Determination of Zearalenone and Trichothecenes, Including Deoxynivalenol and Its Acetylated Derivatives, Nivalenol, T-2 and HT-2 Toxins, in Wheat and Wheat Products by LC-MS/MS: A Collaborative Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Pre-Trial Results
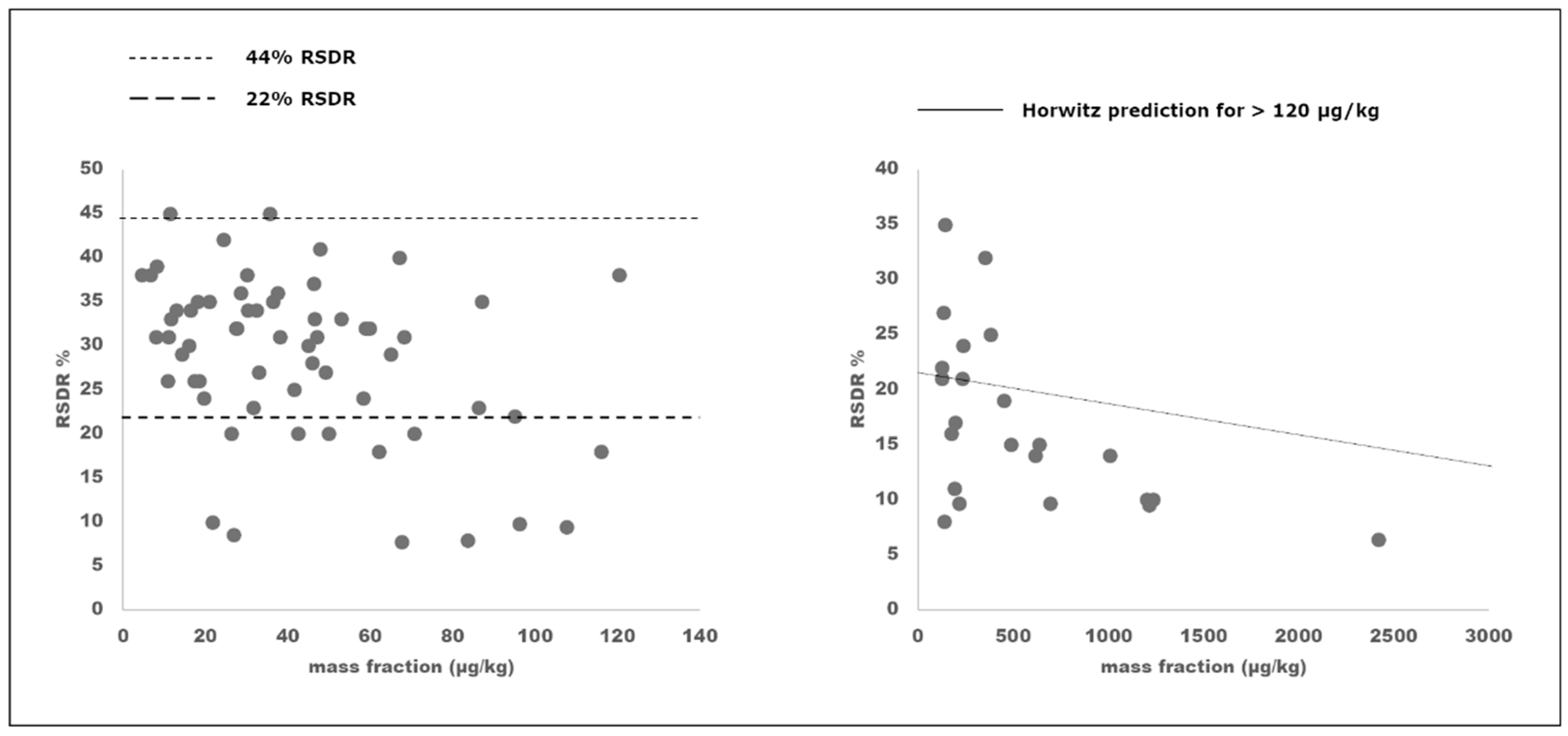
2.2. Full Collaborative Study
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Test Materials
4.2.1. Preparation of Whole Soft Wheat and Soft Wheat Flour Test Materials
4.2.2. Preparation of Wheat Crackers Test Materials
4.2.3. Homogeneity of Test Materials
4.2.4. Stability of Test Materials
4.3. Collaborative Study
4.3.1. Study Layout
- One blank wheat flour sample, to be used for five determinations (two determinations as blank and three determinations for recovery check).
