The Involvement of Central Noradrenergic Pathway in the Analgesic Effect of Bee Venom Acupuncture on Vincristine-Induced Peripheral Neuropathy in Rats
Abstract
:1. Introduction
2. Results
2.1. Cold and Mechanical Hypersensitivity Following Vincristine Administrations in Rats
2.2. Comparison of Alleviative Effects of BVA, BvPLA2, and Melittin on Vincristine-Induced Behavioral Hypersensitivity
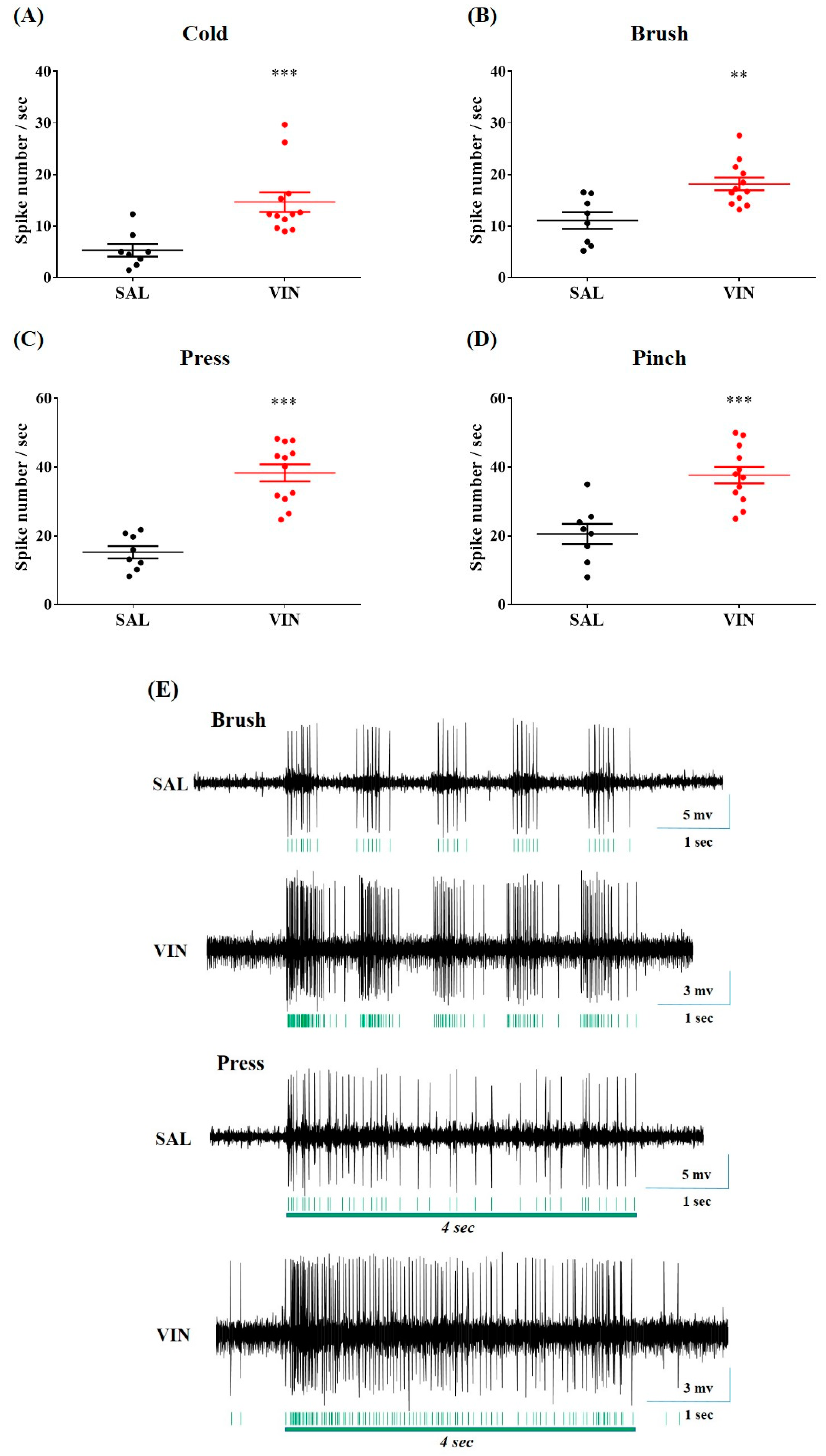
2.3. Vincristine Augmented Cold and Mechanical Sensory Responses of Spinal WDR Neuron in Rats
2.4. BVA Ameliorated Hyperexcitation of Spinal WDR Neuron Following Vincristine Administrations
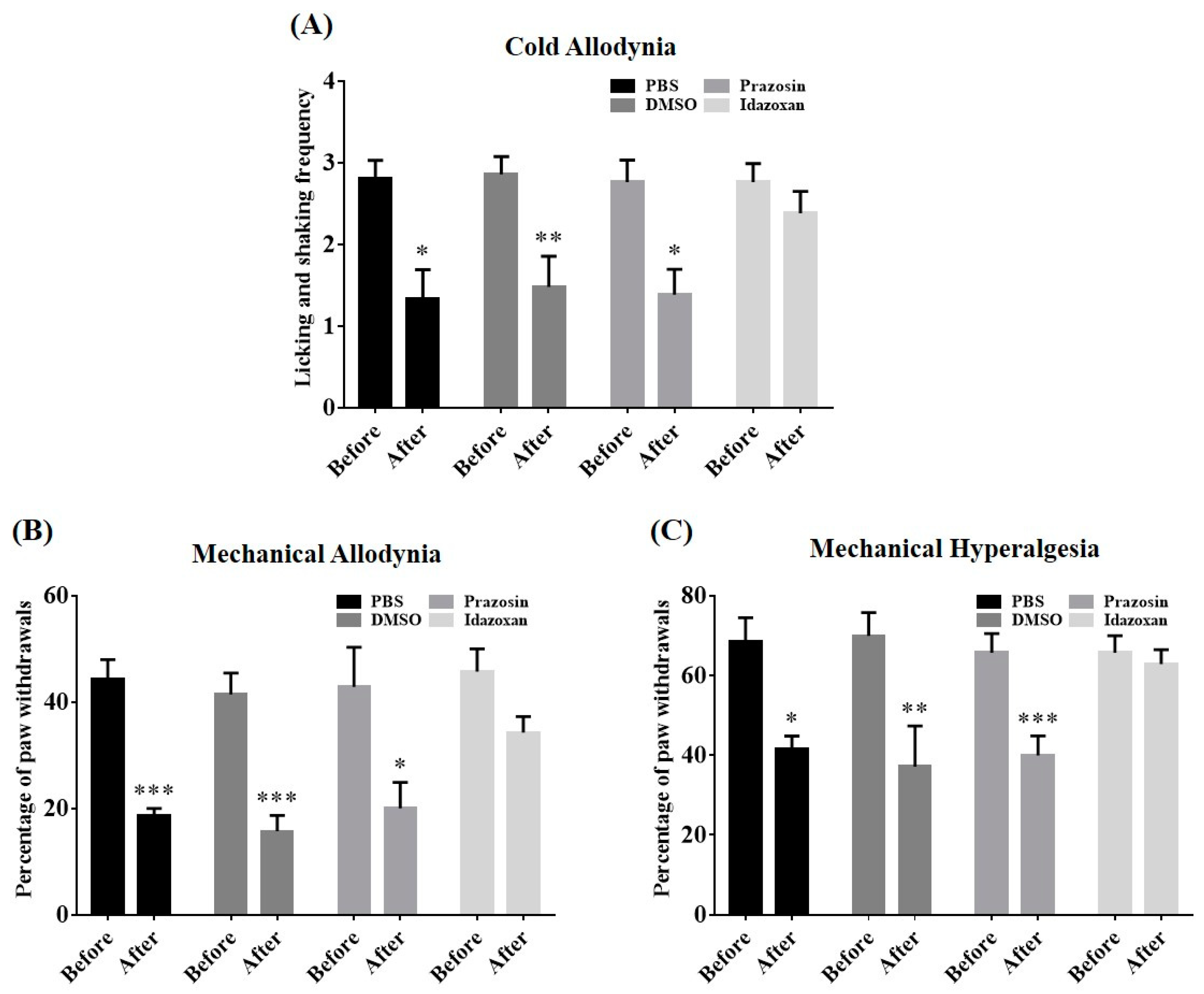
2.5. Antagonism of Spinal α-Adrenergic Receptor Abolished BVA-Induced Analgesia
2.6. Local Blockade of the LC Reversed BVA-Induced Analgesia
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal
5.2. Behavioral Evaluation
5.3. Vincristine Administration
5.4. BV, BvPLA2, or Melittin Acupuncture Treatment
5.5. In Vivo Extracellular Single-Unit Recording
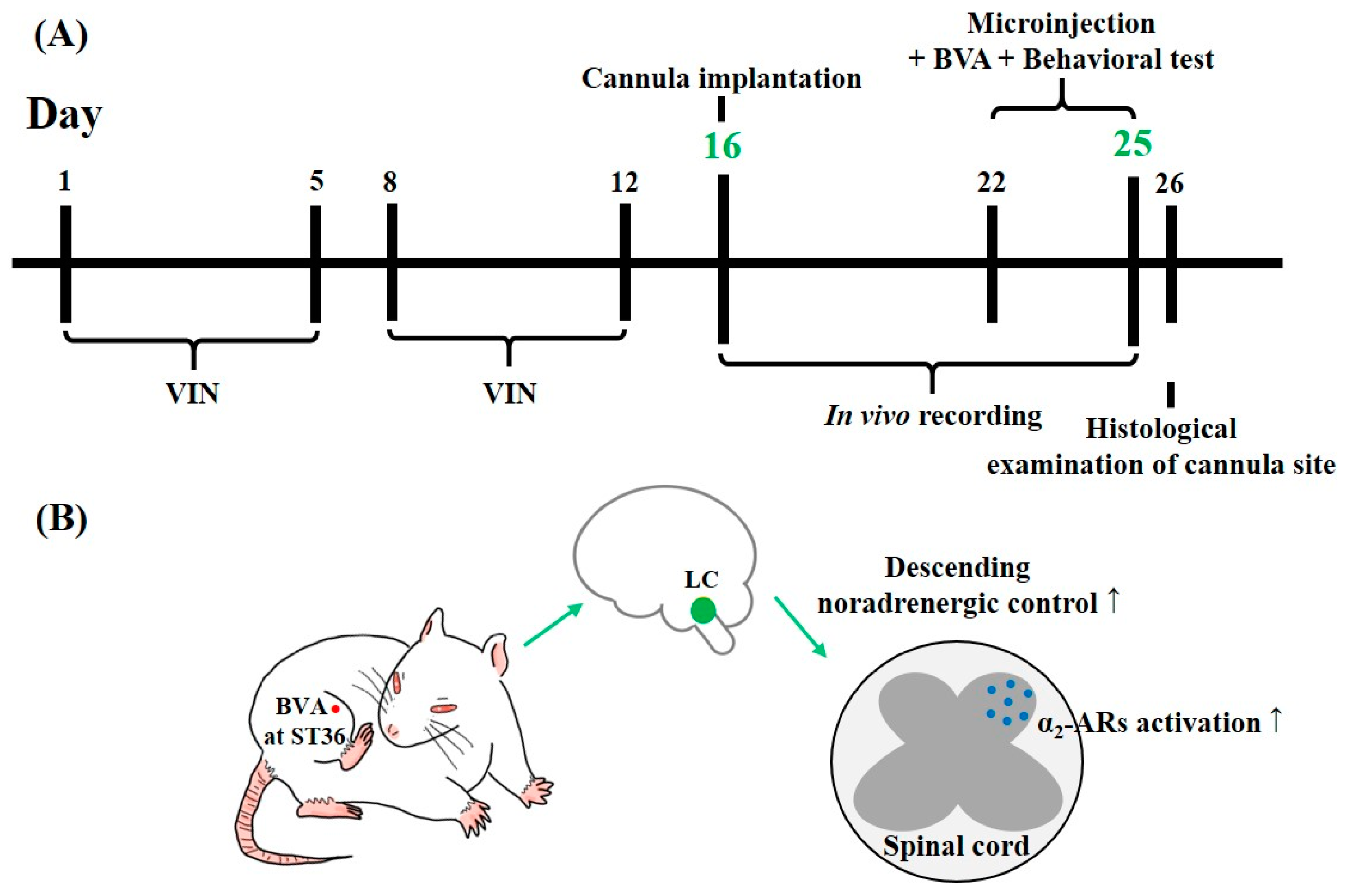
5.6. Cannula Implantation and Drug Microinjection into the LC
5.7. Spinal Antagonist Treatment
5.8. Experimental Schedule and Schematics of the Putative Central Mechanism of BVA-Induced Analgesia
5.9. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mora, E.; Smith, E.M.L.; Donohoe, C.; Hertz, D.L. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am. J. Cancer Res. 2016, 6, 2416. [Google Scholar]
- Dougherty, P.M.; Cata, J.P.; Burton, A.W.; Vu, K.; Weng, H.-R. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J. Pain Symptom Manag. 2007, 33, 166–179. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [Green Version]
- Boyette-Davis, J.A.; Hou, S.; Abdi, S.; Dougherty, P.M. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 2018, 8, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Hsieh, C.-L. Clinical Applications of Bee Venom Acupoint Injection. Toxins 2020, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-K.; Han, K.; Kwon, O.; Jo, D.-J.; Lee, J.