The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review
Abstract
:1. Introduction
2. Objective
3. Results
4. Discussion
4.1. The Gut-Kidney-Axis
4.2. Uremic Toxins
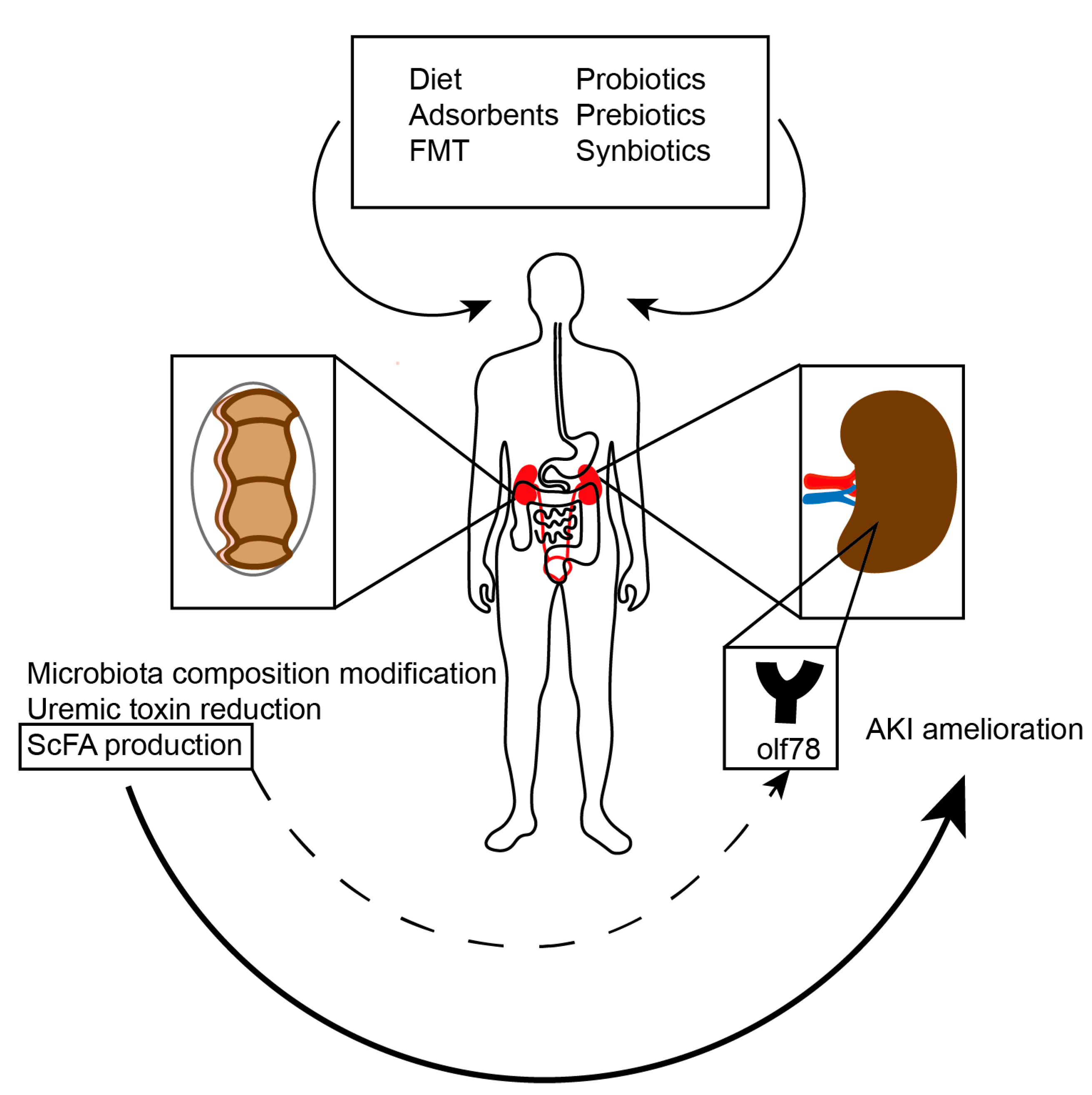
4.3. Potential Interventions
5. Conclusions
6. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NIH Human Microbiome Portfolio Analysis Team. A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007–2016. Microbiome 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2013, 25, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.R.; Gandolfo, M.T.; Ko, G.J.; Satpute, S.; Racusen, L.; Rabb, H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am. J. Physiol. Physiol. 2009, 297, F1457–F1465. [Google Scholar] [CrossRef] [Green Version]
- Nandi, D.K.; Samanta, A.; Patra, A.; Mandal, S.; Roy, S.; Das, K.; Kar, S. Hypoxia: A cause of acute renal failure and alteration of gastrointestinal microbial ecology. Saudi J. Kidney Dis. Transplant. 2018, 29, 879–888. [Google Scholar] [CrossRef]
- Long, Y.; Zhen, X.; Zhu, F.; Hu, Z.; Lei, W.; Li, S.; Zha, Y.; Nie, J. Hyperhomocysteinemia Exacerbates Cisplatin-induced Acute Kidney Injury. Int. J. Biol. Sci. 2017, 13, 219–231. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Moturi, K.; Wang, L.; Zhang, K.; Yu, C. Gut derived-endotoxin contributes to inflammation in severe ischemic acute kidney injury. BMC Nephrol. 2019, 20, 16. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Peng, X.; Yang, J.; Giovanni, V.; Wang, C. Impacts of functional oligosaccharide on intestinal immune modulation in immunosuppressive mice. Saudi J. Biol. Sci. 2020, 27, 233–241. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Popkov, V.A.; Klimenko, N.; Tyakht, A.; Zakharova, E.Y.; Frolova, O.Y.; Zorova, L.D.; Pevzner, I.B.; Zorov, D.B.; Plotnikov, E.Y. Microbiome-Metabolome Signature of Acute Kidney Injury. Metabolites 2020, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Mishima, E.; Ichijo, M.; Kawabe, T.; Kikuchi, K.; Akiyama, Y.; Toyohara, T.; Suzuki, T.; Suzuki, C.; Asao, A.; Ishii, N.; et al. Germ-Free Conditions Modulate Host Purine Metabolism, Exacerbating Adenine-Induced Kidney Damage. Toxins 2020, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Knoflach, A.; Binswanger, U. Serum hippuric acid concentration in renal allograft rejection, ureter obstruction, and tubular necrosis. Transpl. Int. 1994, 7, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Carron, C.; De Barros, J.-P.P.; Gaiffe, E.; Deckert, V.; Adda-Rezig, H.; Roubiou, C.; Laheurte, C.; Masson, D.; Simula-Faivre, D.; Louvat, P.; et al. End-Stage Renal Disease-Associated Gut Bacterial Translocation: Evolution and Impact on Chronic Inflammation and Acute Rejection After Renal Transplantation. Front. Immunol. 