Mycotoxin and Gut Microbiota Interactions
Abstract
:1. Introduction
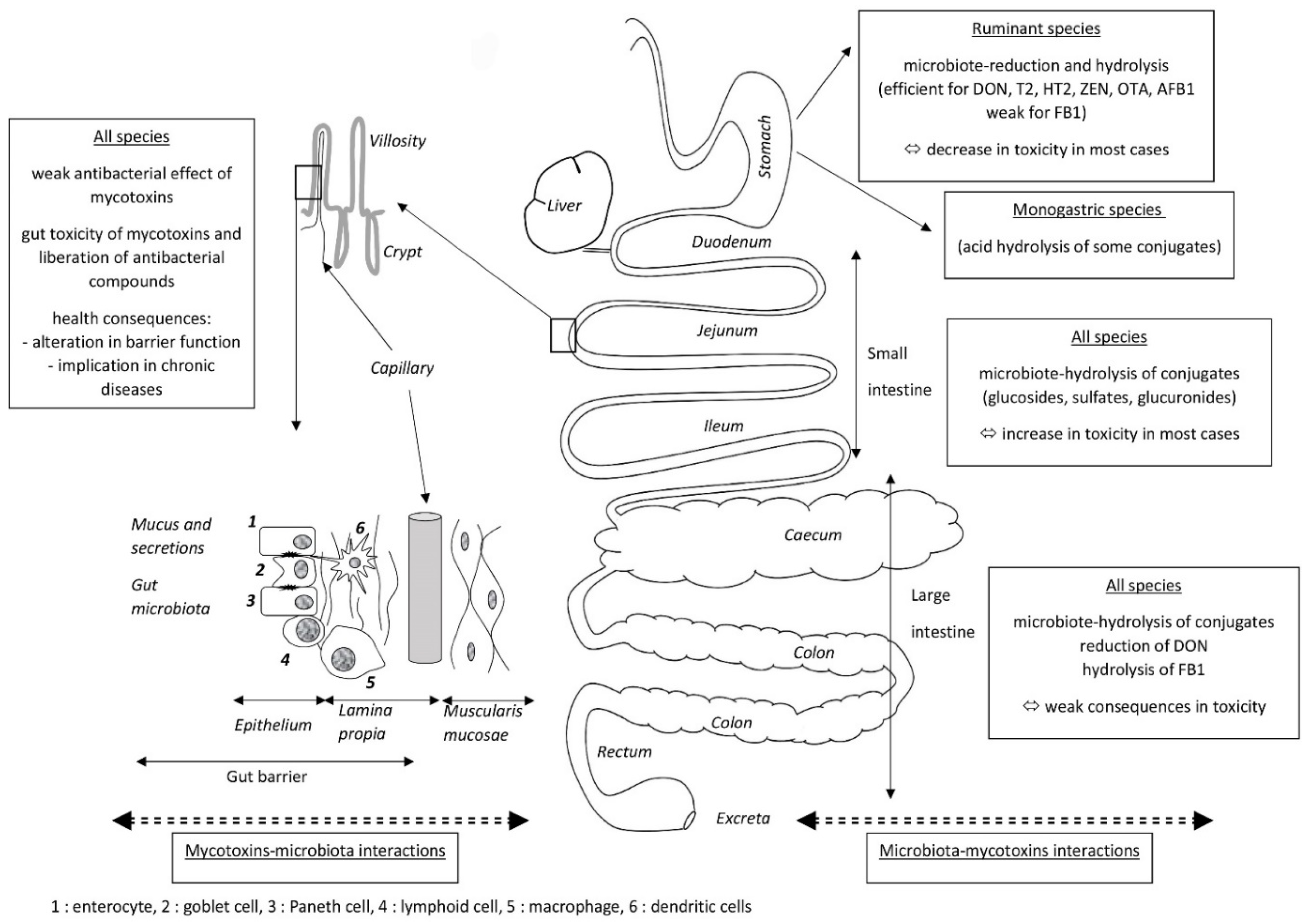
2. Effects of the Gut Microbiota on Mycotoxins
2.1. Biotransformation of Mycotoxins
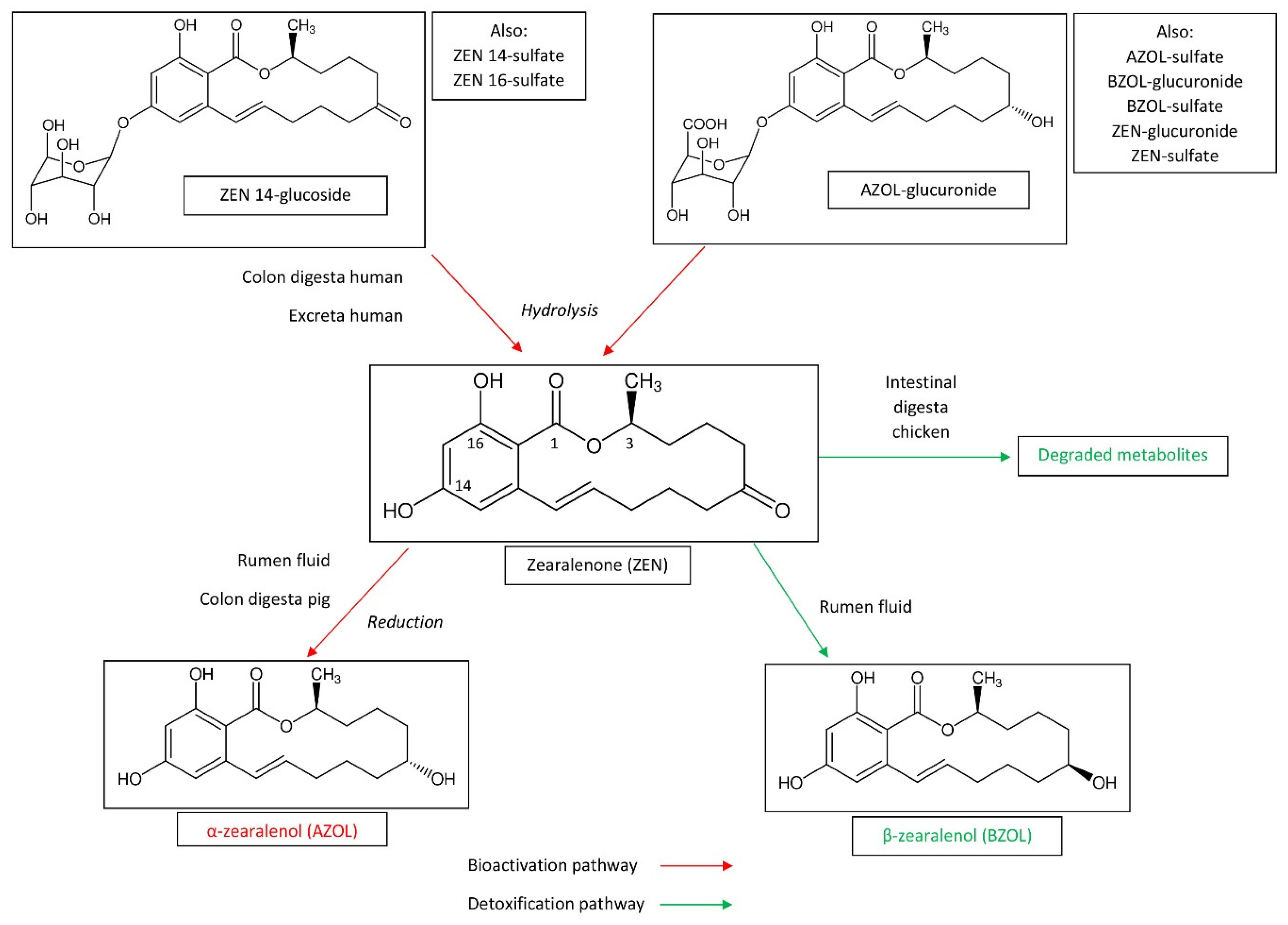
2.1.1. Degradation of Mycotoxins
2.1.2. Bioactivation of Masked Mycotoxins
2.1.3. Microbial Degradation of Mycotoxin and Feed Additives
2.2. Adsorption of Mycotoxins
3. Effects of Mycotoxins on the Gut Microbiota
3.1. Antimicrobial Properties of Mycotoxins
3.2. Cell Toxicity and Leakage of Antimicrobial Products (AMPs)
3.3. Changes in the Gut Microbiota Secondary to Mycotoxin Exposure
4. Combined Effects
4.1. Alteration of the Capacity of Defense against Pathogens
4.2. Other Effects on Heath
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef] [PubMed]
- Buszewska-Forajta, M. Mycotoxins, invisible danger of feedstuff with toxic effect on animals. Toxicon 2020, 182, 34–53. [Google Scholar] [CrossRef] [PubMed]
- FDA Mycotoxin Regulatory Guidance, August 2011. Available online: https://www.ngfa.org/wp-content/uploads/NGFAComplianceGuide-FDARegulatoryGuidanceforMycotoxins8-2011.pdf (accessed on 11 August 2016).
