and Propagation of Uremic VC
3. Uremic Toxins as a Significant Contributor to VC
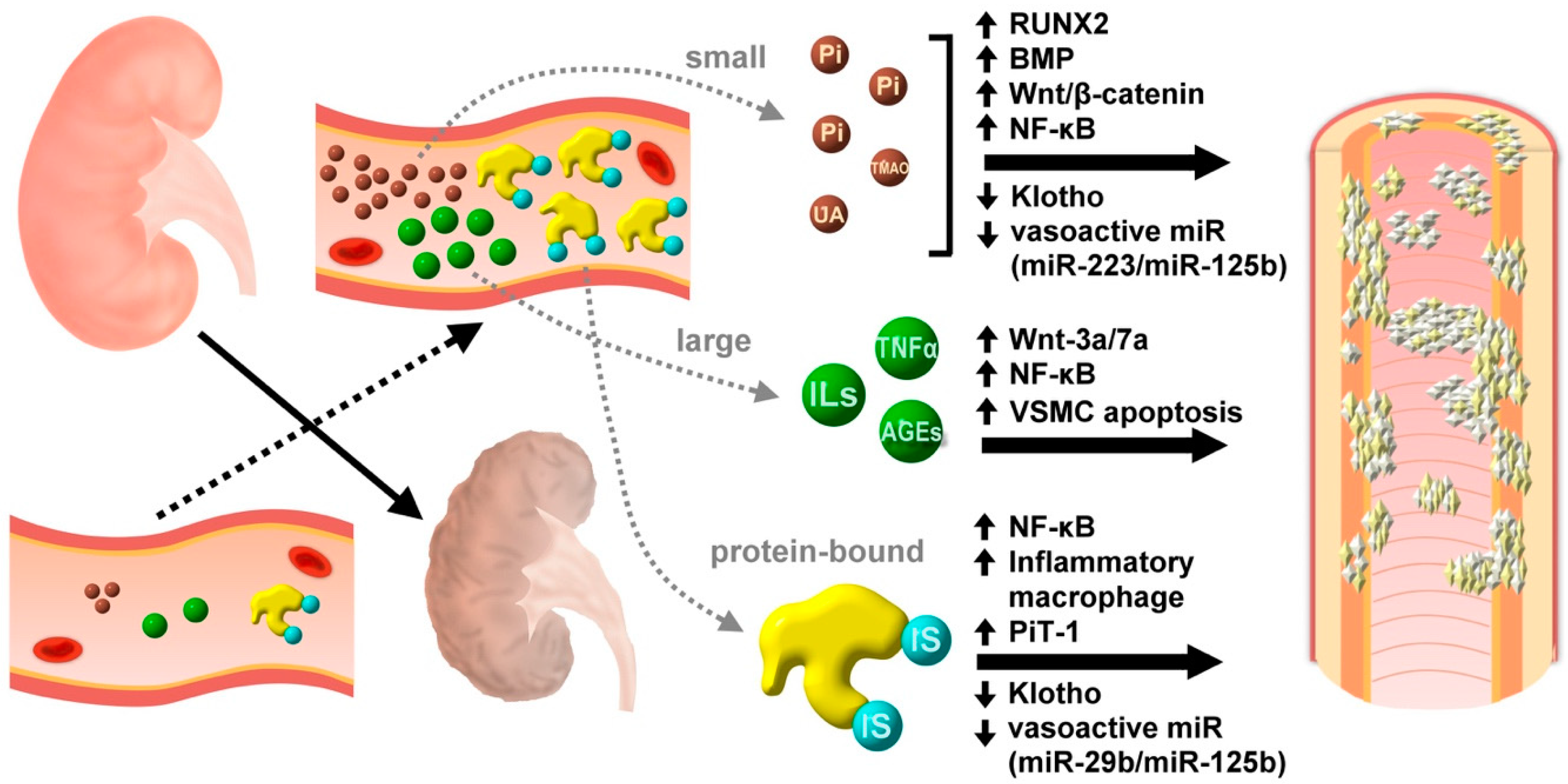
4. Classification of Uremic Toxins
4.1. Small Molecular Eeight Less Than 500 Da
Small Molecular Uremic Toxins and Uremic VC
4.2. Large Molecular (Middle Molecule) Uremic Toxins
Large Molecular (Middle Molecule) Uremic Toxins and Uremic VC
4.3. Protein-Bound Uremic Toxins
Protein-Bound Uremic Toxins and Uremic VC
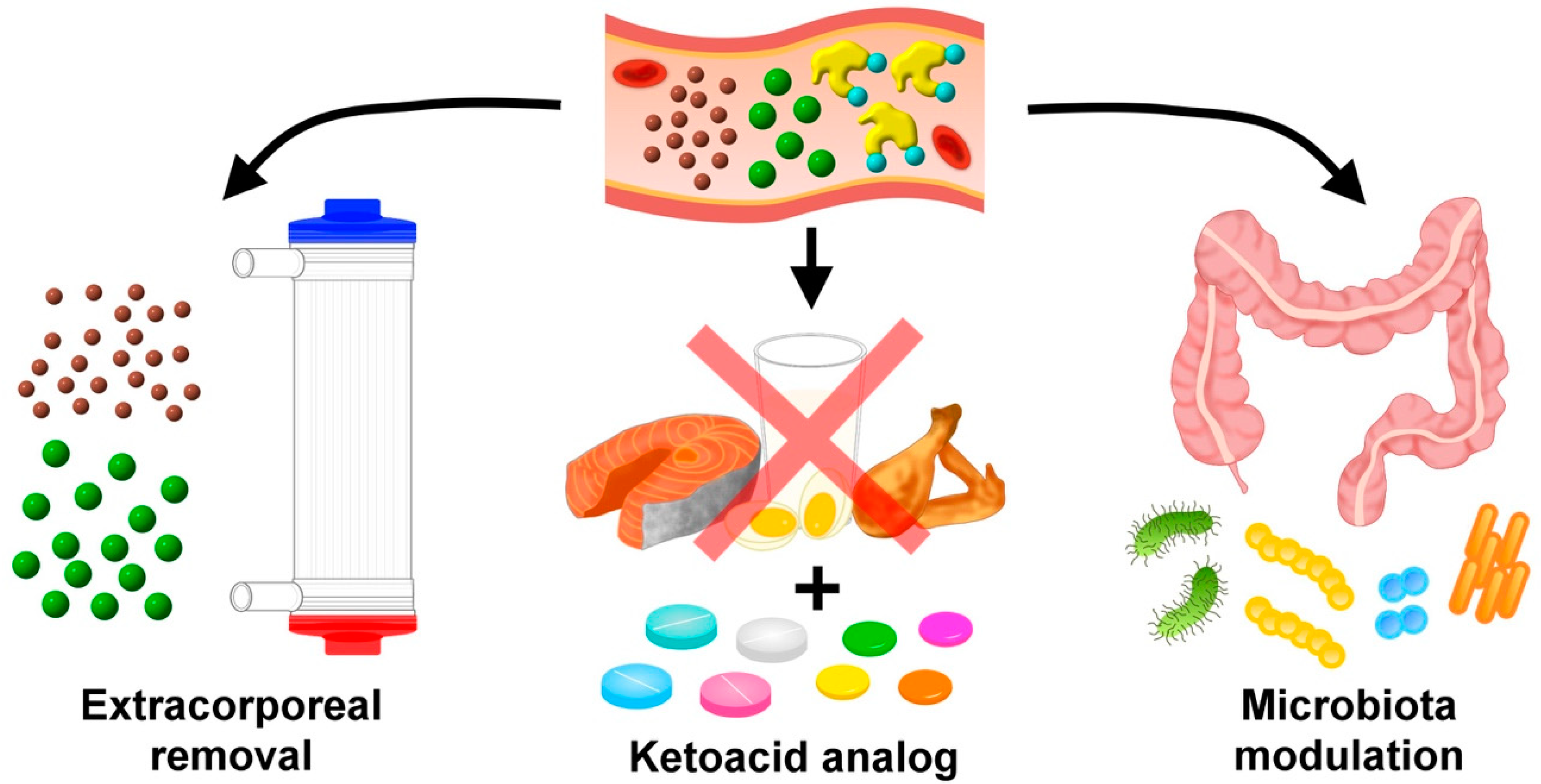
5. Existing Options of Therapeutic Uremic Toxin Reduction for Managing VC
5.1. Extracorporeal Toxin Removal
5.2. Dietary Modification with Ketoacid Supplementation
5.3. Interventions through the Gastrointestinal Tract: An Emerging Approach
6. Gastrointestinal Decontamination for Toxin Removal and VC Counteraction
6.1. Gastrointestinal Phosphate Unloading
6.1.1. Phosphate Binders
6.1.2. Inhibitors of Intestinal Phosphate Absorption
6.2. Reduce Gastrointestinal Calcium Exposure
6.3. Magnesium Competition: A Value-Added Approach
6.4. Oral-Activated Charcoal Administration
6.5. Gut Microbiota Manipulation
7. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Chao, C.T.; Yeh, H.Y.; Tsai, Y.T.; Chuang, P.H.; Yuan, T.H.; Huang, J.W.; Chen, H.W. Natural and non-natural antioxidative compounds: Potential candidates for treatment of vascular calcification. Cell Death Discov. 2019, 5, 145. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Scialla, J.J.; Dobre, M.A.; Chen, J.; Hsu, C.-Y.; Leonard, M.B.; Go, A.S.; Rao, P.S.; Lash, J.P.; et al. Serum calcification propensity and coronary artery calcification among patients with CKD: The CRIC (Chronic Renal Insufficiency Cohort) study. Am. J. Kidney Dis. 2019, 73, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wang, C.; Zhang, Q.; Ma, S.; Gui, B.; Duan, C. Prevalence of abdominal artery calcification in dialysis patients with end-stage renal disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2017, 49, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Rodriguez, M.; Ketteler, M. Laboratory abnormalities in CKD-MBD: Markers, predictors, or mediators of disease? Semin. Nephrol. 2014, 34, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J. Am. Soc. Nephrol. 2014, 25, 339–348. [Google Scholar] [CrossRef]
- Chao, C.T.; Liu, Y.P.; Su, S.F.; Yeh, H.Y.; Chen, H.Y.; Lee, P.J.; Chen, W.J.; Lee, Y.M.; Huang, J.W.; Chiang, C.K.; et al. Circulating MicroRNA-125b predicts the presence and progression of uremic vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1402–1414. [Google Scholar] [CrossRef]
- Chao, C.T.; Yuan, T.H.; Yeh, H.Y.; Chen, H.Y.; Huang, J.W.; Chen, H.W. Risk factors associated with Altered circulating micro RNA-125b and their influences on uremic vascular calcification among patients with end-stage renal disease. J. Am. Heart Assoc. 2019, 8, e010805. [Google Scholar] [CrossRef]