2019, 10, 1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Hao, G.; Pan, Y.; Ma, S.; Yang, T.; Shi, P.; Zhu, Q.; Xie, Y.; Ma, S.; Zhang, Q.; et al. Serum indoxyl sulfate is associated with mortality in hospital-acquired acute kidney injury: A prospective cohort study. BMC Nephrol. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Veldeman, L.; Vanmassenhove, J.; Van Biesen, W.; Massy, Z.A.; Liabeuf, S.; Glorieux, G.; Vanholder, R. Evolution of protein-bound uremic toxins indoxyl sulphate and p-cresyl sulphate in acute kidney injury. Int. Urol. Nephrol. 2019, 51, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.A.; Constantino, L.D.S.; Tomasi, C.D.; Rojas, H.A.; Vuolo, F.S.; Vitto, M.F.; Cesconetto, P.A.; De Souza, C.T.; Ritter, C.; Dal-Pizzol, F. Sodium butyrate decreases the activation of NF-kappaB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol. Dial. Transplant. 2012, 27, 3136–3140. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, B.; Hong, X.; Zhang, X.; Kong, X. Histone deacetylase inhibitor, sodium butyrate, attenuates gentamicin-induced nephrotoxicity by increasing prohibitin protein expression in rats. Eur. J. Pharmacol. 2013, 707, 147–154. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.; De Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef]
- Fujii, H.; Yonekura, Y.; Yamashita, Y.; Kono, K.; Nakai, K.; Goto, S.; Sugano, M.; Goto, S.; Fujieda, A.; Ito, Y.; et al. Anti-oxidative effect of AST-120 on kidney injury after myocardial infarction. Br. J. Pharmacol. 2016, 173, 1302–1313. [Google Scholar] [CrossRef] [Green Version]
- Emal, D.; Rampanelli, E.; Stroo, I.; Butter, L.M.; Teske, G.J.; Claessen, N.; Stokman, G.; Florquin, S.; Leemans, J.C.; Dessing, M.C. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2016, 28, 1450–1461. [Google Scholar] [CrossRef] [Green Version]
- Nakade, Y.; Iwata, Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R.; Miyake, T.; Sakai, N.; Kitajima, S.; Toyama, T.; et al. Gut microbiota–derived D-serine protects against acute kidney injury. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Harbi, N.O.; Nadeem, A.; Ahmad, S.F.; Alotaibi, M.R.; Alasmari, A.F.; Alanazi, W.A.; Al-Harbi, M.M.; El-Sherbeeny, A.M.; Ibrahim, K.E. Short chain fatty acid, acetate ameliorates sepsis-induced acute kidney injury by inhibition of NADPH oxidase signaling in T cells. Int. Immunopharmacol. 2018, 58, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Park, D.; Kim, Y.J.; Lee, I.; Kim, S.; Oh, C.; Kim, J.; Yang, J.; Jo, S.