- Commission Directive 2003/100/EC. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003L0100&rid=2 (accessed on 11 August 2016).
- Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for animal Feeding. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006H0576&from=EN (accessed on 11 August 2016).
- Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013H0165&from=EN (accessed on 11 August 2016).
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.A.; Nachman, M.W. Spatial Heterogeneity of Gut Microbial Composition along the Gastrointestinal Tract in Natural Populations of House Mice. PLoS ONE 2016, 11, e0163720. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Chen, H.; Mao, B.; Yang, Q.; Zhao, J.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Microbial Biogeography and Core Microbiota of the Rat Digestive Tract. Sci. Rep. 2017, 7, 45840. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W.; et al. The Dynamic Distribution of Porcine Microbiota across Different Ages and Gastrointestinal Tract Segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef] [Green Version]
- Mancabelli, L.; Ferrario, C.; Milani, C.; Mangifesta, M.; Turroni, F.; Duranti, S.; Lugli, G.A.; Viappiani, A.; Ossiprandi, M.C.; van Sinderen, D.; et al. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016, 18, 4727–4738. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, M.; Liu, J.; Zhu, W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: Membership and potential function. Sci. Rep. 2015, 5, 16116. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Bertero, A.; Fossati, P.; Tedesco, D.E.A.; Caloni, F. Beauvericin and Enniatins: In Vitro Intestinal Effects. Toxins 2020, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of Mycotoxins through Biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.R.; McQueen, R.E.; Levesque, D.; Greenhalgh, R. Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms. J. Agric. Food Chem. 1984, 32, 1181–1183. [Google Scholar] [CrossRef]
- Kiessling, K.H.; Pettersson, H.; Sandholm, K.; Olsen, M. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 1984, 47, 1070. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, T.; Cote, L.-M.; Swanson, S.P.; Buck, W.B. Confirmation of DOM-1, a Deepoxidation Metabolite of Deoxynivalenol, in Biological Fluids of Lactating Cows. Agric. Biol. Chem. 1986, 50, 227–229. [Google Scholar] [CrossRef]
- Dänicke, S.; Brezina, U. Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: Consequences for diagnosis of exposure and intoxication and carry over. Food Chem. Toxicol. 2013, 60, 58–75. [Google Scholar] [CrossRef]
- Maresca, M. From the Gut to the Brain: Journey and Pathophysiological Effects of the Food-Associated Trichothecene Mycotoxin Deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Böhm, J.; Zentek, J. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit. Contam. Part A 2010, 27, 510–520. [Google Scholar] [CrossRef]
- He, P.; Young, L.G.; Forsberg, C. Microbial transformation of deoxynivalenol (vomitoxin). Appl. Environ. Microbiol. 1992, 58, 3857–3863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Long, M. The biological detoxification of deoxynivalenol: A review. Food Chem. Toxicol. 2020, 145, 111649. [Google Scholar] [CrossRef] [PubMed]
- Westlake, K.; Mackie, R.I.; Dutton, M.F. In vitro metabolism of mycotoxins by bacterial, protozoal and ovine ruminal fluid preparations. Anim. Feed Sci. Technol. 1989, 25, 169–178. [Google Scholar] [CrossRef]
- Hedman, R.; Pettersson, H. Transformation of nivalenol by gastrointestinal microbes. Arch. Für Tierernaehrung 1997, 50, 321–329. [Google Scholar] [CrossRef]
- Debevere, S.; Cools, A.; De Baere, S.; Haesaert, G.; Rychlik, M.; Croubels, S.; Fievez, V. In Vitro Rumen Simulations Show a Reduced Disappearance of Deoxynivalenol, Nivalenol and Enniatin B at Conditions of Rumen Acidosis and Lower Microbial Activity. Toxins 2020, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, G.S.; Pettersson, H.; Johnsen, K.; Lindberg, J.E. Transformation of trichothecenes in ileal digesta and faeces from pigs. Arch. Anim. Nutr. 2002, 56, 263–274. [Google Scholar] [CrossRef]
- Kollarczik, B.; Gareis, M.; Hanelt, M. In vitro transformation of the Fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Nat. Toxins 1994, 2, 105–110. [Google Scholar] [CrossRef]
- Sundstøl Eriksen, G.; Pettersson, H. Lack of de-epoxidation of type B trichothecenes in incubates with human faeces. Food Addit. Contam. 2003, 20, 579–582. [Google Scholar] [CrossRef]
- Swanson, S.P.; Helaszek, C.; Buck, W.B.; Rood, H.D.; Haschek, W.M. The role of intestinal microflora in the metabolism of trichothecene mycotoxins. Food Chem. Toxicol. 1988, 26, 823–829. [Google Scholar] [CrossRef]
- Young, J.C.; Zhou, T.; Yu, H.; Zhu, H.; Gong, J. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 2007, 45, 136–143. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Pettersson, H.; Lindberg, J.E. Absorption, metabolism and excretion of 3-acetyl DON in pigs. Arch. Anim. Nutr. 2003, 57, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Poapolathep, A.; Sugita-Konishi, Y.; Doi, K.; Kumagai, S. The fates of trichothecene mycotoxins, nivalenol and fusarenon-X, in mice. Toxicon 2003, 41, 1047–1054. [Google Scholar] [CrossRef]
- Poapolathep, A.; Poapolathep, S.; Sugita-Konishi, Y.; Imsilp, K.; Tassanawat, T.; Sinthusing, C.; Itoh, Y.; Kumagai, S. Fate of Fusarenon-X in Broilers and Ducks. Poult. Sci. 2008, 87, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Saengtienchai, T.; Poapolathep, S.; Isariyodom, S.; Ikenaka, Y.; Ishizuka, M.; Poapolathep, A. Toxicokinetics and tissue depletion of Fusarenon-X and its metabolite nivalenol in piglets. Food Chem. Toxicol. 2014, 66, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.; Currie, V.; Duncan, G.; Farquharson, F.; Yoshinari, T.; Louis, P.; Gratz, S.W. Prevalent Human Gut Bacteria Hydrolyse and Metabolise Important Food-Derived Mycotoxins and Masked Mycotoxins. Toxins 2020, 12, 654. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed]
- Peles, F.; Sipos, P.; Győri, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse Effects, Transformation and Channeling of Aflatoxins Into Food Raw Materials in Livestock. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Jezkova, A.; Yuan, Z.; Pavlikova, L.; Dohnal, V.; Kuca, K. Biological degradation of aflatoxins. Drug Metab. Rev. 2009, 41, 1–7. [Google Scholar] [CrossRef]
- Mobashar, M.; Hummel, J.; Blank, R.; Südekum, K.-H. Ochratoxin A in Ruminants—A Review on Its Degradation by Gut Microbes and Effects on Animals. Toxins 2010, 2, 809–839. [Google Scholar] [CrossRef] [Green Version]
- Hult, K.; Teiling, A.; Gatenbeck, S. Degradation of Ochratoxin A by a Ruminant. Appl. Environ. Microbiol. 1976, 32, 443–444. [Google Scholar] [CrossRef] [Green Version]
- Mobashar, M.; Blank, R.; Hummel, J.; Westphal, A.; Tholen, E.; Südekum, K.-H. Ruminal ochratoxin A degradation—Contribution of the different microbial populations and influence of diet. Anim. Feed Sci. Technol. 2012, 171, 85–97. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Sung, H.G.; Lee, C.H.; Lee, S.Y.; Kim, S.W.; Cho, K.J.; Ha, J.K. Comparative study on the aflatoxin B1 degradation ability of rumen fluid from Holstein steers and Korean native goats. J. Vet. Sci. 2009, 10, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantaya, D.; Morgavi, D.P.; Silberberg, M.; Chaucheyras-Durand, F.; Martin, C.; Wiryawan, K.G.; Boudra, H. Bioavailability of aflatoxin B1 and ochratoxin A, but not fumonisin B1 or deoxynivalenol, is increased in starch-induced low ruminal pH in nonlactating dairy cows. J. Dairy Sci. 2016, 99, 9759–9767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höhler, D.; Südekum, K.-H.; Wolffram, S.; Frohlich, A.A.; Marquardt, R.R. Metabolism and excretion of ochratoxin A fed to sheep. J. Anim. Sci. 1999, 77, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Song, J.Y.; Park, M.A.; Seo, J.K.; Yang, L.; Lee, C.H.; Cho, K.J.; Ha, J.K. Isolation, Screening and Identification of Swine Gut Microbiota with Ochratoxin A Biodegradation Ability. Asian-Australas. J. Anim. Sci. 2012, 25, 114–121. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Knutsen, H.-K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; EFSA Panel on Contaminants in the Food Chain (CONTAM); et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, e04851. [Google Scholar] [CrossRef] [Green Version]