- Sag, A.A.; Covic, A.; London, G.; Vervloet, M.; Goldsmith, D.; Gorriz, J.L.; Kanbay, M. Clinical imaging of vascular disease in chronic kidney disease. Int. Urol. Nephrol. 2016, 48, 827–837. [Google Scholar] [CrossRef]
- Chen, N.X.; Moe, S.M. Pathophysiology of vascular calcification. Curr. Osteoporos. Rep. 2015, 13, 372–380. [Google Scholar] [CrossRef]
- Hou, Y.C.; Lu, C.L.; Yuan, T.H.; Liao, M.T.; Chao, C.T.; Lu, K.C. The epigenetic landscape of vascular calcification: An integrative perspective. Int. J. Mol. Sci. 2020, 21, 980. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Chen, J.; Yang, W.; Budoff, M.; Go, A.S.; Grunwald, J.E.; Kallem, R.R.; Post, W.S.; Reilly, M.; Ricardo, A.C.; et al. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis 2018, 271, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; He, F.; Wu, X.; Peng, F.; Huang, F.; Yu, X. Relationship between serum uric acid and all-cause and cardiovascular mortality in patients treated with peritoneal dialysis. Am. J. Kidney Dis. 2014, 64, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum trimethylamine-N-Oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. 2016, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Asami, M.; Tanabe, K.; Ito, S.; Yoshida, E.; Aoki, J.; Tanimoto, S.; Horiuchi, Y.; Yoshida, M. Impact of indoxyl sulfate on coronary plaques in patients on hemodialysis. Int. Heart J. 2018, 59, 489–496. [Google Scholar] [CrossRef]
- Yavuz, A.; Tetta, C.; Ersoy, F.F.; D’intini, V.; Ratanarat, R.; De Cal, M.; Bonello, M.; Bordoni, V.; Salvatori, G.; Andrikos, E.; et al. Reviews: Uremic toxins: A new focus on an old subject. Semin. Dial. 2005, 18, 203–211. [Google Scholar] [CrossRef]
- Lau, W.L.; Vaziri, N.D. Urea, a true uremic toxin: The empire strikes back. Clin. Sci. 2016, 131, 3–12. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Chen, Y.X.; Huang, C.; Duan, Z.B.; Xu, C.Y.; Chen, Y. Klotho/FGF23 axis mediates high phosphate-induced vascular calcification in vascular smooth muscle cells via Wnt7b/β-catenin pathway. Kaohsiung J. Med. Sci. 2019, 35, 393–400. [Google Scholar] [CrossRef]
- Chao, C.T.; Yeh, H.Y.; Yuan, T.H.; Chiang, C.K.; Chen, H.W. MicroRNA-125b in vascular diseases: An updated systematic review of pathogenetic implications and clinical applications. J. Cell. Mol. Med. 2019, 23, 5884–5894. [Google Scholar] [CrossRef] [PubMed]
- M’Baya-Moutoula, E.; Louvet, L.; Metzinger-Le Meuth, V.; Massy, Z.A.; Metzinger, L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim. Biophys. Acta 2015, 1852, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-oxide promotes vascular calcification through activation of NLRP3 (Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (Nuclear factor κB) signals. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, D.; Zhu, J.; Pang, X. The effects of hyperuricemia on the differentiation and proliferation of osteoblasts and vascular smooth muscle cells are implicated in the elevated risk of osteopenia and vascular calcification in gout: An in vivo and in vitro analysis. J. Cell. Biochem. 2019, 120, 19660–19672. [Google Scholar] [CrossRef]