-K. Lactobacillus salivarius BP121 prevents cisplatin-induced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and p-cresol sulfate via alleviating dysbiosis. Int. J. Mol. Med. 2020, 45, 1130–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.-W.; Pan, P.; Chen, K.-W.; Fan, J.-X.; Li, C.-X.; Cheng, H.; Zhang, X.-Z. An orally delivered microbial cocktail for the removal of nitrogenous metabolic waste in animal models of kidney failure. Nat. Biomed. Eng. 2020, 4, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Aronsohn, A.; Reddy, K.G.; Te, H.S. Rifaximin Decreases the Incidence and Severity of Acute Kidney Injury and Hepatorenal Syndrome in Cirrhosis. Dig. Dis. Sci. 2016, 61, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.D. The coming-of-age of the hygiene hypothesis. Respir. Res. 2001, 2, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.; Wong, J.; Pahl, M.V.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitching, A.R.; Holdsworth, S.R. The Emergence of Th17 Cells as Effectors of Renal Injury. J. Am. Soc. Nephrol. 2011, 22, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Meek, B.; Doi, Y.; Muramatsu, M.; Chiba, T.; Honjo, T.; Fagarasan, S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. USA 2004, 101, 1981–1986. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, A.J.; Gatto, D.; Sainsbury, E.; Harriman, G.R.; Hengartner, H.; Zinkernagel, R.M. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 2000, 288, 2222–2226. [Google Scholar] [CrossRef]
- Kiryluk, K.; Li, Y.; Scolari, F.; Sanna-Cherchi, S.; Choi, M.; Verbitsky, M.; Fasel, D.A.; Lata, S.; Prakash, S.; Shapiro, S.; et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat. Genet. 2014, 46, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Kortman, G.A.M.; Reijnders, D.; Swinkels, D.W. Oral iron supplementation: Potential implications for the gut microbiome and metabolome in patients with CKD. Hemodial. Int. 2017, 21, S28–S36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortman, G.A.M.; Dutilh, B.E.; Maathuis, A.J.H.; Engelke, U.F.; Boekhorst, J.; Keegan, K.P.; Nielsen, F.G.G.; Betley, J.R.; Weir, J.C.; Kingsbury, Z.; et al. Microbial Metabolism Shifts Towards an Adverse Profile with Supplementary Iron in the TIM-2 In vitro Model of the Human Colon. Front. Microbiol. 2016, 6, 1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, M.; Hayashi, H.; Watanabe, M.; Ueda, K.; Yamato, H.; Yoshioka, T.; Motojima, M. Uremic Toxins Overload Accelerates Renal Damage in a Rat Model of Chronic Renal Failure. Nephron Exp. Nephrol. 2003, 95, e111–e118. [Google Scholar] [CrossRef]