- One wheat sample (to be analyzed as blind duplicate) contaminated with 1298 µg/kg DON; 58 µg/kg HT-2; 8.3 µg/kg T-2; 148 µg/kg ZEN.
- A mixed stock solution (stock solution B, see Section 4.3.2) and a mixed standard solution in acetonitrile to be used for spiking purposes and calibrants preparation, respectively, and a mixed isotopically labeled internal standard (ISTD) solution in acetonitrile (mixed ISTD, see Section 4.3.2) to be used as internal standard.
- Columns for solid phase extraction (SPE) clean-up.
- Method protocol in SOP (Standard Operating Procedure) format and reporting sheets.
- Two blank materials per each commodity (wheat, wheat flour, wheat crackers), about 15 g each, to be used for two independent determinations as spiked sample for recovery checking. Participants were asked to fortify the material with the respective spiking solution with an evaporation time of approximately 1 h before the determination.
- Blind duplicates of 1 blank material and 3 contaminated materials (low, medium and high level see Table 1) per each commodity. Test material size (15 g) was sufficient to perform a single determination. Materials were coded in a random pattern.
- Three mixed mycotoxin stock solutions to spike the 3 target commodities respectively; a mixed standard solution for calibrant preparation; a mixed ISTD solution.
- Thirty (+ 2 extra) solid phase extraction (SPE) columns.
- Material receipt form.
- Standard operating protocol (SOP).
- Reporting sheets.
4.3.2. Mycotoxin Solutions
- Mixed stock solution A, to be used for wheat spiking: NIV, 12.5 μg/mL; DON, 62.5 μg/mL; 3-AcDON, 7.5 μg/mL; 15-AcDON, 7.5 μg/mL; T-2, 2.5, μg/mL; HT-2, 2.5 μg/mL; ZEA, 5.0 μg/mL.
- Mixed stock solution B, to be used for wheat flour spiking: NIV, 7.5 μg/mL; DON, 37.5 μg/mL; 3-AcDON, 3.75 μg/mL; 15-AcDON, 3.75 μg/mL; T-2, 1.25, μg/mL; HT-2, 1.25 μg/mL; ZEA, 3.75 μg/mL.
- Mixed stock solution C, to be used for wheat crackers spiking: NIV, 5 μg/mL; DON, 25 μg/mL; 3-AcDON, 2.5 μg/mL; 15-AcDON, 2.5 μg/mL; T-2, 0.625, μg/mL; HT-2, 0.625 μg/mL; ZEA, 2.5 μg/mL.
- Mixed standard solution, prepared by 10 times dilution with acetonitrile of the multi-toxin stock solution A. This solution was used to prepare calibrants (see Table 2).
- Mixed internal standard (ISTD) solution, prepared by mixing the commercial individual ISTD solutions to obtain a mixture containing 13C15-NIV, 1.25 µg/mL; 13C15-DON, 6.25 µg/mL; 13C17-3-AcDON, 0.75 µg/mL; 13C22-HT-2, 0.25 µg/mL; 13C24-T-2, 0.25 µg/mL; 13C18-ZEN, 0.5 µg/mL.