- Caloni, F.; Spotti, M.; Auerbach, H.; Op den Camp, H.; Fink Gremmels, J.; Pompa, G. In Vitro Metabolism of Fumonisin B1 by Ruminal Microflora. Vet. Res. Commun. 2000, 24, 379–387. [Google Scholar] [CrossRef]
- Dang, H.A.; Zsolnai, A.; Kovács, M.; Bors, I.; Bónai, A.; Bóta, B.; Szabó-Fodor, J. In vitro Interaction between Fumonisin B1 and the Intestinal Microflora of Pigs. Pol. J. Microbiol. 2017, 66, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Gurung, N.K.; Rankins, D.L.J.; Shelby, R.A. In vitro ruminal disappearance of fumonisin B1 and its effects on in vitro dry matter disappearance. Vet. Hum. Toxicol. 1999, 41, 196–199. [Google Scholar]
- Gallo, A.; Giuberti, G.; Bertuzzi, T.; Moschini, M.; Masoero, F. Study of the effects of PR toxin, mycophenolic acid and roquefortine C on in vitro gas production parameters and their stability in the rumen environment. J. Agric. Sci. 2015, 153, 163–176. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Boudra, H.; Jouany, J.P. Michalet-Doreau, the former B. Effect and stability of gliotoxin, an Aspergillus fumigatus toxin, on in vitro rumen fermentation. Food Addit. Contam. 2004, 21, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, N.; Devreese, M.; De Baere, S.; De Backer, P.; Croubels, S. Modified Fusarium mycotoxins unmasked: From occurrence in cereals to animal and human excretion. Food Chem. Toxicol. 2015, 80, 17–31. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [Green Version]
- De Nijs, M.; Van den Top, H.J.; Portier, L.; Oegema, G.; Kramer, E.; Van Egmond, H.P.; Hoogenboom, L.A.P. Digestibility and absorption of deoxynivalenol-3-ß-glucoside in in vitro models. World Mycotoxin J. 2012, 5, 319–324. [Google Scholar] [CrossRef]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked Mycotoxins Are Efficiently Hydrolyzed by Human Colonic Microbiota Releasing Their Aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef]
- De Angelis, E.; Monaci, L.; Visconti, A. Investigation on the stability of deoxynivalenol and DON-3 glucoside during gastro-duodenal in vitro digestion of a naturally contaminated bread model food. Food Control 2014, 43, 270–275. [Google Scholar] [CrossRef]
- Gratz, S.W.; Duncan, G.; Richardson, A.J. The Human Fecal Microbiota Metabolizes Deoxynivalenol and Deoxynivalenol-3-Glucoside and May Be Responsible for Urinary Deepoxy-Deoxynivalenol. Appl. Environ. Microbiol. 2013, 79, 1821–1825. [Google Scholar] [CrossRef] [Green Version]
- Nagl, V.; Schwartz, H.; Krska, R.; Moll, W.-D.; Knasmüller, S.; Ritzmann, M.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012, 213, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagl, V.; Woechtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.-D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekaert, N.; Devreese, M.; van Bergen, T.; Schauvliege, S.; De Boevre, M.; De Saeger, S.; Vanhaecke, L.; Berthiller, F.; Michlmayr, H.; Malachová, A.; et al. In vivo contribution of deoxynivalenol-3-β-d-glucoside to deoxynivalenol exposure in broiler chickens and pigs: Oral bioavailability, hydrolysis and toxicokinetics. Arch. Toxicol. 2017, 91, 699–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratz, S.W.; Currie, V.; Richardson, A.J.; Duncan, G.; Holtrop, G.; Farquharson, F.; Louis, P.; Pinton, P.; Oswald, I.P. Porcine Small and Large Intestinal Microbiota Rapidly Hydrolyze the Masked Mycotoxin Deoxynivalenol-3-Glucoside and Release Deoxynivalenol in Spiked Batch Cultures In Vitro. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plasencia, J.; Mirocha, C.J. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Appl. Environ. Microbiol. 1991, 57, 146–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gareis, M.; Bauer, J.; Thiem, J.; Plank, G.; Grabley, S.; Gedek, B. Cleavage of Zearalenone-Glycoside, a “Masked” Mycotoxin, during Digestion in Swine. J. Vet. Med. Ser. B 1990, 37, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Veršilovskis, A.; Geys, J.; Huybrechts, B.; Goossens, E.; De Saeger, S.; Callebaut, A. Simultaneous determination of masked forms of deoxynivalenol and zearalenone after oral dosing in rats by LC-MS/MS. World Mycotoxin J. 2012, 5, 303–318. [Google Scholar] [CrossRef]
- Kovalsky Paris, M.P.; Schweiger, W.; Hametner, C.; Stückler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A New Masked Mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef]
- Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of Zearalenone and Its Major Modified Forms in Pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Catteuw, A.; Broekaert, N.; De Baere, S.; Lauwers, M.; Gasthuys, E.; Huybrechts, B.; Callebaut, A.; Ivanova, L.; Uhlig, S.; De Boevre, M.; et al. Insights into In Vivo Absolute Oral Bioavailability, Biotransformation, and Toxicokinetics of Zearalenone, α-Zearalenol, β-Zearalenol, Zearalenone-14-glucoside, and Zearalenone-14-sulfate in Pigs. J. Agric. Food Chem. 2019, 67, 3448–3458. [Google Scholar] [CrossRef]
- Lei, Y.P.; Zhao, L.H.; Ma, Q.G.; Zhang, J.Y.; Zhou, T.; Gao, C.Q.; Ji, C. Degradation of zearalenone in swine feed and feed ingredients by Bacillus subtilis ANSB01G. World Mycotoxin J. 2014, 7, 143–151. [Google Scholar] [CrossRef]
- Tardieu, D.; Travel, A.; Metayer, J.-P.; Le Bourhis, C.; Guerre, P. Zearalenone and Metabolites in Livers of Turkey Poults and Broiler Chickens Fed with Diets Containing Fusariotoxins. Toxins 2020, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Y. Review on microbial degradation of zearalenone and aflatoxins. Grain Oil Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Afshar, P.; Shokrzadeh, M.; Raeisi, S.N.; Ghorbani-HasanSaraei, A.; Nasiraii, L.R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon 2020, 178, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.; Horvath, E.M.; Schatzmayr, G.; Ellend, N.; Danner, H.; Krska, R.; Braun, R. Screening for Deoxynivalenol-Detoxifying Anaerobic Rumen Microorganisms. Cereal Res. Commun. 1997, 25, 343–346. [Google Scholar] [CrossRef]
- Fuchs, E.; Binder, E.M.; Heidler, D.; Krska, R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 2002, 19, 379–386. [Google Scholar] [CrossRef]
- Awad, W.A.; Böhm, J.; Razzazi-Fazeli, E.; Hulan, H.W.; Zentek, J. Effects of deoxynivalenol on general performance and electrophysiological properties of intestinal mucosa of broiler chickens. Poult. Sci. 2004, 83, 1964–1972. [Google Scholar] [CrossRef]
- Awad, W.A.; Böhm, J.; Razzazi-Fazeli, E.; Ghareeb, K.; Zentek, J. Effect of Addition of a Probiotic Microorganism to Broiler Diets Contaminated with Deoxynivalenol on Performance and Histological Alterations of Intestinal Villi of Broiler Chickens. Poult. Sci. 2006, 85, 974–979. [Google Scholar] [CrossRef]
- Tso, K.-H.; Ju, J.-C.; Fan, Y.-K.; Chiang, H.-I. Enzyme Degradation Reagents Effectively Remove Mycotoxins Deoxynivalenol and Zearalenone from Pig and Poultry Artificial Digestive Juices. Toxins 2019, 11, 599. [Google Scholar] [CrossRef] [Green Version]