-H. Efficacy of bee venom acupuncture for chronic low back pain: A randomized, double-blinded, sham-controlled trial. Toxins 2017, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.-R.; Lin, L.-T.; Xiao, L.-Y.; Zhou, P.; Shi, G.-X.; Liu, C.-Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P.; Govorushko, S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon 2018, 154, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Lee, G.; Lee, C.; Ye, M.; Chung, H.-S.; Kim, H.; Sung-joo, S.B.; Hwang, D.-S.; Bae, H. Bee venom phospholipase A2, a novel Foxp3+ regulatory T cell inducer, protects dopaminergic neurons by modulating neuroinflammatory responses in a mouse model of Parkinson’s disease. J. Immunol. 2015, 195, 4853–4860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aufschnaiter, A.; Kohler, V.; Khalifa, S.; El-Wahed, A.; Du, M.; El-Seedi, H.; Büttner, S. Apitoxin and its components against cancer, neurodegeneration and rheumatoid arthritis: Limitations and possibilities. Toxins 2020, 12, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Jeon, C.; Lee, J.H.; Jang, J.U.; Quan, F.S.; Lee, K.; Kim, W.; Kim, S.K. Suppressive Effects of Bee Venom Acupuncture on Paclitaxel-Induced Neuropathic Pain in Rats: Mediation by Spinal α2-Adrenergic Receptor. Toxins 2017, 9, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Chae, H.K.; Heo, H.; Hahm, D.-H.; Kim, W.; Kim, S.K. Analgesic Effect of Melittin on Oxaliplatin-Induced Peripheral Neuropathy in Rats. Toxins 2019, 11, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Yoo, J.H.; Kim, S.K. Long-Lasting and Additive Analgesic Effects of Combined Treatment of Bee Venom Acupuncture and Venlafaxine on Paclitaxel-Induced Allodynia in Mice. Toxins 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Pertovaara, A.; Almeida, A. Descending inhibitory systems. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 81, pp. 179–192. [Google Scholar]
- Kim, W.; Kim, M.J.; Go, D.; Min, B.-I.; Na, H.S.; Kim, S.K. Combined effects of bee venom acupuncture and morphine on oxaliplatin-induced neuropathic pain in mice. Toxins 2016, 8, 33. [Google Scholar] [CrossRef]