4.3.3. Sample Preparation
4.3.4. LC-MS Analysis
4.3.5. Calculations
- R was the peak area ratio of the relevant analyte and the corresponding internal standard in the sample test solution;
- a was the slope of the calibration curve from calibration data, in µg−1;
- b was the intercept of the calibration curve from calibration data;
- V1 was the volume of the reconstituted extract after clean-up, here: 0.4 mL;
- V2 was the injection volume of the reconstituted sample extract, in milliliters;
- 1000 is a conversion factor;
- mSPE was the sample equivalent weight purified on SPE column, here: 1 g.
- m was the mass of the extracted test portion, here: 10 g;
- V3 was the volume of the extraction mixture, here: 50 mL;
- V4 was the volume of filtered extract dried before clean-up, here: 5 mL.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, J.D. Mycotoxins in small grains and maize: Old problems, new challenges. Food Addit. Contam. 2008, 25, 219–230. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Panel on Contaminants in the Food Chain. Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA J. 2013, 11, 3262. [Google Scholar] [CrossRef]
- IARC. Monographs on the Valuation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 1993; Volume 56, (Suppl. 7), Available online: https://monographs.iarc.fr/list-of-classifications (accessed on 5 November 2020).
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- European Food Safety Authority (EFSA). Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, 4972. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Appropriateness to set a group health based guidance value for nivalenol and its modified forms. EFSA J. 2017, 15, 4751. [Google Scholar] [CrossRef]
- Pascale, M.; De Girolamo, A.; Lippolis, V.; Stroka, J.; Mol, G.J.H.; Lattanzio, V.M.T. Performance evaluation of LC-MS Methods for Multimycotoxin Determination. J. AOAC Int. 2019, 102, 1708–1720. [Google Scholar] [CrossRef]
- De Girolamo, A.; Ciasca, B.; Stroka, J.; Bratinova, S.; Pascale, M.; Visconti, A.; Lattanzio, V.M.T. Performance evaluation of LC-MS/MS methods for multi-mycotoxin determination in maize and wheat by means of international Proficiency Testing. TRAC-Trend Anal. Chem. 2017, 86, 222–234. [Google Scholar] [CrossRef]
- Bessaire, T.; Mujahid, C.; Mottier, P.; Desmarchelier, A. Multiple Mycotoxins determination in Food by LC-MS/MS: An International Collaborative Study. Toxins 2019, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- EN 16924:2017. Foodstuffs—Determination of Zearalenone in Edible Vegetable Oils by LC-FLD or LC-MS/MS; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- EN 16923:2017. Foodstuffs—Determination of T-2 Toxin and HT-2 Toxin in Cereals and Cereal Products for Infants and Young Children by LC-MS/MS after SPE Cleanup; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- European Union. Mandate for Standardisation Addressed to CEN for Methods of Analysis for Mycotoxins in Food. Available online: https://law.resource.org/pub/eu/mandates/m520.pdf (accessed on 5 November 2020).
- EN 17280:2019 Foodstuffs—Determination of Zearalenone and Trichothecenes Including Deoxynivalenol and Its Acetylated Derivatives (3-Acetyl-deoxynivalenol and 15-Acetyl-deoxynivalenol), Nivalenol T-2 Toxin and HT-2 Toxin in Cereals and Cereal Products by LC-MS/MS; European Committee for Standardization: Brussels, Belgium, 2019.
- Horwitz, W.; Kamps, L.R.; Boyer, K.W. Quality assurance in the analysis of foods and trace constituents. J. AOAC Int. 1980, 63, 1344–1354. [Google Scholar] [CrossRef]
- Thompson, M. Recent trends in inter-laboratory precision at ppb and sub-ppb concentrations in relation to fitness for purpose criteria in proficiency testing. Analyst 2000, 125, 385–386. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. [Google Scholar]
- European Commission. Commission Regulation of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off. J. Eur. Union 2014, L147, 29–43. [Google Scholar]
- AOAC International. Appendix D: Guidelines for Collaborative Study Procedures to Validate Characteristics of a Method of Analysis. 2005. Available online: http://www.eoma.aoac.org/appendices.asp (accessed on 16 October 2020).