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.-C.; Fremy, J.-M.; Massimi, C.; Prigent, P.; Debongnie, P.; Pussemier, L.; Boudra, H. Review of mycotoxin-detoxifying agents used as feed additives: Mode of action, efficacy and feed/food safety. EFSA Support. Publ. 2009, 6, 22E. [Google Scholar] [CrossRef]
- Carere, J.; Hassan, Y.I.; Lepp, D.; Zhou, T. The enzymatic detoxification of the mycotoxin deoxynivalenol: Identification of DepA from the DON epimerization pathway. Microb. Biotechnol. 2018, 11, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.; Efremenko, E. Enzymes for Detoxification of Various Mycotoxins: Origins and Mechanisms of Catalytic Action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, H.; Zhu, Z.; Ji, F.; Yin, X.; Hong, Q.; Shi, J. Isolation and characterization of Bacillus amyloliquefaciens ZDS-1: Exploring the degradation of Zearalenone by Bacillus spp. Food Control 2016, 68, 244–250. [Google Scholar] [CrossRef]
- Lee, A.; Cheng, K.-C.; Liu, J.-R. Isolation and characterization of a Bacillus amyloliquefaciens strain with zearalenone removal ability and its probiotic potential. PLoS ONE 2017, 12, e0182220. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-W.; Wang, H.-T.; Shih, W.-Y.; Ciou, Y.-A.; Chang, Y.-Y.; Ananda, L.; Wang, S.-Y.; Hsu, J.-T. Application of Zearalenone (ZEN)-Detoxifying Bacillus in Animal Feed Decontamination through Fermentation. Toxins 2019, 11, 330. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-C.; Hsu, T.-C.; Cheng, K.-C.; Liu, J.-R. Expression of the Clonostachys rosea lactonohydrolase gene by Lactobacillus reuteri to increase its zearalenone-removing ability. Microb. Cell Factories 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Stander, M.A.; Bornscheuer, U.T.; Henke, E.; Steyn, P.S. Screening of Commercial Hydrolases for the Degradation of Ochratoxin A. J. Agric. Food Chem. 2000, 48, 5736–5739. [Google Scholar] [CrossRef]
- Chang, X.; Wu, Z.; Wu, S.; Dai, Y.; Sun, C. Degradation of ochratoxin A by Bacillus amyloliquefaciens ASAG1. Food Addit. Contam. Part A 2015, 32, 564–571. [Google Scholar] [CrossRef]
- Wei, W.; Qian, Y.; Wu, Y.; Chen, Y.; Peng, C.; Luo, M.; Xu, J.; Zhou, Y. Detoxification of ochratoxin A by Lysobacter sp. CW239 and characteristics of a novel degrading gene carboxypeptidase cp4. Environ. Pollut. 2020, 258, 113677. [Google Scholar] [CrossRef]
- Politis, I.; Fegeros, K.; Nitsch, S.; Schatzmayr, G.; Kantas, D. Use of Trichosporon mycotoxinivorans to suppress the effects of ochratoxicosis on the immune system of broiler chicks. Br. Poult. Sci. 2005, 46, 58–65. [Google Scholar] [CrossRef]
- Ouethrani, M.; Van de Wiele, T.; Verbeke, E.; Bruneau, A.; Carvalho, M.; Rabot, S.; Camel, V. Metabolic fate of ochratoxin A as a coffee contaminant in a dynamic simulator of the human colon. Food Chem. 2013, 141, 3291–3300. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa, L.; Paterson, R.R.M.; Venâncio, A. Biodegradation of Ochratoxin A for Food and Feed Decontamination. Toxins 2010, 2, 1078–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Heinl, S.; Hartinger, D.; Thamhesl, M.; Vekiru, E.; Krska, R.; Schatzmayr, G.; Moll, W.-D.; Grabherr, R. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 2010, 145, 120–129. [Google Scholar] [CrossRef]
- Antonissen, G.; De Baere, S.; Novak, B.; Schatzmayr, D.; den Hollander, D.; Devreese, M.; Croubels, S. Toxicokinetics of Hydrolyzed Fumonisin B1 after Single Oral or Intravenous Bolus to Broiler Chickens Fed a Control or a Fumonisins-Contaminated Diet. Toxins 2020, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Fodor, J.; Balogh, K.; Weber, M.; Miklós, M.; Kametler, L.; Pósa, R.; Mamet, R.; Bauer, J.; Horn, P.; Kovács, F.; et al. Absorption, distribution and elimination of fumonisin B(1) metabolites in weaned piglets. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2008, 25, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Masching, S.; Naehrer, K.; Schwartz-Zimmermann, H.-E.; Sărăndan, M.; Schaumberger, S.; Dohnal, I.; Nagl, V.; Schatzmayr, D. Gastrointestinal Degradation of Fumonisin B1 by Carboxylesterase FumD Prevents Fumonisin Induced Alteration of Sphingolipid Metabolism in Turkey and Swine. Toxins 2016, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Grenier, B.; Schwartz-Zimmermann, H.E.; Gruber-Dorninger, C.; Dohnal, I.; Aleschko, M.; Schatzmayr, G.; Moll, W.D.; Applegate, T.J. Enzymatic hydrolysis of fumonisins in the gastrointestinal tract of broiler chickens. Poult. Sci. 2017, 96, 4342–4351. [Google Scholar] [CrossRef]
- Grenier, B.; Bracarense, A.-P.F.L.; Schwartz, H.E.; Lucioli, J.; Cossalter, A.-M.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Biotransformation Approaches To Alleviate the Effects Induced by Fusarium Mycotoxins in Swine. J. Agric. Food Chem. 2013, 61, 6711–6719. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef]
- Gallo, A.; Masoero, F. In vitro models to evaluate the capacity of different sequestering agents to adsorb aflatoxins. Ital. J. Anim. Sci. 2010, 9, e21. [Google Scholar] [CrossRef] [Green Version]
- Moschini, M.; Gallo, A.; Piva, G.; Masoero, F. The effects of rumen fluid on the in vitro aflatoxin binding capacity of different sequestering agents and in vivo release of the sequestered toxin. Anim. Feed Sci. Technol. 2008, 147, 292–309. [Google Scholar] [CrossRef]
- Diaz, D.E.; Hagler, W.M.; Blackwelder, J.T.; Eve, J.A.; Hopkins, B.A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin Binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.R.; Ledoux, D.R.; Naehrer, K.; Berthiller, F.; Applegate, T.J.; Grenier, B.; Phillips, T.D.; Schatzmayr, G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015, 94, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Chlebicz, A.; Śliżewska, K. In vitro detoxification of aflatoxin B 1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Nagatomi, Y.; Uyama, A.; Mochizuki, N. Degradation of Aflatoxin B1 during the Fermentation of Alcoholic Beverages. Toxins 2013, 5, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- Jouany, J.P.; Yiannikouris, A.; Bertin, G. The chemical bonds between mycotoxins and cell wall components of Saccharomyces cerevisiae have been identified. Arch. Zootech. 2005, 8, 26–50. [Google Scholar]
- Keller, L.; Abrunhosa, L.; Keller, K.; Rosa, C.A.; Cavaglieri, L.; Venâncio, A. Zearalenone and Its Derivatives α-Zearalenol and β-Zearalenol Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Toxins 2015, 7, 3297–3308. [Google Scholar] [CrossRef] [Green Version]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Walczak, J.; Buszewski, B. Microbiology neutralization of zearalenone using Lactococcus lactis and Bifidobacterium sp. Anal. Bioanal. Chem. 2018, 410, 943–952. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Walczak, J.; Railean-Plugaru, V.; Rudnicka, J.; Buszewski, B. Investigation of Zearalenone Adsorption and Biotransformation by Microorganisms Cultured under Cellular Stress Conditions. Toxins 2019, 11, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Złoch, M.; Rogowska, A.; Pomastowski, P.; Railean-Plugaru, V.; Walczak-Skierska, J.; Rudnicka, J.; Buszewski, B. Use of Lactobacillus paracasei strain for zearalenone binding and metabolization. Toxicon 2020, 181, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.U.; Li, M.; Jiang, W.; Sims, P.A.; Leong, K.W.; Wang, H.H. Spatial metagenomic characterization of microbial biogeography in the gut. Nat. Biotechnol. 2019, 37, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Ito, T.; Koyama, Y. Antimicrobial activity of aflatoxins. J. Bacteriol. 1967, 93, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Mojtahedi, M.; Mesgaran, M.D.; Vakili, S.A.; Hayati-Ashtiani, M. Effect of Aflatoxin B1 on in vitro Rumen Microbial Fermentation Responses Using Batch Culture. Annu. Res. Rev. Biol. 2013, 3, 686–693. [Google Scholar]