- Clark, W.R.; Dehghani, N.L.; Narsimhan, V.; Ronco, C. Uremic toxins and their relation to dialysis efficiency. Blood. Purif. 2019, 48, 299–314. [Google Scholar] [CrossRef]
- Wolley, M.J.; Hutchison, C.A. Large uremic toxins: An unsolved problem in end-stage kidney disease. Nephrol. Dial. Transpl. 2018, 33, 11. [Google Scholar] [CrossRef]
- Florens, N.; Juillard, L. Large middle molecule and albumin removal: Why should we not rest on our laurels? Contrib. Nephrol. 2017, 191, 178–187. [Google Scholar]
- Kizu, A.; Shioi, A.; Jono, S.; Koyama, H.; Okuno, Y.; Nishizawa, Y. Statins inhibit in vitro calcification of human vascular smooth muscle cells induced by inflammatory mediators. J. Cell. Biochem. 2004, 93, 1011–1019. [Google Scholar] [CrossRef]
- Al-Aly, Z. Arterial calcification: A tumor necrosis factor-alpha mediated vascular Wnt-opathy. Transl. Res. 2008, 151, 233–239. [Google Scholar] [CrossRef]
- Koike, S.; Yano, S.; Tanaka, S.; Sheikh, A.M.; Nagai, A.; Sugimoto, T. Advanced glycation end-products induce apoptosis of vascular smooth muscle cells: A mechanism for vascular calcification. Int. J. Mol. Sci. 2016, 17, 1567. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. P-cresyl sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; D’Haese, P.C.; Verhulst, A. Molecular and cellular mechanisms that induce arterial calcification by indoxyl sulfate and P-cresyl sulfate. Toxins 2020, 12, 58. [Google Scholar] [CrossRef]
- Hénaut, L.; Mary, A.; Chillon, J.M.; Kamel, S.; Massy, Z.A. The impact of uremic toxins on vascular smooth muscle cell function. Toxins 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Zhang, H.; Liu, T.; Zhang, H.; Teng, J.; Ji, J.; Ding, X. Indoxyl sulfate enhance the hypermethylation of klotho and promote the process of vascular calcification in chronic kidney disease. Int. J. Biol. Sci. 2016, 12, 1236–1246. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Shen, Z.; Gu, Y.; Xu, L.; Hu, J.; Zhang, X.; Ding, X. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/β-catenin signaling. Toxicol. Lett. 2018, 284, 29–36. [Google Scholar] [CrossRef]
- He, X.; Jiang, H.; Gao, F.; Liang, S.; Wei, M.; Chen, L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-κB signaling pathway. Microsc. Res. Tech. 2019, 82, 2000–2006. [Google Scholar] [CrossRef]
- Wu, Y.; Han, X.; Wang, L.; Diao, Z.; Liu, W. Indoxyl sulfate promotes vascular smooth muscle cell calcification via the JNK/Pit-1 pathway. Ren. Fail. 2016, 38, 1702–1710. [Google Scholar] [CrossRef]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.; Kum, A.S.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-Notch signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef]