- Ye, J.; Wu, Y.; Guo, Q.; Lu, M.; Wang, S.; Xin, Y.; Xie, G.; Zhang, Y.; Mariappan, M.; Wang, S. Development and Interlaboratory Study of a Liquid Chromatography Tandem Mass Spectrometric Method for the Determination of Multiple Mycotoxins in Cereals Using Stable Isotope Dilution. J. AOAC Int. 2018, 101, 667–676. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; Della Gatta, S.; Suman, M.; Visconti, A. Development and in house validation of a robust and sensitive solid phase extraction: LC-MS/MS method for quantitative determination of aflatoxins B1, B2, G1, G2, ochratoxin A, deoxynivalenol, zearalenone, T-2 and HT-2 toxins in cereal-based foods. Rapid Commun. Mass Spectrom. 2011, 25, 1869–1880. [Google Scholar] [CrossRef]
- MacDonald, S.J.; Chan, D.; Brereton, P.; Damant, A.; Wood, R. Determination of deoxynivalenol in cereals and cereal products by immunoaffinity column cleanup with liquid chromatography: Interlaboratory Study. J. AOAC Int. 2005, 88, 1197–1204. [Google Scholar] [CrossRef]
- MacDonald, S.J.; Anderson, S.; Brereton, P.; Wood, R.; Damant, A. Determination of zearalenone in barley, maize and wheat flour, polenta, and maize-based baby food by immunoaffinity column cleanup with liquid chromatography: Interlaboratory study. J. AOAC Int. 2005, 88, 1733–1740. [Google Scholar] [CrossRef]
- Pascale, M.; Panzarini, G.; Visconti, A. Determination of HT-2 and T-2 toxins in oats and wheat by ultra-performance liquid chromatography with photodiode array detection. Talanta 2012, 89, 231–236. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO Guide 35:2006 Reference Materials—General and Statistical Principles for Certification; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- International Organization for Standardization. ISO 13528:2015 Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparison; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Lamberty, A.; Schimmel, H.; Pauwels, J. The study of the stability of reference materials by isochronus measurements. Fresenius J. Anal. Chem. 1998, 360, 359–361. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry. Protocol for the design, conduct and interpretation of collaborative studies. Pure Appl. Chem. 1988, 60, 855–864. [Google Scholar] [CrossRef]