- Jiang, Y.H.; Yang, H.J.; Lund, P. Effect of aflatoxin B1 on in vitro ruminal fermentation of rations high in alfalfa hay or ryegrass hay. Anim. Feed Sci. Technol. 2012, 175, 85–89. [Google Scholar] [CrossRef]
- Auerbach, H.; Maas, R.F.M.; Op Den Camp, H.J.M.; Pol, A.; Fink Gremmels, J. Biodegradation of aflatoxin B1 by bovine rumen microorganisms in vitro and its effects on rumen fermentation. In Proceedings of the Mycotox 98. Mycotoxins in Food Chain: Processing and toxicological aspects (Mycotox 98. Les Mycotoxines dans la Chaine Alimentaire: Aspects toxicologiques et technologiques), Toulouse, France, 2–4 July 1998. [Google Scholar]
- Tapia, M.O.; Stern, M.D.; Soraci, A.L.; Meronuck, R.; Olson, W.; Gold, S.; Koski-Hulbert, R.L.; Murphy, M.J. Patulin-producing molds in corn silage and high moisture corn and effects of patulin on fermentation by ruminal microbes in continuous culture. Anim. Feed Sci. Technol. 2005, 119, 247–258. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Martin, C.; Boudra, H. Fungal secondary metabolites from Monascus spp. reduce rumen methane production in vitro and in vivo. J. Anim. Sci. 2013, 91, 848–860. [Google Scholar] [CrossRef]
- May, H.D.; Wu, Q.; Blake, C.K. Effects of the Fusarium spp. mycotoxins fusaric acid and deoxynivalenol on the growth of Ruminococcus albus and Methanobrevibacter ruminantium. Can. J. Microbiol. 2000, 46, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S.; Lee, J.H.; Simizu, Y.; Tazaki, H.; Itabashi, H.; Kimura, N. Effects of the Fusarium mycotoxin deoxynivalenol on in vitro rumen fermentation. Anim. Feed Sci. Technol. 2010, 162, 144–148. [Google Scholar] [CrossRef]
- Seeling, K.; Boguhn, J.; Strobel, E.; Dänicke, S.; Valenta, H.; Ueberschär, K.H.; Rodehutscord, M. On the effects of Fusarium toxin contaminated wheat and wheat chaff on nutrient utilisation and turnover of deoxynivalenol and zearalenone in vitro (Rusitec). Toxicol. In Vitro 2006, 20, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Boguhn, J.; Neumann, D.; Helm, A.; Strobel, E.; Tebbe, C.C.; Dänicke, S.; Rodehutscord, M. Effects of concentrate proportion in the diet with or without Fusarium toxin-contaminated triticale on ruminal fermentation and the structural diversity of rumen microbial communities in vitro. Arch. Anim. Nutr. 2010, 64, 467–483. [Google Scholar] [CrossRef] [Green Version]
- Cario, E. Bacterial Interactions with Cells of the intestinal mucosa: Toll-like receptors and nod2. Gut 2005, 54, 1182–1193. [Google Scholar] [CrossRef]
- Liew, W.-P.-P.; Mohd-Redzwan, S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007. [Google Scholar] [CrossRef] [Green Version]
- Szathmary, C.I.; Mirocha, C.J.; Palyusik, M.; Pathre, S.V. Identification of mycotoxins produced by species of Fusarium and Stachybotrys obtained from Eastern Europe. Appl. Environ. Microbiol. 1976, 32, 579. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.P. Effects of T-2 mycotoxin on gastrointestinal tissues: A Review ofin vivo andin vitro models. Arch. Environ. Contam. Toxicol. 1989, 18, 374–387. [Google Scholar] [CrossRef]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health B Crit. Rev. 2000, 3, 109–143. [Google Scholar] [CrossRef]
- Van Kol, S.W.M.; Hendriksen, P.J.M.; van Loveren, H.; Peijnenburg, A. The effects of deoxynivalenol on gene expression in the murine thymus. Toxicol. Appl. Pharmacol. 2011, 250, 299–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Górniak, S.L. Zearalenone, an Estrogenic Mycotoxin, Is an Immunotoxic Compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, T.; Suzuki, H.; Ishigami, N.; Shinozuka, J.; Uetsuka, K.; Nakayama, H.; Doi, K. Development of apoptosis and changes in lymphocyte subsetsin thymus, mesenteric lymph nodes and Peyer’s patches of mice orally inoculated with T-2 toxin. Exp. Toxicol. Pathol. 2001, 53, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Tigran, H.; Galina, H.; Nelli, B.; Arsen, A.; Rouben, A. The genotoxic and cytotoxic effects of ochratoxin A and T-2 toxin in rats bone marrow and blood cells. Toxicon 2019, 159, S28. [Google Scholar] [CrossRef]
- Ostaff, M.J.; Stange, E.F.; Wehkamp, J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 2013, 5, 1465. [Google Scholar] [CrossRef] [PubMed]
- Sivieri, K.; Bassan, J.; Peixoto, G.; Monti, R. Gut microbiota and antimicrobial peptides. Curr. Opin. Food Sci. 2017, 13, 56–62. [Google Scholar] [CrossRef]
- Xu, Z.; Takizawa, F.; Casadei, E.; Shibasaki, Y.; Ding, Y.; Sauters, T.J.C.; Yu, Y.; Salinas, I.; Sunyer, J.O. Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Huang, K.; Chen, S.; Qi, X.; He, X.; Cheng, W.-H.; Luo, Y.; Xia, K.; Xu, W. Combination of Metagenomics and Culture-Based Methods to Study the Interaction Between Ochratoxin A and Gut Microbiota. Toxicol. Sci. 2014, 141, 314–323. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Glenn, T.C.; Wang, J.-S. Aflatoxin B1 Induced Compositional Changes in Gut Microbial Communities of Male F344 Rats. Toxicol. Sci. 2016, 150, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Liew, W.-P.-P.; Mohd-Redzwan, S.; Than, L.T.L. Gut Microbiota Profiling of Aflatoxin B1-Induced Rats Treated with Lactobacillus casei Shirota. Toxins 2019, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Saint-Cyr, M.J.; Perrin-Guyomard, A.; Houée, P.; Rolland, J.-G.; Laurentie, M. Evaluation of an Oral Subchronic Exposure of Deoxynivalenol on the Composition of Human Gut Microbiota in a Model of Human Microbiota-Associated Rats. PLoS ONE 2013, 8, e80578. [Google Scholar] [CrossRef] [PubMed]
- Miró-Abella, E.; Torrell, H.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Ras, R.; Fontanals, N. Monitoring and evaluation of the interaction between deoxynivalenol and gut microbiota in Wistar rats by mass spectrometry-based metabolomics and next-generation sequencing. Food Chem. Toxicol. 2018, 121, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Dobrindt, U.; Martin, P.; Secher, T.; Bracarense, A.P.F.L.; Boury, M.; Laffitte, J.; Pinton, P.; Oswald, E.; Oswald, I.P. The Food Contaminant Deoxynivalenol Exacerbates the Genotoxicity of Gut Microbiota. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Liu, L.; Chen, J.; Xiao, A. Response of Intestinal Bacterial Flora to the Long-Term Feeding of Aflatoxin B1 (AFB1) in Mice. Toxins 2017, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Sun, Y.; Mu, P.; Zheng, T.; Mu, H.; Deng, F.; Deng, Y.; Wen, J. Lactobacillus rhamnosus GG supplementation modulates the gut microbiota to promote butyrate production, protecting against deoxynivalenol exposure in nude mice. Biochem. Pharmacol. 2020, 175, 113868. [Google Scholar] [CrossRef]
- Wang, J.-J.; Zhang, R.-Q.; Zhai, Q.-Y.; Liu, J.-C.; Li, N.; Liu, W.-X.; Li, L.; Shen, W. Metagenomic analysis of gut microbiota alteration in a mouse model exposed to mycotoxin deoxynivalenol. Toxicol. Appl. Pharmacol. 2019, 372, 47–56. [Google Scholar] [CrossRef]
- Vignal, C.; Djouina, M.; Pichavant, M.; Caboche, S.; Waxin, C.; Beury, D.; Hot, D.; Gower-Rousseau, C.; Body-Malapel, M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch. Toxicol. 2018, 92, 2327–2338. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, Y.; Wang, K.; Wei, H.; Shen, L. Isolation and characterization of the Bacillus cereus BC7 strain, which is capable of zearalenone removal and intestinal flora modulation in mice. Toxicon 2018, 155, 9–20. [Google Scholar] [CrossRef]
- Li, P.; Yang, S.; Zhang, X.; Huang, S.; Wang, N.; Wang, M.; Long, M.; He, J. Zearalenone Changes the Diversity and Composition of Caecum Microbiota in Weaned Rabbit. Available online: https://www.hindawi.com/journals/bmri/2018/3623274/ (accessed on 23 September 2020).