- Jaggi, A.S.; Kaur, G.; Bali, A.; Singh, N. Pharmacological investigations on mast cell stabilizer and histamine receptor antagonists in vincristine-induced neuropathic pain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1087–1096. [Google Scholar] [CrossRef]
- Flatters, S.J.; Bennett, G.J. Ethosuximide reverses paclitaxel-and vincristine-induced painful peripheral neuropathy. Pain 2004, 109, 150–161. [Google Scholar] [CrossRef]
- Weng, H.-R.; Cordella, J.; Dougherty, P.M. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. PAIN® 2003, 103, 131–138. [Google Scholar] [CrossRef]
- Roh, D.-H.; Kwon, Y.-B.; Kim, H.-W.; Ham, T.-W.; Yoon, S.-Y.; Kang, S.-Y.; Han, H.-J.; Lee, H.-J.; Beitz, A.J.; Lee, J.-H. Acupoint stimulation with diluted bee venom (apipuncture) alleviates thermal hyperalgesia in a rodent neuropathic pain model: Involvement of spinal alpha2-adrenoceptors. J. Pain 2004, 5, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lee, Y.; Kim, W.; Lee, K.; Bae, H.; Kim, S.K. Analgesic effects of bee venom derived phospholipase A2 in a mouse model of oxaliplatin-induced neuropathic pain. Toxins 2015, 7, 2422–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Park, D.S. Antinociceptive effect and the mechanism of bee venom acupuncture (Apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by α2-Adrenoceptors. Brain Res. 2006, 1073, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Eze, O.; Nwodo, O.; Ogugua, V.N. Therapeutic effect of honey bee venom. Proteins (enzymes) 2016, 1, 2. [Google Scholar]
- Yin, C.S.; Jeong, H.-S.; Park, H.-J.; Baik, Y.; Yoon, M.-H.; Choi, C.-B.; Koh, H.G. A proposed transpositional acupoint system in a mouse and rat model. Res. Vet. Sci. 2008, 84, 159–165. [Google Scholar] [CrossRef]