- SANTE/12089/2016. Guidance Document on Identification of Mycotoxins in Food and Feed; European Commission Directorate General for Health and Food Safety: Brussels, Belgium, 2016. [Google Scholar]
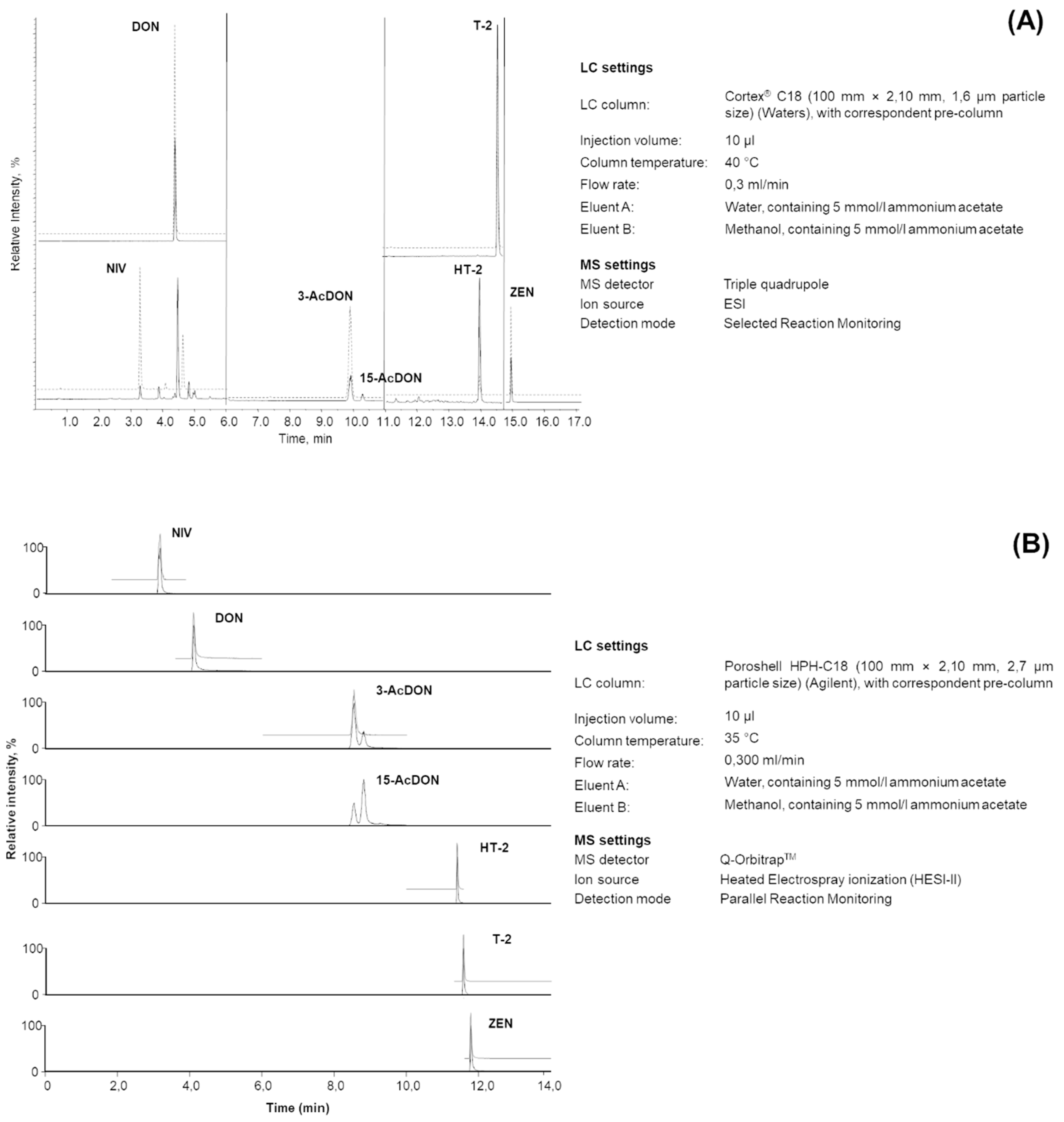
| Material Description | No. of Labs a | Mean (µg/kg) | Recovery (%) | RSDr (%) | RSDR(%) | HorRat b | |
|---|---|---|---|---|---|---|---|
| NIV | Wheat, 250 µg/kg spike | 14 | 194.1 | 78 | 5.8 | 17 | 0.8 |
| Wheat, contaminated A | 12 | 87.12 | n.a. | 33 | 35 | 1.6 | |
| Wheat, contaminated B | 12 | 189.4 | n.a. | 11 | 11 | 0.6 | |
| Wheat, contaminated C | 13 | 377.8 | n.a. | 14 | 25 | 1.4 | |
| Wheat flour, 150 µg/kg spike | 11 | 116.0 | 77 | 6.1 | 18 | 0.8 | |
| Wheat flour, contaminated A | 14 | 52.89 | n.a. | 34 | 35 | 1.5 | |
| Wheat flour, contaminated B | 11 | 107.7 | n.a. | 4.2 | 9.4 | 0.4 | |
| Wheat flour, contaminated C | 11 | 214.8 | n.a. | 3.5 | 9.7 | 0.5 | |
| Wheat crackers, 100 µg/kg spike | 14 | 70.71 | 71 | 8.3 | 20 | 0.9 | |
| Wheat crackers, contaminated A | 12 | 27.69 | n.a. | 15 | 32 | 1.5 | |
| Wheat crackers, contaminated B | 13 | 64.87 | n.a. | 6.2 | 29 | 1.3 | |
| Wheat crackers, contaminated C | 13 | 123.8 | n.a. | 5.8 | 22 | 1.0 | |
| DON | Wheat, 1250 µg/kg spike | 11 | 1212 | 97 | 3.7 | 9.5 | 0.6 |
| Wheat, contaminated A | 12 | 635.4 | n.a. | 3.0 | 15 | 0.9 | |
| Wheat, contaminated B | 12 | 1201 | n.a. | 11 | 10 | 0.6 | |
| Wheat, contaminated C | 10 | 2420 | n.a. | 2.2 | 6.4 | 0.5 | |
| Wheat flour, 750 µg/kg spike | 11 | 692.5 | 92 | 5.0 | 9.7 | 0.6 | |
| Wheat flour, contaminated A | 14 | 351.0 | n.a. | 6.7 | 32 | 1.7 | |
| Wheat flour, contaminated B | 13 | 613.0 | n.a. | 3.8 | 14 | 0.8 | |
| Wheat flour, contaminated C | 11 | 1234 | n.a. | 3.3 | 10 | 0.7 | |
| Wheat crackers, 500 µg/kg spike | 13 | 448.7 | 90 | 2.8 | 19 | 1.0 | |
| Wheat crackers, contaminated A | 13 | 233.9 | n.a. | 14 | 24 | 1.2 | |
| Wheat crackers, contaminated B | 12 | 487.6 | n.a. | 5.0 | 15 | 0.8 | |
| Wheat crackers, contaminated C | 12 | 1005 | n.a. | 13 | 14 | 0.9 | |