- Wang, C.; Huang, L.; Wang, P.; Liu, Q.; Wang, J. The Effects of Deoxynivalenol on the Ultrastructure of the Sacculus Rotundus and Vermiform Appendix, as Well as the Intestinal Microbiota of Weaned Rabbits. Toxins 2020, 12, 569. [Google Scholar] [CrossRef]
- Waché, Y.J.; Valat, C.; Postollec, G.; Bougeard, S.; Burel, C.; Oswald, I.P.; Fravalo, P. Impact of Deoxynivalenol on the Intestinal Microflora of Pigs. Int. J. Mol. Sci. 2009, 10, 1–17. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Chu, X.-H.; Ma, R.; Wang, Y.-W.; Liu, Q.; Zhang, N.-Y.; Karrow, N.A.; Sun, L.-H. Effects of deoxynivalenol on the porcine growth performance and intestinal microbiota and potential remediation by a modified HSCAS binder. Food Chem. Toxicol. 2020, 141, 111373. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Zhang, B.; Zhang, L.; Wu, K.; Yang, A.; Li, C.; Wang, Y.; Zhang, J.; Qi, D. Potential Link between Gut Microbiota and Deoxynivalenol-Induced Feed Refusal in Weaned Piglets. J. Agric. Food Chem. 2019, 67, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Śliżewska, K.; Nowak, A.; Zielonka, Ł.; Żakowska, Z.; Gajęcka, M.; Gajęcki, M. The Effect of Experimental Fusarium Mycotoxicosis on Microbiota Diversity in Porcine Ascending Colon Contents. Toxins 2014, 6, 2064–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.E.; Jeong, J.Y.; Song, J.; Lee, Y.; Lee, H.-J.; Kim, D.-W.; Jung, H.J.; Kim, K.H.; Kim, M.; Oh, Y.K.; et al. Colon Microbiome of Pigs Fed Diet Contaminated with Commercial Purified Deoxynivalenol and Zearalenone. Toxins 2018, 10, 374. [Google Scholar] [CrossRef] [Green Version]
- Cieplińska, K.; Gajęcka, M.; Dąbrowski, M.; Rykaczewska, A.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Zielonka, Ł.; Gajęcki, M.T. Time-Dependent Changes in the Intestinal Microbiome of Gilts Exposed to Low Zearalenone Doses. Toxins 2019, 11, 296. [Google Scholar] [CrossRef] [Green Version]
- Le Sciellour, M.; Zemb, O.; Serviento, A.-M.; Renaudeau, D. Transient effect of single or repeated acute deoxynivalenol and zearalenone dietary challenge on fecal microbiota composition in female finishing pigs. Anim. Int. J. Anim. Biosci. 2020, 1–11. [Google Scholar] [CrossRef]
- Burel, C.; Tanguy, M.; Guerre, P.; Boilletot, E.; Cariolet, R.; Queguiner, M.; Postollec, G.; Pinton, P.; Salvat, G.; Oswald, I.P.; et al. Effect of Low Dose of Fumonisins on Pig Health: Immune Status, Intestinal Microbiota and Sensitivity to Salmonella. Toxins 2013, 5, 841–864. [Google Scholar] [CrossRef] [Green Version]
- Mateos, I.; Combes, S.; Pascal, G.; Cauquil, L.; Barilly, C.; Cossalter, A.-M.; Laffitte, J.; Botti, S.; Pinton, P.; Oswald, I.P. Fumonisin-Exposure Impairs Age-Related Ecological Succession of Bacterial Species in Weaned Pig Gut Microbiota. Toxins 2018, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Wang, J.Q.; Jia, S.C.; Chen, Y.K.; Wang, J.P. Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B1 and necrotic enteritis. Poult. Sci. 2018, 97, 477–484. [Google Scholar] [CrossRef]
- Jahanian, E.; Mahdavi, A.H.; Asgary, S.; Jahanian, R. Effect of dietary supplementation of mannanoligosaccharides on growth performance, ileal microbial counts, and jejunal morphology in broiler chicks exposed to aflatoxins. Livestock Sci. 2016, 190, 123–130. [Google Scholar] [CrossRef]
- Jahanian, E.; Mahdavi, A.H.; Asgary, S.; Jahanian, R.; Tajadini, M.H. Effect of dietary supplementation of mannanoligosaccharides on hepatic gene expressions and humoral and cellular immune responses in aflatoxin-contaminated broiler chicks. Prev. Vet. Med. 2019, 168, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Galarza-Seeber, R.; Latorre, J.D.; Bielke, L.R.; Kuttappan, V.A.; Wolfenden, A.D.; Hernandez-Velasco, X.; Merino-Guzman, R.; Vicente, J.L.; Donoghue, A.; Cross, D.; et al. Leaky Gut and Mycotoxins: Aflatoxin B1 Does Not Increase Gut Permeability in Broiler Chickens. Front. Vet. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Lucke, A.; Böhm, J.; Zebeli, Q.; Metzler-Zebeli, B.U. Dietary Deoxynivalenol Contamination and Oral Lipopolysaccharide Challenge Alters the Cecal Microbiota of Broiler Chickens. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonissen, G.; Croubels, S.; Pasmans, F.; Ducatelle, R.; Eeckhaut, V.; Devreese, M.; Verlinden, M.; Haesebrouck, F.; Eeckhout, M.; De Saeger, S.; et al. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet. Res. 2015, 46, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Śliżewska, K.; Markowiak-Kopeć, P.; Sip, A.; Lipiński, K.; Mazur-Kuśnirek, M. The Effect of Using New Synbiotics on the Turkey Performance, the Intestinal Microbiota and the Fecal Enzymes Activity in Turkeys Fed Ochratoxin A Contaminated Feed. Toxins 2020, 12, 578. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, S.; Xia, Y.; Wang, H.; Ruan, D.; Zhou, T.; Zhu, Y.; Zhang, H.; Zhang, M.; Ye, H.; et al. Ochratoxin A induces liver inflammation: Involvement of intestinal microbiota. Microbiome 2019, 7. [Google Scholar] [CrossRef]
- Wos-Oxley, M.L.; Bleich, A.; Oxley, A.P.A.; Kahl, S.; Janus, L.M.; Smoczek, A.; Nahrstedt, H.; Pils, M.C.; Taudien, S.; Platzer, M.; et al. Comparative evaluation of establishing a human gut microbial community within rodent models. Gut Microb. 2012, 3, 234–249. [Google Scholar] [CrossRef] [Green Version]
- Gresse, R.; Chaucheyras Durand, F.; Dunière, L.; Blanquet-Diot, S.; Forano, E. Microbiota Composition and Functional Profiling Throughout the Gastrointestinal Tract of Commercial Weaning Piglets. Microorganisms 2019, 7, 343. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Camilleri, M.; Lyle, B.J.; Madsen, K.L.; Sonnenburg, J.; Verbeke, K.; Wu, G.D. Role for diet in normal gut barrier function: Developing guidance within the framework of food-labeling regulations. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G17–G39. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-H.; Kim, D.; Kim, J.; Moon, Y. Effects of Mycotoxins on Mucosal Microbial Infection and Related Pathogenesis. Toxins 2015, 7, 4484–4502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resta-Lenert, S.; Barrett, K.E. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: Role of iNOS and COX-2. Gastroenterology 2002, 122, 1070–1087. [Google Scholar] [CrossRef]
- Farfán-García, A.E.; Ariza-Rojas, S.C.; Vargas-Cárdenas, F.A.; Vargas-Remolina, L.V. Virulence mechanisms of enteropathogenic Escherichia coli. Rev. Chil. Infectologia Organo Of. Soc. Chil. Infectologia 2016, 33, 438–450. [Google Scholar] [CrossRef] [Green Version]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Cuff, C.F.; Pestka, J. Modulation of Murine Host Response to Enteric Reovirus Infection by the Trichothecene Deoxynivalenol. Toxicol. Sci. 2005, 87, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Cuff, C.F.; Pestka, J.J. T-2 toxin impairment of enteric reovirus clearance in the mouse associated with suppressed immunoglobulin and IFN-γ responses. Toxicol. Appl. Pharmacol. 2006, 214, 318–325. [Google Scholar] [CrossRef]
- Shrestha, A.; Uzal, F.A.; McClane, B.A. Enterotoxic Clostridia: Clostridium perfringens Enteric Diseases. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Antonissen, G.; Immerseel, F.V.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F.; Timbermont, L.; Verlinden, M.; Janssens, G.P.J.; Eeckhaut, V.; Eeckhout, M.; et al. The Mycotoxin Deoxynivalenol Predisposes for the Development of Clostridium perfringens-Induced Necrotic Enteritis in Broiler Chickens. PLoS ONE 2014, 9, e108775. [Google Scholar] [CrossRef] [Green Version]