- Taguchi, K.; Elias, B.C.; Brooks, C.R.; Ueda, S.; Fukami, K. Uremic toxin–targeting as a therapeutic strategy for preventing cardiorenal syndrome. Circ. J. 2020, 84, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Eloot, S.; Ledebo, I.; Ward, R.A. Extracorporeal removal of uremic toxins: Can we still do better? Semin. Nephrol. 2014, 34, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; de Oliveira, M.C.; Fouque, D. Ketoacid analogues supplementation in chronic kidney disease and future perspectives. Nutrients 2019, 11, 2071. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Xu, H.; Carrero, J.J.; Pascoe, E.; French, C.; Campbell, K.L. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 860–865. [Google Scholar] [CrossRef]
- Locatelli, F.; Del Vecchio, L.; Aicardi, V. Nutritional issues with incremental dialysis: The role of low-protein diets. Semin. Dial. 2017, 30, 246–250. [Google Scholar] [CrossRef]
- Evenepoel, P.; Dejongh, S.; Verbeke, K.; Meijers, B. The role of gut dysbiosis in the bone-vascular axis in chronic kidney disease. Toxins 2020, 12, 285. [Google Scholar] [CrossRef]
- Muteliefu, G.; Enomoto, A.; Jiang, P.; Takahashi, M.; Niwa, T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol. Dial. Transpl. 2009, 24, 2051–2058. [Google Scholar] [CrossRef]
- Chao, C.T.; Yeh, H.Y.; Tsai, Y.T.; Chiang, C.K.; Chen, H.W. A combined microRNA and taregt protein-based panel for predicting the probability and severity of uremic vascular calcification. Cardiovasc. Res. 2020, cvaa255. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Centron, P.; Barrows, I.; Dwivedi, R.; Raj, D.S. Gut microbiota and cardiovascular uremic toxicities. Toxins 2018, 10, 287. [Google Scholar] [CrossRef]
- Kumari, R.; Palaniyandi, S.; Hildebrandt, G.C. Microbiome: An emerging new frontier in graft-versus-host disease. Dig. Dis. Sci. 2019, 64, 669–677. [Google Scholar] [CrossRef]
- Kesecioglu, J.; Eggimann, P. What is new in selective decontamination of the digestive tract? Intensive Care Med. 2016, 42, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lezana, T.; Raurell, I.; Bravo, M.; Torres-Arauz, M.; Salcedo, M.T.; Santiago, A.; Schoenenberger, A.; Manichanh, C.; Genesca, J.; Martell, M.; et al. Restoration of a healthy intestinal microbiota normalized portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology 2018, 67, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Ruospo, M.; Palmer, S.C.; Natale, P.; Craig, J.C.; Vecchio, M.; Elder, G.J.; Strippoli, G.F. Phosphate binder for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane. Database. Syst. Rev. 2018, 8, CD006023. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Del Vecchio, L.; Violo, L.; Pontoriero, G. Phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: A comparison of safety profiles. Expert. Opin. Durg. Saf. 2014, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.H.; Chung, C.H.; Chien, W.C.; Chu, P. Effects of calcium-containing phosphate binders on cardiovascular events and mortality in predialysis CKD stage 5 patients. PLoS ONE 2020, 15, e0241435. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Kono, K.; Nakai, K.; Goto, S.; Nishii, T.; Kono, A.; Nishi, S. Effects of lanthanum carbonate on coronary artery calcification and cardiac abnormalities after initiating hemodialysis. Calcif. Tissue. Int. 2018, 102, 310–320. [Google Scholar] [CrossRef]