| 3AcDON | Wheat, 150 µg/kg spike | 10 | 136.5 | 91 | 5.7 | 8.0 | 0.4 |
| Wheat, contaminated A | 11 | 49.10 | n.a. | 3.0 | 27 | 1.2 | |
| Wheat, contaminated B | 10 | 83.68 | n.a. | 9.4 | 7.9 | 0.4 | |
| Wheat, contaminated C | 9 | 67.68 | n.a. | 6.4 | 7.7 | 0.4 | |
| Wheat flour, 75 µg/kg spike | 11 | 67.01 | 89 | 8.6 | 40 | 1.8 | |
| Wheat flour, contaminated A | 11 | 30.22 | n.a. | 6.9 | 34 | 1.5 | |
| Wheat flour, contaminated B | 11 | 58.95 | n.a. | 10 | 32 | 1.4 | |
| Wheat flour, contaminated C | 9 | 96.22 | n.a. | 5.3 | 9.8 | 0.4 | |
| Wheat crackers, 50 µg/kg spike | 13 | 44.96 | 90 | 5.9 | 30 | 1.4 | |
| Wheat crackers, contaminated A | 12 | 18.47 | n.a. | 17 | 26 | 1.2 | |
| Wheat crackers, contaminated B | 12 | 26.33 | n.a. | 6.7 | 20 | 0.9 | |
| Wheat crackers, contaminated C | 12 | 49.96 | n.a. | 12 | 20 | 0.9 | |
| 15AcDON | Wheat, 150 µg/kg spike | 11 | 141.8 | 95 | 8.5 | 35 | 1.6 |
| Wheat, contaminated A | 8 | 35.64 | n.a | 5.8 | 45 | 2.0 | |
| Wheat, contaminated B | 8 | 41.48 | n.a | 9.3 | 25 | 1.1 | |
| Wheat, contaminated C | 8 | 30.14 | n.a | 7.1 | 38 | 1.7 | |
| Wheat flour, 75 µg/kg spike | 9 | 68.25 | 91 | 6.0 | 31 | 1.4 | |
| Wheat flour, contaminated A | 8 | 12.87 | n.a | 10 | 34 | 1.5 | |
| Wheat flour, contaminated B | 9 | 24.38 | n.a | 14 | 42 | 1.9 | |
| Wheat flour, contaminated C | 9 | 28.64 | n.a | 9.6 | 36 | 1.7 | |
| Wheat crackers, 50 µg/kg spike | 12 | 46.24 | 93 | 8.6 | 37 | 1.7 | |
| Wheat crackers, contaminated A | 9 | 11.41 | n.a | 25 | 45 | 2.0 | |
| Wheat crackers, contaminated B | 10 | 16.42 | n.a | 10 | 34 | 1.5 | |
| Wheat crackers, contaminated C | 11 | 32.34 | n.a | 5.9 | 34 | 1.5 | |
| HT-2 | Wheat, 50 µg/kg spike | 14 | 46.40 | 93 | 7.3 | 33 | 1.5 |
| Wheat, contaminated A | 12 | 17.33 | n.a. | 6.6 | 26 | 1.2 | |
| Wheat, contaminated B | 14 | 36.29 | n.a. | 31 | 35 | 1.6 | |
| Wheat, contaminated C | 12 | 133.8 | n.a. | 10 | 27 | 1.2 | |
| Wheat flour, 25 µg/kg spike | 11 | 21.60 | 86 | 6.1 | 9.9 | 0.5 | |
| Wheat flour, contaminated A | 14 | 6.629 | n.a. | 26 | 38 | 1.7 | |
| Wheat flour, contaminated B | 13 | 14.18 | n.a. | 8.9 | 29 | 1.3 | |