- Zeng, Y.-J.; Lin, Y.-H.; Wang, Y.-C.; Chang, J.-H.; Wu, J.-H.; Hsu, S.-F.; Tsai, S.-Y.; Lin, C.-H.; Wen, Y.-R. Laser acupuncture-induced analgesic effect and molecular alterations in an incision pain model: A comparison with electroacupuncture-induced effects. Lasers Med. Sci. 2018, 33, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.-W.; Hsu, C.-K.; Hsieh, C.-L.; Yang, J.; Lin, Y.-W. Probing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36–ST37 acupoints on CFA-induced inflammatory pain. Sci. Rep. 2016, 6, 22123. [Google Scholar] [CrossRef]
- Zuo, C.-Y.; Lv, P.; Zhang, C.-S.; Lei, R.-X.; Zhou, W.; Wu, Q.-F.; Luo, L.; Tang, Y.; Yin, H.-Y.; Yu, S.-G. Ipsi-and contralateral moxibustion generate similar analgesic effect on inflammatory pain. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Le Bars, D.; Cadden, S. What is a wide-dynamic-range cell? In The Senses: A Comprehensive Reference; Academic Press: Cambridge, MA, USA, 2008; pp. 331–338. [Google Scholar]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Baron, R. Mechanisms of disease: Neuropathic pain—A clinical perspective. Nat. Clin. Pract. Neurol. 2006, 2, 95–106. [Google Scholar] [CrossRef]
- Renn, C.L.; Carozzi, V.A.; Rhee, P.; Gallop, D.; Dorsey, S.G.; Cavaletti, G. Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice. Mol. Pain 2011, 7, 1744–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazemi, S.; Manaheji, H.; Noorbakhsh, S.M.; Zaringhalam, J.; Sadeghi, M.; Mohammad-Zadeh, M.; Haghparast, A. Inhibition of microglial activity alters spinal wide dynamic range neuron discharge and reduces microglial Toll-like receptor 4 expression in neuropathic rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Tan, H.Y.; Nam, T.S.; Paik, K.S.; Hulsebosch, C.E.; Leem, J.W. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J. Neurotrauma 2006, 23, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kucharczyk, M.; Montagut-Bordas, C.; Lockwood, S.; Dickenson, A.H. Neuropathy following spinal nerve injury shares features with the irritable nociceptor phenotype: A back-translational study of oxcarbazepine. Eur. J. Pain 2019, 23, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wang, W.; Li, L.; Qin, Q.; Yu, Y.; Liu, K.; Zhao, Y.; Rong, P.; Zhu, B. Inhibition of electroacupuncture on nociceptive responses of dorsal horn neurons evoked by noxious colorectal distention in an intensity-dependent manner. J. Pain Res. 2019, 12, 231. [Google Scholar] [CrossRef] [Green Version]