- Bouma-de Krijger, A.; van Ittersum, F.J.; Hoekstra, T.; Ter Wee, P.M.; Vervloet, M.G. Short-term effects of sevelamer-carbonate on fibroblast growth factor 23 and pulse wave velocity in patients with normophosphatemic chronic kidney disease stage 3. Clin. Kidney. J. 2019, 12, 678–685. [Google Scholar] [CrossRef]
- Di Lorio, B.; Bellasi, A.; Russo, D. Independent study investigators. Mortality in kidney disease patients treated with phosphate binders: A randomized study. Clin. J. Am. Soc. Nephrol. 2012, 7, 487–493. [Google Scholar] [CrossRef]
- Toussaint, N.D.; Pedagogos, E.; Lioufas, N.M.; Elder, G.J.; Pascoe, E.M.; Badve, S.V.; Valks, A.; Block, G.A.; Boudville, N.; Cameron, J.D.; et al. A randomized trial on the effect of phosphate reduction on vascular end points in CKD (IMPROVE-CKD). J. Am. Soc. Nephrol. 2020, 31, 2653–2666. [Google Scholar] [CrossRef]
- Lida, A.; Kemmochi, Y.; Kakimoto, K.; Tanimoto, M.; Mimura, T.; Shinozaki, Y.; Uemura, A.; Matsuo, A.; Matsushita, M.; Miyamoto, K. Ferric citrate hydrate, a new phosphate binder, prevents the complications of secondary hyperparathyroidism and vascular calcification. Am. J. Nephrol. 2013, 37, 346–358. [Google Scholar]
- Ciceri, P.; Falleni, M.; Tosi, D.; Martinelli, C.; Bulfamante, G.; Block, G.A.; Messa, P.; Cozzolino, M. High-phosphate induced vascular calcification is reduced by iron citrate through inhibition of extracellular matrix osteo-chondrogenic shift in VSMCs. Int. J. Cardiol. 2019, 297, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Neradova, A.; Schumacher, S.P.; Hubeek, I.; Lux, P.; Schurgers, L.J.; Vervloet, M.G. Phosphate binders affect vitamin K concentration by undesired binding, an in vitro study. BMC Nephrol. 2017, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.G.; Biancorosso, N.; Brilli, E.; Tarantino, G.; Adorni, M.P.; Vivian, G.; Salvalaio, M.; Dall’Acqua, S.; Sut, S.; Neutel, C.; et al. Cholesterol-lowering action of a novel nutraceutical combination in uremic rats: Insights into the molecular mechanism in a hepatoma cell line. Nutrients 2020, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Vervloet, M.; Ketteler, M. Targeting gastrointestinal transport proteins to control hyperphosphatemia in chronic kidney disease. Drugs 2018, 78, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Tanaka, A.; Nakamura, T.; Fukuwatari, T.; Shibata, J.; Shimada, N.; Ebihara, I.; Koide, H. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney. Int. 2004, 65, 1099–1104. [Google Scholar] [CrossRef]