| Wheat flour, contaminated C | 14 | 32.97 | n.a. | 9.2 | 27 | 1.2 | |
| Wheat crackers, 12.5 µg/kg spike | 12 | 10.73 | 86 | 4.7 | 26 | 1.2 | |
| Wheat crackers, contaminated A | 14 | 7.988 | n.a. | 14 | 31 | 1.4 | |
| Wheat crackers, contaminated B | 14 | 19.59 | n.a. | 15 | 24 | 1.1 | |
| Wheat crackers, contaminated C | 14 | 38.02 | n.a. | 11 | 31 | 1.4 | |
| T-2 | Wheat, 50 µg/kg spike | 12 | 45.89 | 92 | 12 | 28 | 1.3 |
| Wheat, contaminated A | 12 | 27.50 | n.a. | 11 | 32 | 1.5 | |
| Wheat, contaminated B | 13 | 47.79 | n.a. | 34 | 41 | 1.9 | |
| Wheat, contaminated C | 12 | 18.01 | n.a. | 12 | 35 | 1.6 | |
| Wheat flour, 25 µg/kg spike | 13 | 20.85 | 83 | 6.3 | 35 | 1.6 | |
| Wheat flour, contaminated A | 13 | 11.53 | n.a. | 7.3 | 33 | 1.5 | |
| Wheat flour, contaminated B | 10 | 26.85 | n.a. | 6.3 | 8.5 | 0.4 | |
| Wheat flour, contaminated C | 13 | 37.57 | n.a. | 7.2 | 36 | 1.7 | |
| Wheat crackers, 12.5 µg/kg spike | 13 | 11.05 | 88 | 7.0 | 31 | 1.4 | |
| Wheat crackers, contaminated A | 13 | 4.530 | n.a. | 12 | 38 | 1.7 | |
| Wheat crackers, contaminated B | 14 | 8.091 | n.a. | 10 | 39 | 1.8 | |
| Wheat crackers, contaminated C | 13 | 15.88 | n.a. | 4.9 | 30 | 1.4 | |
| ZEN | Wheat, 100 µg/kg spike | 10 | 95.12 | 95 | 6.5 | 22 | 1.0 |
| Wheat, contaminated A | 11 | 59.85 | n.a. | 14 | 32 | 1.5 | |
| Wheat, contaminated B | 10 | 125.1 | n.a. | 12 | 21 | 1.0 | |
| Wheat, contaminated C | 10 | 229.7 | n.a. | 11 | 21 | 1.0 | |
| Wheat flour, 75 µg/kg spike | 11 | 62.03 | 83 | 13 | 18 | 0.8 | |
| Wheat flour, contaminated A | 11 | 42.49 | n.a. | 13 | 20 | 0.9 | |
| Wheat flour, contaminated B | 11 | 86.38 | n.a. | 24 | 23 | 1.1 | |
| Wheat flour, contaminated C | 11 | 171.7 | n.a. | 8.3 | 16 | 0.8 | |
| Wheat crackers, 50 µg/kg spike | 12 | 47.13 | 94 | 7.4 | 31 | 1.4 | |
| Wheat crackers, contaminated A | 10 | 31.62 | n.a. | 17 | 23 | 1.1 | |
| Wheat crackers, contaminated B | 9 | 58.26 | n.a. | 16 | 24 | 1.1 | |
| Wheat crackers, contaminated C | 11 | 120.4 | n.a. | 7.3 | 38 | 1.7 |
| Mass Concentration of Calibration Solutions | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Calibration Solution | Mixed Standard Solution | Mixed ISTD Solution | NIV | DON | 3-Ac DON | 15-Ac DON | HT-2 | T-2 | ZEN |