- You, H.-J.; Morch, C.D.; Chen, J.; Arendt-Nielsen, L. Simultaneous recordings of wind-up of paired spinal dorsal horn nociceptive neuron and nociceptive flexion reflex in rats. Brain Res. 2003, 960, 235–245. [Google Scholar] [CrossRef]
- Viisanen, H.; Pertovaara, A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience 2007, 146, 1785–1794. [Google Scholar] [CrossRef]
- Hickey, L.; Li, Y.; Fyson, S.J.; Watson, T.C.; Perrins, R.; Hewinson, J.; Teschemacher, A.G.; Furue, H.; Lumb, B.M.; Pickering, A.E. Optoactivation of locus ceruleus neurons evokes bidirectional changes in thermal nociception in rats. J. Neurosci. 2014, 34, 4148–4160. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Eisenach, J.C. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Res. 2003, 970, 110–118. [Google Scholar] [CrossRef]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef]
- Clark, F.M.; Proudfit, H.K. The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res. 1991, 538, 231–245. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Yan, C.-Q.; Hu, S.-Q.; Huo, J.-W.; Wang, Z.-Y.; Zhou, P.; Liu, C.-H.; Liu, C.-Z. Different mechanisms of contralateral-or ipsilateral-acupuncture to modulate the brain activity in patients with unilateral chronic shoulder pain: A pilot fMRI study. J. Pain Res. 2018, 11, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, M.; Furue, H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J. Pharmacol. Sci. 2006, 101, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Yamada, A.; Kim, W.; Kim, S.K.; Furue, H. Noradrenergic inhibition of spinal hyperexcitation elicited by cutaneous cold stimuli in rats with oxaliplatin-induced allodynia: Electrophysiological and behavioral assessments. J. Physiol. Sci. 2017, 67, 431–438. [Google Scholar] [CrossRef]