- Kaesler, N.; Goettsch, C.; Weis, D.; Schurgers, L.; Hellmann, B.; Floege, J.; Kramann, R. Magnesium but not nicotinamide prevents vascular calcification in experimental uraemia. Nephrol. Dial. Transpl. 2020, 35, 65–73. [Google Scholar] [CrossRef]
- King, A.J.; Siegel, M.; He, Y.; Nie, B.; Wang, J.; Koo-McCoy, S.; Minassian, N.A.; Jafri, Q.; Pan, D.; Kohler, J.; et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci. Transl. Med. 2018, 10, eaam6474. [Google Scholar] [CrossRef]
- Block, G.A.; Rosenbaum, D.P.; Leonsson-Zachrisson, M.; Astrand, M.; Johansson, S.; Knutsson, M.; Langkilde, A.M.; Chertow, G.M. Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 1933–1942. [Google Scholar] [CrossRef]
- Block, G.A.; Rosenbaum, D.P.; Yan, A.; Chertow, G.M. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: A randomized phase 3 trial. J. Am. Soc. Nephrol. 2019, 30, 641–652. [Google Scholar] [CrossRef]
- Markham, A. Tenapanor: First approval. Drugs 2019, 79, 1897–1903. [Google Scholar] [CrossRef]
- Derici, U.; El Nahas, A.M. Vascular calcifications in uremia: Old concepts and new insights. Semin. Dial. 2006, 19, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Salusky, I.B.; Goodman, W.G. Cardiovascular calcification in end-stage renal disease. Nephrol. Dial. Transplant. 2002, 17, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, A.; Sonou, T.; Ohya, M.; Yashiro, M.; Nakashima, Y.; Okuda, K.; Iwashita, Y.; Mima, T.; Negi, S.; Shigematsu, T. Calcium overload accelerates phosphate-induced calcification via Pit-1, but not the calcium-sensing receptor. J. Atheroscler. Thromb. 2017, 24, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.D.; Gomez, L.M.; Marchioni, D.M.L.; Dos Anjos, F.S.N.; Molina, M.D.C.B.; Lotufo, P.A.; Bensenor, I.J.M.; de Oliveira Titan, S.M. Association between dietary intake and coronary artery calcification in non-dialysis chronic kidney disease: The PROGREDIR study. Nutrients 2018, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Leenders, N.H.; Vervloet, M.G. Magnesium: A magic bullet for cardiovascular disease in chronic kidney disease? Nutrients 2019, 11, 455. [Google Scholar] [CrossRef]
- De Schutter, T.M.; Behets, G.J.; Geryl, H.; Peter, M.E.; Steppan, S.; Gundlach, K.; Passlick-Deetjen, J.; D’Haese, P.C.; Neven, E. Effect of a magnesium-based phosphate binder on medial calcification in a rat model of uremia. Kidney. Int. 2013, 83, 1109–1117. [Google Scholar] [CrossRef]
- Ciceri, P.; Volpi, E.; Brenna, I.; Elli, F.; Borghi, E.; Brancaccio, D.; Cozzolino, M. The combination of lanthanum chloride and the calcimimetic calindol delays the progression of vascular smooth muscle cells calcification. Biochem. Biophys. Res. Commun. 2012, 418, 770–773. [Google Scholar] [CrossRef]
- Ishikawa, I.; Araya, M.; Hayama, T.; Sugano, M.; Yamato, H.; Ise, M. Effect of oral adsorbent (AST-120) on renal function, acquired renal cysts and aortic calcification in rats with adriamycin nephropathy. Nephron 2002, 92, 399–406. [Google Scholar] [CrossRef]