- Devlin, A.S.; Marcobal, A.; Dodd, D.; Nayfach, S.; Plummer, N.; Meyer, T.; Pollard, K.S.; Sonnenburg, J.L.; Fischbach, M. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe 2016, 20, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Meijers, B.; Bammens, B.; De Moor, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008, 73, 1174–1180. [Google Scholar] [CrossRef] [Green Version]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, J.A.; Wheeler, D.C. The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease. Kidney Int. 2017, 92, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Shafi, T.; Powe, N.R.; Meyer, T.W.; Hwang, S.; Hai, X.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Hostetter, T.H. Trimethylamine N-Oxide and Cardiovascular Events in Hemodialysis Patients. J. Am. Soc. Nephrol. 2016, 28, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Nygård, O.; Nordrehaug, J.E.; Refsum, H.; Ueland, P.M.; Farstad, M.; Vollset, S.E. Plasma Homocysteine Levels and Mortality in Patients with Coronary Artery Disease. N. Engl. J. Med. 1997, 337, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ponte, B.; Pruijm, M.; Marques-Vidal, P.; Martin, P.-Y.; Burnier, M.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Bochud, M. Determinants and burden of chronic kidney disease in the population-based CoLaus study: A cross-sectional analysis. Nephrol. Dial. Transplant. 2013, 28, 2329–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamison, R.L.; Hartigan, P.; Kaufman, J.S.; Goldfarb, D.S.; Warren, S.R.; Guarino, P.D.; Gaziano, J.M.; For the Veterans Affairs Site Investigators. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: A randomized controlled trial. JAMA 2007, 298, 1163–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalim, S.; Clish, C.B.; Deferio, J.J.; Ortiz, G.; Moffett, A.S.; Gerszten, R.E.; Thadhani, R.I.; Rhee, E.P. Cross-sectional examination of metabolites and metabolic phenotypes in uremia. BMC Nephrol. 2015, 16, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulman, G.; Agarwal, R.; Acharya, M.; Berl, T.; Blumenthal, S.; Kopyt, N. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am. J. Kidney Dis. 2006, 47, 565–577. [Google Scholar] [CrossRef]
- Schulman, G.; Berl, T.; Beck, G.J.; Remuzzi, G.; Ritz, E.; Arita, K.; Kato, A.; Shimizu, M. Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKD. J. Am. Soc. Nephrol. 2015, 26, 1732–1746. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bliss, D.Z.; Stein, T.P.; Schleifer, C.R.; Settle, R.G. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am. J. Clin. Nutr. 1996, 63, 392–398. [Google Scholar] [CrossRef]
- Effect of Probiotics and Prebiotics in Renal Function in Septic Acute Kidney Injury Patients. Available online: https://ClinicalTrials.gov/show/NCT03877081 (accessed on 12 October 2020).