- Song, Z.; Ansah, O.; Meyerson, B.; Pertovaara, A.; Linderoth, B. Exploration of supraspinal mechanisms in effects of spinal cord stimulation: Role of the locus coeruleus. Neuroscience 2013, 253, 426–434. [Google Scholar] [CrossRef]
- Ren, K.; Randich, A.; Gebhart, G. Electrical stimulation of cervical vagal afferents. I. Central relays for modulation of spinal nociceptive transmission. J. Neurophysiol. 1990, 64, 1098–1114. [Google Scholar] [CrossRef]
- Safari, M.-S.; Haghparast, A.; Semnanian, S. Effect of lidocaine administration at the nucleus locus coeruleus level on lateral hypothalamus-induced antinociception in the rat. Pharmacol. Biochem. Behav. 2009, 92, 629–634. [Google Scholar] [CrossRef]
- Brightwell, J.J.; Taylor, B.K. Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience 2009, 160, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Moon, D.-O.; Park, S.-Y.; Heo, M.-S.; Kim, K.-C.; Park, C.; Ko, W.S.; Choi, Y.H.; Kim, G.-Y. Key regulators in bee venom-induced apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells through downregulation of ERK and Akt. Int. Immunopharmacol. 2006, 6, 1796–1807. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, H.; Bae, Y.; Shin, K.; Kang, S.; Kim, H.; Oh, J.; Bae, H. Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J. Immunother. Cancer 2019, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yu, M.; He, Y.; Xiao, L.; Wang, F.; Song, C.; Sun, S.; Ling, C.; Xu, Z. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology 2008, 47, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Soliman, C.; Eastwood, S.; Truong, V.K.; Ramsland, P.A.; Elbourne, A. The membrane effects of melittin on gastric and colorectal cancer. PLoS ONE 2019, 14, e0224028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Li, D.; Lee, J.H.; Choi, C.W.; Kim, J.; Kim, S.K.; Kim, W. The Analgesic Effect of Venlafaxine and Its Mechanism on Oxaliplatin-Induced Neuropathic Pain in Mice. Int. J. Mol. Sci. 2019, 20, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, C.; Wook, Y.Y.; Sik, N.H.; Ho, K.S.; Mo, C.J. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994, 59, 369–376. [Google Scholar] [CrossRef]