- Yamamoto, S.; Zuo, Y.; Ma, J.; Yancey, P.G.; Hunley, T.E.; Motojima, M.; Fogo, A.B.; Linton, M.F.; Fazio, S.; Ichikawa, I.; et al. Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol. Dial. Transpl. 2011, 26, 2491–2497. [Google Scholar] [CrossRef]
- Muteliefu, G.; Shimizu, H.; Enomoto, A.; Nishijima, F.; Takahashi, M.; Niwa, T. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelamin A through oxidative stress. Am. J. Physiol. Cell Physiol. 2012, 303, 126–134. [Google Scholar] [CrossRef]
- Kuwahara, M.; Bannai, K.; Segawa, H.; Miyamoto, K.I.; Yamato, H. Cardiac remodeling associated with protein increase and lipid accumulation in early-stage chronic kidney disease in rats. Biochim. Biophys. Acta 2014, 1842, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Inami, Y.; Hamada, C.; Seto, T.; Hotta, Y.; Aruga, S.; Inuma, J.; Azuma, K.; Io, H.; Kaneko, K.; Watada, H.; et al. Effect of AST-120 on endothelial dysfunction in adenine-induced Uremic rats. Int. J. Nephrol. 2014, 2014, 164125. [Google Scholar] [CrossRef] [PubMed]
- Six, I.; Gross, P.; Rémond, M.C.; Chillon, J.M.; Poirot, S.; Drueke, T.B.; Massy, Z.A. Deleterious vascular effects of indoxyl sulfate and reversal by oral adsorbent AST-120. Atherosclerosis 2015, 243, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Nakada, Y.; Onoue, K.; Nakano, T.; Ishihara, S.; Kumazawa, T.; Nakagawa, H.; Ueda, T.; Nishida, T.; Soeda, T.; Okayama, S.; et al. AST-120, an oral carbon absorbent, protects against the progression of atherosclerosis in a mouse chronic renal failure model by preserving sFlt-1 expression levels. Sci. Rep. 2019, 9, 15571. [Google Scholar] [CrossRef]
- Goto, S.; Kitamura, K.; Kono, K.; Nakai, K.; Fujii, H.; Nishi, S. Association between AST-120 and abdominal aortic calcification in predialysis patients with chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 365–371. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, G.; Li, Y.; Lv, C.; Wang, Z. Effects of oral activated charcoal on hyperphosphatemia and vascular calcification in Chinese patients with stage 3-4 chronic kidney disease. J. Nephrol. 2019, 32, 265–272. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Hamano, T.; Obi, Y.; Monden, C.; Oka, T.; Yamaguchi, S.; Matsui, I.; Hashimoto, N.; Matsumoto, A.; Shimada, K.; et al. A Randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J. Am. Soc. Nephrol. 2019, 30, 1073–1085. [Google Scholar] [CrossRef]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Backhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, 1–63. [Google Scholar] [CrossRef]