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.-X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guida, B.; Germanò, R.; Trio, R.; Russo, D.; Memoli, B.; Grumetto, L.; Barbato, F.; Cataldi, M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.-C.; McWhinney, B.C.; Ungerer, J.P.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Zhang, S.; Omede, F.; Stubbs, J. Rifaximin effects on serum trimethylamine-n-oxide in chronic kidney disease. FASEB J. 2018. [Google Scholar] [CrossRef]
- Kimber, C.; Zhang, S.; Johnson, C.; West, R.E., III; Prokopienko, A.J.; Mahnken, J.D.; Yu, A.S.; Hoofngale, A.N.; Ir, D.; Robertson, C.E.; et al. Randomized, Placebo-controlled Trial of Rifaximin Therapy for Lowering Gut- derived Cardiovascular Toxins and Inflammation in Chronic Kidney Disease. Kidney 2020, 360. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Gonzalez, H.U.D.J.E. Fecal Microbiota Transplantation as a Therapeutic Strategy in the Progression of Chronic Kidney Disease. 2018. Available online: https://ClinicalTrials.gov/show/NCT04361097 (accessed on 12 October 2020).
- Innovative Approach to Fecal Microbiota Transplantation (FMT) Applied for Chronic Kidney Disease (CKD). Available online: https://ClinicalTrials.gov/show/NCT04222153 (accessed on 12 October 2020).
- Mackowiak, P.A. Recycling Metchnikoff: Probiotics, the Intestinal Microbiome and the Quest for Long Life. Front. Public Health 2013, 1, 52. [Google Scholar] [CrossRef]


| Author | Year | Population | Uremic Toxin/Parameter Assessed | Outcome | Results and Key Observations |
|---|---|---|---|---|---|
| Experimental | |||||
| Jang et al. [5] | 2009 | germ-free mice, afterwards fed bacteria-rich diet | numbers and phenotypes of T cells and NK cells, panel of cytokines | extent of renal injury and functional decline after IRI | microbial stimuli influence the phenotype of renal lymphocytes and ameliorate the extent of renal injury |
| Samanta et al. [6] | 2017 | Wistar rats | microbiota composition | hypoxia induced AKI | hypobaric hypoxia causes both AKI and affects gut microbial population |
| Long et al. [7] | 2017 | C57BL/6 mice | influence of elevated Hcy levels | cisplatin-induced AKI | cisplatin induces more severe tubular injury, tubular cell apoptosis and lower proliferation in hyperHcy mice |
| Li et al. [8] | 2019 | Sprague-Dawley rats | gut-derived endotoxin | increased renal mRNA of TLR4 and proinflammatory mediators (Il-6 and MCP-1) | endotoxin increases intrarenal inflammatory response |
| Yang et al. [9] | 2020 | C57BL/6 mice and germ-free C57BL/6 mice | microbiota composition | severity of IRI | intestinal dysbiosis, inflammation and leaky gut are consequences of AKI but also determine its severity |
| Andrianova et al. [10] | 2020 | Wistar rats | microbiota composition, levels of selected toxins (acylcarnitines) | severity of IRI, creatinine and urea levels | specific bacteria in the gut may ameliorate or aggravate IRI and affect toxin levels |
| Mishima et al. [11] | 2020 | germ-free mice and mice with microbiota | metabolome analysis | extent of kidney damage in adenine-induced AKI | germ-free mice enhanced host purine metabolism and exacerbated kidney damage |
| Human | |||||
| Knoflach et al. [12] | 1994 | retrospective | hippuric acid concentration | acute kidney allograft rejection | hippuric acid concentration was higher in patients with acute allograft rejection and fell after antirejection treatment |
| Carron et al. [13] | 2019 | prospective observational design: 146 kidney transplant recipients | circulating lipopolysaccharide | chronic inflammation and acute rejection episodes | chronic exposure to LPS in the period before transplantation can promote endotoxin tolerance and those patients are less prompt to develop acute rejection after transplantation |
| Wang et al. [14] | 2019 | prospective observational design: 262 patients with hospital-acquired AKI | serum indoxyl sulfate levels | 90-day mortality | serum indoxyl sulfate levels were elevated in patients with AKI and associated with a worse prognosis |