- Zheng, F.; Xiao, W.-H.; Bennett, G. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 2011, 176, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Chung, G.; Kim, C.Y.; Yun, Y.-C.; Yoon, S.H.; Kim, M.-H.; Kim, Y.K.; Kim, S.J. Upregulation of prefrontal metabotropic glutamate receptor 5 mediates neuropathic pain and negative mood symptoms after spinal nerve injury in rats. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Muto, Y.; Sakai, A.; Sakamoto, A.; Suzuki, H. Activation of NK1 receptors in the locus coeruleus induces analgesia through noradrenergic-mediated descending inhibition in a rat model of neuropathic pain. Br. J. Pharmacol. 2012, 166, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- De la Calle, J.L.; Paíno, C.L. A procedure for direct lumbar puncture in rats. Brain Res. Bull. 2002, 59, 245–250. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Chung, G.; Kim, S.K. The Involvement of Central Noradrenergic Pathway in the Analgesic Effect of Bee Venom Acupuncture on Vincristine-Induced Peripheral Neuropathy in Rats. Toxins 2020, 12, 775. https://doi.org/10.3390/toxins12120775
Li D, Chung G, Kim SK. The Involvement of Central Noradrenergic Pathway in the Analgesic Effect of Bee Venom Acupuncture on Vincristine-Induced Peripheral Neuropathy in Rats. Toxins. 2020; 12(12):775. https://doi.org/10.3390/toxins12120775
Chicago/Turabian StyleLi, Daxian, Geehoon Chung, and Sun Kwang Kim. 2020. "The Involvement of Central Noradrenergic Pathway in the Analgesic Effect of Bee Venom Acupuncture on Vincristine-Induced Peripheral Neuropathy in Rats" Toxins 12, no. 12: 775. https://doi.org/10.3390/toxins12120775
APA StyleLi, D., Chung, G., & Kim, S. K. (2020). The Involvement of Central Noradrenergic Pathway in the Analgesic Effect of Bee Venom Acupuncture on Vincristine-Induced Peripheral Neuropathy in Rats. Toxins, 12(12), 775. https://doi.org/10.3390/toxins12120775