- Vila, J.; Sáez-López, E.; Johnson, J.R.; Römling, U.; Dobrindt, U.; Cantón, R.; Giske, C.G.; Naas, T.; Carattoli, A.; Martínez-Medina, M.; et al. Escherichia coli: An old friend with new tidings. FEMS Microbiol. Rev. 2016, 40, 437–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baines, D.; Sumarah, M.; Kuldau, G.; Juba, J.; Mazza, A.; Masson, L. Aflatoxin, Fumonisin and Shiga Toxin-Producing Escherichia coli Infections in Calves and the Effectiveness of Celmanax®/Dairyman’s ChoiceTM Applications to Eliminate Morbidity and Mortality Losses. Toxins 2013, 5, 1872–1895. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jindal, N.; Shukla, C.L.; Asrani, R.K.; Ledoux, D.R.; Rottinghaus, G.E. Pathological changes in broiler chickens fed ochratoxin A and inoculated with Escherichia coli. Avian Pathol. 2004, 33, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Ledoux, D.R.; Bermudez, A.J.; Fritsche, K.L.; Rottinghaus, G.E. The individual and combined effects of fumonisin B1 and moniliformin on performance and selected immune parameters in turkey poults. Poult. Sci. 2000, 79, 871–878. [Google Scholar] [CrossRef]
- Oswald, I.P.; Desautels, C.; Laffitte, J.; Fournout, S.; Peres, S.Y.; Odin, M.; Le Bars, P.; Le Bars, J.; Fairbrother, J.M. Mycotoxin Fumonisin B1 Increases Intestinal Colonization by Pathogenic Escherichia coli in Pigs. Appl. Environ. Microbiol. 2003, 69, 5870–5874. [Google Scholar] [CrossRef] [Green Version]
- Devriendt, B.; Gallois, M.; Verdonck, F.; Wache, Y.; Bimczok, D.; Oswald, I.P.; Goddeeris, B.M.; Cox, E. The food contaminant fumonisin B1 reduces the maturation of porcine CD11R1+ intestinal antigen presenting cells and antigen-specific immune responses, leading to a prolonged intestinal ETEC infection. Vet. Res. 2009, 40. [Google Scholar] [CrossRef] [Green Version]
- Hurley, D.; McCusker, M.P.; Fanning, S.; Martins, M. Salmonella–Host Interactions-Modulation of the Host Innate Immune System. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ramírez, J.O.; Nava-Ramírez, M.J.; Merino-Guzmán, R.; Téllez-Isaías, G.; Vázquez-Durán, A.; Méndez-Albores, A. The effect of moderate-dose aflatoxin B1 and Salmonella Enteritidis infection on intestinal permeability in broiler chickens. Mycotoxin Res. 2020, 36, 31–39. [Google Scholar] [CrossRef]
- Hara-Kudo, Y.; Sugita-Konishi, Y.; Kasuga, F.; Kumagai, S. Effects of deoxynivalenol on Salmonella enteritidis infection. Mycotoxins 1996, 1996, 51–55. [Google Scholar] [CrossRef]
- Vandenbroucke, V.; Croubels, S.; Martel, A.; Verbrugghe, E.; Goossens, J.; Van Deun, K.; Boyen, F.; Thompson, A.; Shearer, N.; De Backer, P.; et al. The mycotoxin deoxynivalenol potentiates intestinal inflammation by Salmonella typhimurium in porcine ileal loops. PLoS ONE 2011, 6, e23871. [Google Scholar] [CrossRef] [Green Version]
- Tai, J.H.; Pestka, J.J. Impaired murine resistance to Salmonella typhimurium following oral exposure to the trichothecene T-2 toxin. Food Chem. Toxicol. 1988, 26, 691–698. [Google Scholar] [CrossRef]
- Tai, J.-H.; Pestka, J.J. T-2 toxin impairment of murine response to Salmonella typhimurium: A histopathologic assessment. Mycopathologia 1990, 109, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ziprin, R.L.; Elissalde, M.H. Effect of T-2 toxin on resistance to systemic Salmonella typhimurium infection of newly hatched chickens. Am. J. Vet. Res. 1990, 51, 1869–1872. [Google Scholar] [PubMed]
- Kubena, L.F.; Bailey, R.H.; Byrd, J.A.; Young, C.R.; Corrier, D.E.; Stanker, L.H.; Rottinghaust, G.E. Cecal Volatile Fatty Acids and Broiler Chick Susceptibility to Salmonella typhimurium Colonization as Affected by Aflatoxins and T-2 Toxin1. Poult. Sci. 2001, 80, 411–417. [Google Scholar] [CrossRef]
- Fukata, T.; Sasai, K.; Baba, E.; Arakawa, A. Effect of ochratoxin A on Salmonella typhimurium-challenged layer chickens. Avian Dis. 1996, 40, 924–926. [Google Scholar] [CrossRef]
- Deshmukh, S.; Asrani, R.K.; Jindal, N.; Ledoux, D.R.; Rottinghaus, G.E.; Sharma, M.; Singh, S.P. Effects of Fusarium moniliforme culture material containing known levels of fumonisin B1 on progress of Salmonella Gallinarum infection in Japanese quail: Clinical signs and hematologic studies. Avian Dis. 2005, 49, 274–280. [Google Scholar] [CrossRef]
- Vandenbroucke, V.; Croubels, S.; Verbrugghe, E.; Boyen, F.; De Backer, P.; Ducatelle, R.; Rychlik, I.; Haesebrouck, F.; Pasmans, F. The mycotoxin deoxynivalenol promotes uptake of Salmonella Typhimurium in porcine macrophages, associated with ERK1/2 induced cytoskeleton reorganization. Vet. Res. 2009, 40, 64. [Google Scholar] [CrossRef] [Green Version]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 1–17. [Google Scholar] [CrossRef]
- Alhinai, E.A.; Walton, G.E.; Commane, D.M. The Role of the Gut Microbiota in Colorectal Cancer Causation. Int. J. Mol. Sci. 2019, 20, 5295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federici, E.; Prete, R.; Lazzi, C.; Pellegrini, N.; Moretti, M.; Corsetti, A.; Cenci, G. Bacterial Composition, Genotoxicity, and Cytotoxicity of Fecal Samples from Individuals Consuming Omnivorous or Vegetarian Diets. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, F.; Dinu, M.; Pagliai, G.; Pierre, F.; Gueraud, F.; Bowman, J.; Gerard, P.; Longo, V.; Giovannelli, L.; Caderni, G.; et al. Fecal microbiome as determinant of the effect of diet on colorectal cancer risk: Comparison of meat-based versus pesco-vegetarian diets (the MeaTIc study). Trials 2019, 20. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Geneva, Switzerland, 1993; ISBN 978-92-832-1256-0. [Google Scholar]
- Tu, P.; Chi, L.; Bodnar, W.; Zhang, Z.; Gao, B.; Bian, X.; Stewart, J.; Fry, R.; Lu, K. Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics 2020, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef] [Green Version]
- Norris, G.H.; Blesso, C.N. Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. Nutrients 2017, 9, 1180. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.L.; Heaver, S.L.; Waters, J.L.; Kim, B.I.; Bretin, A.; Goodman, A.L.; Gewirtz, A.T.; Worgall, T.S.; Ley, R.E. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 2020, 11, 2471. [Google Scholar] [CrossRef]


| Species | Exposure * | Method of Aanalyze | Sample Analyzed | Result | Reference |
|---|---|---|---|---|---|
| Rat | AFB1: 5, 25, 75 μg/kg BW 28 days | 16S rRNA gene sequencing | excreta | Decreased phylogenic diversity No consistent pattern of increase or decrease at phylum level | [144] |
| Rat | AFB1: 25 μg/kg BW 28 days | 16S rRNA gene sequencing | excreta | Firmicutes (82%), Bacteroidetes (13.5%) the most abundant Proteobacteria (3.3%) Actinobacteria (1.7%) and Saccharibacteria (1%) No effect on microbiota richness Increased abundance of Alloprevotella spp decrease in Prevotella_9. | [145] |
| Rat | DON: 100 μg/kg BW 28 days | RT-PCR | excreta rats inoculated with human fecal flora | Variation of microbiota composition with time Increased concentration of Bacteroides and Prevotella genera on day 10–20 Reduced expression of Escherichia coli on day 27 | [146] |
| Rat | DON: 60, 120 μg/kg BW 40 days | 16S rRNA gene sequencing | cecal digesta | Firmicutes and Bacteroidetes were the most abundant Increase in the relative abundance of Coprococcus genus | [147] |
| Rat | DON: 2, 10 mg/kg feed 28 days | 16S rRNA gene sequencing | excreta | No significant alteration of the composition or diversity of the microbiota | [148] |
| Rat | OTA: 70, 210 μg/kg BW 28 days | 16S rRNA gene sequencing | excreta | Reduced within-subject diversity of the microbiota Increased relative abundance of Lactobacillus Reduced relative abundance of Bacteroides, Dorea, Escherichia, Oribacterium, Ruminococcus, and Syntrophococcus | [143] |
| Mice | AFB1*: 100, 160, 400 μg/kg BW 60 days | 16S rRNA gene sequencing | intestinal contents (from jejunum to rectum) | Lactobacillus and Bacteroides are the dominant flora Differences in the relative abundance of bacterial flora Effects are not dose-dependent | [149] |