- Meijers, B.K.; De Preter, V.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-cresul sulfate serum concentrations in patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transpl. 2010, 25, 219–224. [Google Scholar] [CrossRef]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Takayama, F.; Taki, K.; Niwa, T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am. J. Kidney. Dis. 2003, 41, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.K.; Wu, Y.Y.; Yang, Y.F.; Ting, I.W.; Lin, C.C.; Yen, T.H.; Chen, J.H.; Wang, C.H.; Huang, C.C.; Lin, H.C. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dualysis patients: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2015, 6, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Yeh, H.Y.; Tsai, Y.T.; Yuan, T.H.; Liao, M.T.; Huang, J.W.; Chen, H.W. Astaxanthin counteracts vascular calcification in vitro through an early up-regulation of SOD2 based on a transcriptomic approach. Int. J. Mol. Sci. 2020, 21, 8530. [Google Scholar] [CrossRef] [PubMed]

| Phosphate Binders | Binding Efficacy * | Calcium Load * | Effect on FGF-23 | Effect on PTH | Effect on 1,25-(OH)2-vit D | Effect on Survival | Side Effects |
|---|---|---|---|---|---|---|---|
| Calcium-based (carbonate, acetate, citrate) | + | + ~ +++ | None | Decrease | Decrease | None | Hypercalcemia, ectopic calcification |
| Non-calcium-based | |||||||
| Magnesium-based | + | − | ? | Decrease | Increase (potential) | None | Diarrhea, magnesium overload |
| Iron-based | + ~ ++ | Decrease | Decrease | Increase (potential) | None | Diarrhea, iron overload | |
| Sevelamer | + ~ ++ | − | Decrease | Decrease | Increase | Improve | Constipation, metabolic acidosis (if HCl group) |
| Aluminum hydroxide | +++ | − | ? | Decrease | ? | None | Aluminum toxicity, adynamic bone disease, constipation |
| Lanthanum carbonate | ++++ | − | Decrease | Decrease | None | Improve | Constipation |
| Adsorbent Types | Dose | VC Measurement Methods | Effects | Baseline Renal Function | Number of Patients | Reference |
|---|---|---|---|---|---|---|
| AST-120 | 5.1 ± 1.4 g/d | Abdominal aortic calcifications in abdominal CT | Lower aortic calcification index in users | Stage 4 to 5 (pre-dialysis) | 199 | [85] |
| Activated charcoal | 1.8–3.6 g/d | Coronary artery calcifications in multidetector CT | Lower coronary calcium scores in users | Stage 3 to 4 | 97 | [86] |
| AST-120 | 6 g/d | Coronary artery calcifications and thoracic aorta calcifications in multidetector CT | No differences in coronary calcium scores or aortic calcification | Stage 3 to 4 | 96 | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-T.; Lin, S.-H. Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins. Toxins 2020, 12, 812. https://doi.org/10.3390/toxins12120812
Chao C-T, Lin S-H. Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins. Toxins. 2020; 12(12):812. https://doi.org/10.3390/toxins12120812
Chicago/Turabian StyleChao, Chia-Ter, and Shih-Hua Lin. 2020. "Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins" Toxins 12, no. 12: 812. https://doi.org/10.3390/toxins12120812
APA StyleChao, C.-T., & Lin, S.-H. (2020). Uremic Vascular Calcification: The Pathogenic Roles and Gastrointestinal Decontamination of Uremic Toxins. Toxins, 12(12), 812. https://doi.org/10.3390/toxins12120812