| Veldeman et al. [15] | 2019 | prospective observational design:194 patients with sepsis | serum indoxyl sulfate and p-cresyl sulfate levels | acute kidney injury due to sepsis | serum indoxyl sulfate and p-cresyl sulfate levels were higher in patients with AKI and correlated with AKI course |
| Author | Year | Population | Uremic Toxin/Parameter Assessed | Outcome | Results and Key Observations |
|---|---|---|---|---|---|
| Experimental | |||||
| Machado et al. [16] | 2012 | Wistar rats | SCFA (sodium butyrate) | levels of creatinine, inflammatory markers and histology in contrast induced AKI | SCFA treatment attenuated creatinine levels and histological damage |
| Sun et al. [17] | 2013 | Sprague-Dawley rats | SCFA (sodium butyrate) | levels of creatinine, AKI markers, antioxidant enzymes and histology in gentamicin induced AKI | chronic treatment with SCFA protects from gentamicin-induced nephrotoxicity |
| Andrade-Oliveira et al. [18] | 2015 | C57BL/6 mice | SCFAs (acetate, butyrate, propionate) | levels of creatinine and urea, necrosis score in kidney tubular epithelial cells in IRI | mice treated with acetate-producing bacteria had improved mitochondrial biogenesis and better outcomes |
| Fujii et al. [19] | 2016 | SH rats | AST-120 | myocardial infarction induced kidney damage | treatment with AST-120 may have protective effects (reduced indole levels and urine, serum biomarkers of kidney injury) |
| Emal et al. [20] | 2017 | C57BL/6 wild-type mice | broad-spectrum antibiotics | renal damage and tubular integrity after IRI | depletion of gut microbiota protects against renal injury |
| Nakade et al. [21] | 2018 | germ-free C57BL/6 mice | D-serine | hypoxia-induced tubular damage and kidney function | renoprotective effects of gut-derived D-serine in AKI proven |
| Al-Harbi et al. [22] | 2018 | BALB/c mice | SCFA (sodium acetate) | kidney function/ renal peroxidase activity/kidney tubular structure in sepsis induced AKI | acetate ameliorates sepsis-induced kidney injury by restoration of oxidant–antioxidant balance in T cells |
| Lee et al. [23] | 2020 | Sprague-Dawley rats and Caco-2 cells | Lactobacillus salivarius BP121 | cisplatin-induced AKI occurence | L. salivarius BP121 reduced Caco-2 damage and protected against cisplatin-induced AKI |
| Zheng et al. [24] | 2020 | BALB/c mice and Bama miniature pigs | microbial cocktail (Escherichia, Bacillus, Enterobacter) | urea and creatinine concentration in nephrotoxin-induced AKI (adenine, cisplatin, glycerol) | in both murine and porcine models of AKI the orally delivered cocktail reduced urea and creatinine concentration |
| Human | |||||
| Dong et al. [25] | 2016 | retrospective analysis, 176 cirrhotic adult patients (88 treated with rifaximin | rifaximin | AKI & HRS risk | incidence rate ratio of AKI and HRS, as well as the risk of RRT was lower in the rifaximin group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydzewska-Rosołowska, A.; Sroka, N.; Kakareko, K.; Rosołowski, M.; Zbroch, E.; Hryszko, T. The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review. Toxins 2020, 12, 788. https://doi.org/10.3390/toxins12120788
Rydzewska-Rosołowska A, Sroka N, Kakareko K, Rosołowski M, Zbroch E, Hryszko T. The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review. Toxins. 2020; 12(12):788. https://doi.org/10.3390/toxins12120788
Chicago/Turabian StyleRydzewska-Rosołowska, Alicja, Natalia Sroka, Katarzyna Kakareko, Mariusz Rosołowski, Edyta Zbroch, and Tomasz Hryszko. 2020. "The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review" Toxins 12, no. 12: 788. https://doi.org/10.3390/toxins12120788
APA StyleRydzewska-Rosołowska, A., Sroka, N., Kakareko, K., Rosołowski, M., Zbroch, E., & Hryszko, T. (2020). The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review. Toxins, 12(12), 788. https://doi.org/10.3390/toxins12120788