| µL | µL | µg/mL | µg/mL | µg/mL | µg/mL | µg/mL | µg/mL | µg/mL | |
| 1 | 25 | 100 | 0.078 | 0.391 | 0.047 | 0.047 | 0.016 | 0.016 | 0.031 |
| 2 | 50 | 100 | 0.156 | 0.781 | 0.094 | 0.094 | 0.031 | 0.031 | 0.063 |
| 3 | 100 | 100 | 0.313 | 1.563 | 0.188 | 0.188 | 0.063 | 0.063 | 0.125 |
| 4 | 200 | 100 | 0.625 | 3.125 | 0.375 | 0.375 | 0.125 | 0.125 | 0.250 |
| 5 | 400 | 100 | 1.250 | 6.250 | 0.750 | 0.750 | 0.250 | 0.250 | 0.500 |
| 6 | 600 | 100 | 1.875 | 9.375 | 1.125 | 1.125 | 0.375 | 0.375 | 0.750 |
| Mass Concentration of Isotopically Labelled Analytes (µg/mL) in All Calibration Solutions | |||||||||
| 0.313 | 1.563 | 0.188 | 0.188 | 0.063 | 0.063 | 0.125 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolamo, A.D.; Ciasca, B.; Pascale, M.; Lattanzio, V.M.T. Determination of Zearalenone and Trichothecenes, Including Deoxynivalenol and Its Acetylated Derivatives, Nivalenol, T-2 and HT-2 Toxins, in Wheat and Wheat Products by LC-MS/MS: A Collaborative Study. Toxins 2020, 12, 786. https://doi.org/10.3390/toxins12120786
Girolamo AD, Ciasca B, Pascale M, Lattanzio VMT. Determination of Zearalenone and Trichothecenes, Including Deoxynivalenol and Its Acetylated Derivatives, Nivalenol, T-2 and HT-2 Toxins, in Wheat and Wheat Products by LC-MS/MS: A Collaborative Study. Toxins. 2020; 12(12):786. https://doi.org/10.3390/toxins12120786
Chicago/Turabian StyleGirolamo, Annalisa De, Biancamaria Ciasca, Michelangelo Pascale, and Veronica M.T. Lattanzio. 2020. "Determination of Zearalenone and Trichothecenes, Including Deoxynivalenol and Its Acetylated Derivatives, Nivalenol, T-2 and HT-2 Toxins, in Wheat and Wheat Products by LC-MS/MS: A Collaborative Study" Toxins 12, no. 12: 786. https://doi.org/10.3390/toxins12120786
APA StyleGirolamo, A. D., Ciasca, B., Pascale, M., & Lattanzio, V. M. T. (2020). Determination of Zearalenone and Trichothecenes, Including Deoxynivalenol and Its Acetylated Derivatives, Nivalenol, T-2 and HT-2 Toxins, in Wheat and Wheat Products by LC-MS/MS: A Collaborative Study. Toxins, 12(12), 786. https://doi.org/10.3390/toxins12120786