| Mice | DON: 1, 5 mg/kg BW 14 days | 16S rRNA gene sequencing | excreta | Variation of microbiota composition with time Bacteroidetes, Firmicutes, Proteobacteria and Verrucomicrobia are the dominant bacterial phyla Reduced relative abundance of Bacteroidaceae family and Alistipes genus on day 14. | [150] |
| Mice | DON: 1, 5 mg/kg BW 14 days | shotgun sequencing | cecal digesta | The most abundant genera were Lactobacillus, Mastadenovirus, Bacteroides, Mucispirillum, and Parabacteroides Increased relative abundance of Firmicutes at low doses Increased relative abundance of Bacteroidetes at high doses | [151] |
| Mice | DON: 10 μg/kg BW 280 days | 16S rRNA gene sequencing | excreta | Bacteria of the Firmicutes phyla are the most abundant Increase of Deferribacteres, Proteobacteria, TM7, Verrucomicrobia, Tenericutes, and Cyanobacteria. Reduced abundance of Actinobacteria and Bacteroidetes Several significant differences in taxonomic abundances at the family and genus levels. | [152] |
| Mice | ZEN: 10 mg/kg BW 14 days | 16S rRNA gene sequencing | colon digesta | Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria were the dominant phyla in the colon Reduced diversity of the microbiota Reduced abundance of Firmicutes, Bacteroidetes | [153] |
| Rabbit | ZEN: 400, 800, 1600 µg/kg BW 28 days | 16S rRNA gene sequencing | cecal digesta | Reduced abundance of Actinobacteria and increase the abundance of Cyanobacteria, Synergistetes, and Proteobacteria. Reduced abundance of Adlercreutzia, Blautia, Desulfitobacter, Lactobacillus, Oxalobacter, and p-75-a5. | [154] |
| Rabbit | DON: 1.5 mg/kg BW 24 days | 16S rRNA gene sequencing | ileal, cecal and colon digesta | Reduced abundance and diversity of the microflora, Firmicutes, Bacteroidetes, and Proteobacteria were the dominant phyla. Reduced relative abundance of Proteobacteria, Actinobacteria, and Cyanobacteria in both the ileum and caecum and increased relative abundance of Firmicutes and Bacteroidetes in the ileum and colon.in the ileum and Increased relative abundance of Ruminococcaceae, Bacteriods, and Lachnospiraleaes in the ileum, caecum, and colon. Ruminococcaceae represented the largest number of bacteria in the three intestinal segments at the genus level. | [155] |
| Pig | DON: 2.5 mg/kg feed 28 days | Bacterial culture Capillary electrophoresis | excreta | Variation of total aerobic bacterial flora with time Increase in total aerobic mesophilic bacteria max on day 7 | [156] |
| Pig | DON: 1, 3 mg/kg feed 28 days | 16S rRNA gene sequencing | small intestinal lumen digesta | Firmicutes, Proteobacteria, Cyanobacteria and Actinobacteria were the dominant phyla Reduced abundance of Firmicutes and increased abundance of Actinobacteria in duodenum and ileum Reduced abundance of Proteobacteria and increased abundance of Cyanobacteria in duodenum, jejunum, and ileum Lactobacillus, Cupriavidus, Acinetobacter, Burholderia, Staphylococcus, Ochrobactrum, Corynebacterium, and Streptococcus were the predominant generaReduced abundance of Lactobacillus and Cupriavidus and increased abundance of Staphylococcus Reduced abundance of Burkholderia in the duodenum and jejunum, but increased abundance in the ileum | [157] |
| Pig | DON: 0.61, 1.28, 2.89 mg/kg feed 28 days | 16S rRNA gene sequencing | cecal digesta | Reduced abundances of unclassified f_Lachnospiraceae, Phascolarctobacterium and Ruminococcaceae_UCG-014 Increased Prevotella_9 and norank f_Prevotellaceae | [158] |
| Pig | ZEN: 40 μg/kg BW DON: 12 μg/kg BW ZEN + DON: 40 + 12 μg/kg BW42 days | EcoPlate tests | ascending colon digesta | Variation of total aerobic bacterial flora with time Same effect in nature whatever the toxin Lactic acid bacteria predominant floraDecrease in the number of mesophilic aerobic bacteria Decrease in the level of C. perfringens, E. coli, and Enterobacteriaceae family | [159] |
| Pig | ZEN: 0.8 mg/kg feed DON: 8 mg/kg feed 7 days | 16S rRNA gene sequencing | colon digesta | Firmicutes and Bacteroidetes were the dominant phyla Lactobacillus, Megasphaera, and Faecalibacterium genera, and the unclassified Clostridiaceae family were the most abundant Lactobacillus was particularly more abundant in the DON (7.6%) and ZEN (2.7%) groups than in the control (0.2%). | [160] |
| Pig | ZEN: 5, 10, 15 µg/kg BW 7, 21, 42 days | Bacterial culture | duodenal cap, third duodenum part, jejunum, caecum, descending colon digesta | Microbial counts, mainly E. coli and Enterococcus faecalis, varied from the proximal to the distal segments of the intestinal tract ZEN affected the colony counts of microbiota rather than diversity Increased yeast and mold counts in all intestinal segments, in particular in the colon | [161] |
| Pig | DON + ZEN: 3.02 + 0.76 mg/kg feed 7 days Repeated exposure | 16S rRNA gene sequencing | excreta | Reduced relative abundances of Ruminococcaceae, Streptococcaceae, and Veillonellaceae and increased Erysipelotrichaceae Microbiota returned to the initial state within 3 weeks after the end of a single or repeated DON/ZEN challenge | [162] |
| Pig | FB1+FB2: 8.6 + 3.2 mg/kg feed 63 days | Capillary single-stranded conformation polymorphism analysis | excreta | Variation of total aerobic bacterial flora with time Reversible alteration of the microbiota balance | [163] |
| Pig | FB1: 12 mg/kg feed 0, 8, 15, 22, 29 days | 16S rRNA gene sequencing | excreta | Decrease in the diversity index, and shifts and constraints in the structure and the composition of the microbiota after 15 days of exposure that reached maximum after 22 days of exposure Increased Lactobacillus and reduced Lachnospiraceae, Veillonellaceae families, and particularly the genera Mitsuokella, Faecalibacterium, and Roseburia | [164] |
| Broiler | AFB1: 40 μg/kg feed 21 days | Bacterial culture | ileal digesta | No effect on Lactobacilli, Bifidobacteria, C. perfringens, E. coli | [165] |
| Broiler | Aflatoxins 0.5, 2 mg/kg feed 7 and 28 days | Bacterial culture | ileal digesta | Increased E. coli, Salmonella, Klebsiella, and total Gram- bacteria at day 28 of exposure Changes persisted for 14 days after exposure stopped | [166,167] |
| Broiler | AFB1: 1, 1.5, 2 mg/kg feed 21 days | Bacterial culture | cecal digesta | Increased total aerobic bacteria, total Gram - bacteria, variable effect on total lactic acid bacteria Effects are not always dose-dependent | [168] |
| Broiler | DON: 2.5, 5 and 10 mg/kg feed 35 days | 16S rRNA gene sequencing | cecal digesta | Increased relative abundance of Firmicutes (decreased Oscillospira, Clostridiaceae genus, Clostridium, and Ruminococcaceae genera but increased Clostridiales genus) Reduced relative abundance of Proteobacteria | [169] |
| Broiler | FB1 + FB2: 10.4 + 8.2 mg/kg feed 15 days | 16S rRNA gene sequencing | ileal digesta | Reduced abundance of Candidatus Savagella and Lactobaccilus spp., increased total Clostridium perfringens | [170] |
| Turkey | OTA: 199 to 462 µg/kg feed 21, 42, 63, 105 days | Bacterial culture | jejunum and cecal digesta, excreta | Reduced Lactobacillus spp. and Bifidobacterium spp. in samples of the intestinal content and the excreta after 15 weeks | [171] |
| Duck | OTA: 235 µg/kg BW 14 days | 16S rRNA gene sequencing | excreta | Increased Bacteroidetes (phylum level), Bacteroides (genus level), Bacteroides plebeius (species level) | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerre, P. Mycotoxin and Gut Microbiota Interactions. Toxins 2020, 12, 769. https://doi.org/10.3390/toxins12120769
Guerre P. Mycotoxin and Gut Microbiota Interactions. Toxins. 2020; 12(12):769. https://doi.org/10.3390/toxins12120769
Chicago/Turabian StyleGuerre, Philippe. 2020. "Mycotoxin and Gut Microbiota Interactions" Toxins 12, no. 12: 769. https://doi.org/10.3390/toxins12120769
APA StyleGuerre, P. (2020). Mycotoxin and Gut Microbiota Interactions. Toxins, 12(12), 769. https://doi.org/10.3390/toxins12120769





