Presence of 19 Mycotoxins in Human Plasma in a Region of Northern Spain
Abstract
:1. Introduction
2. Results
2.1. Control of the Analytical Sequences
2.2. Re-Validation of the Methodology after Enzymatic Treatment
2.3. Plasma Samples
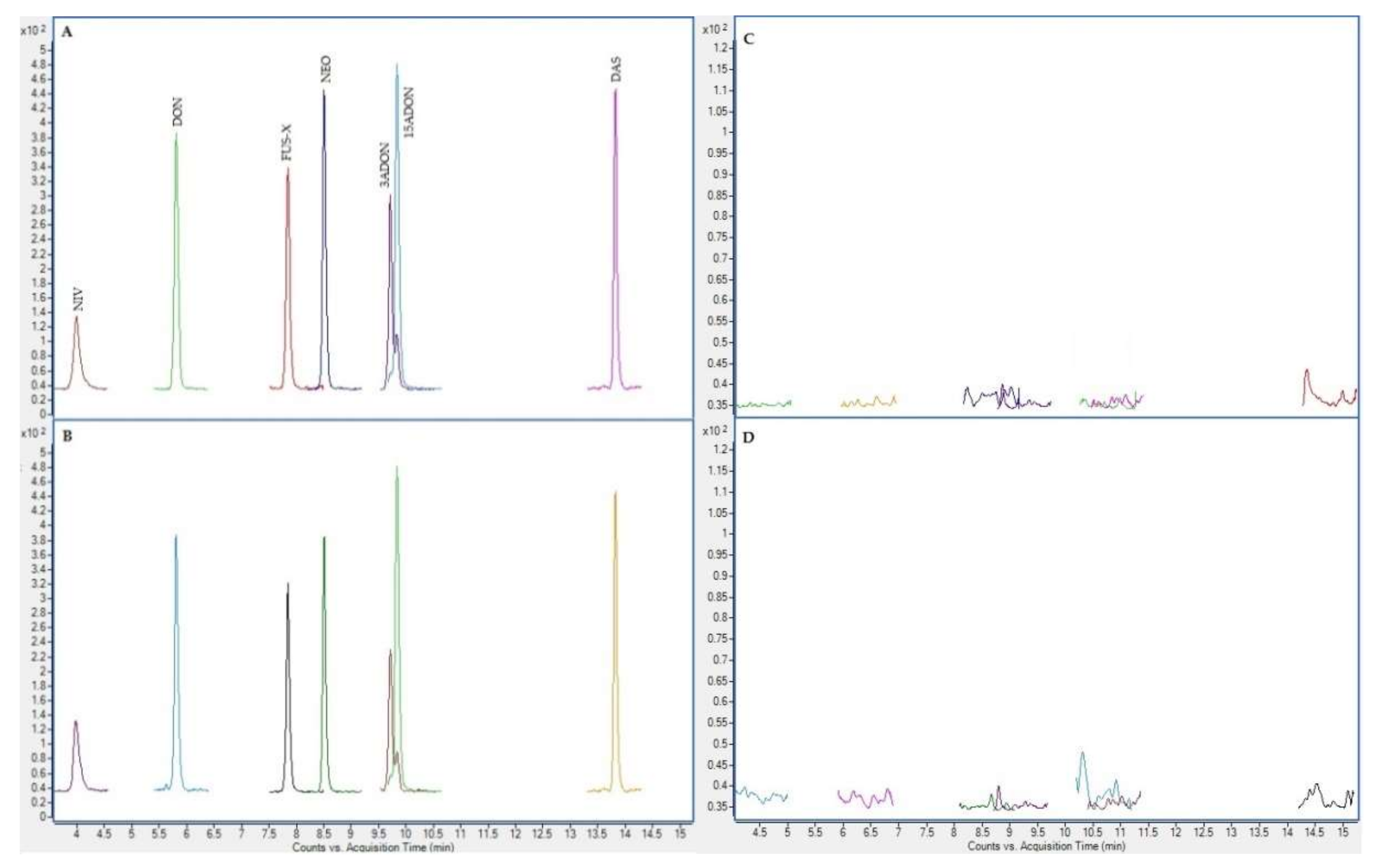
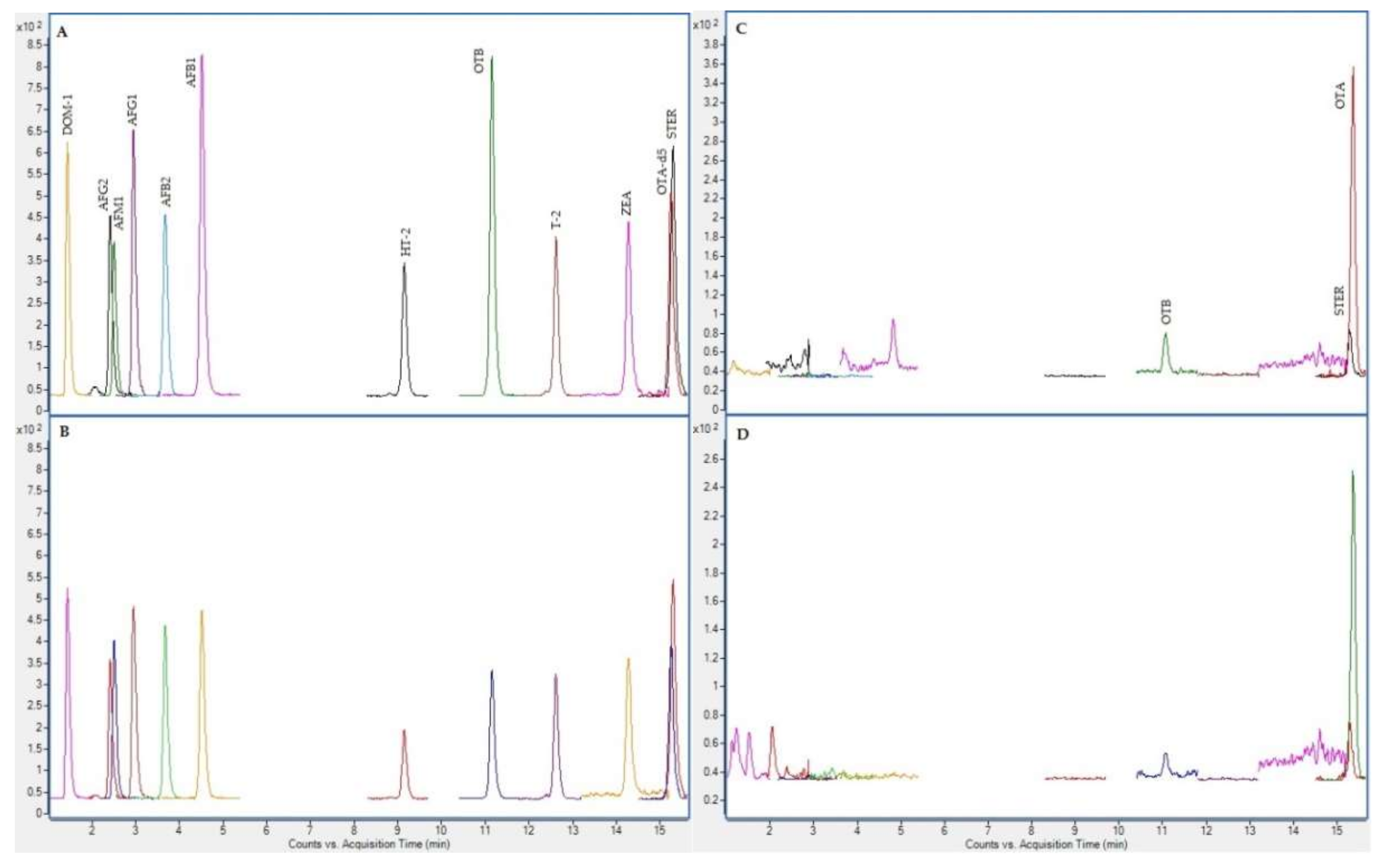
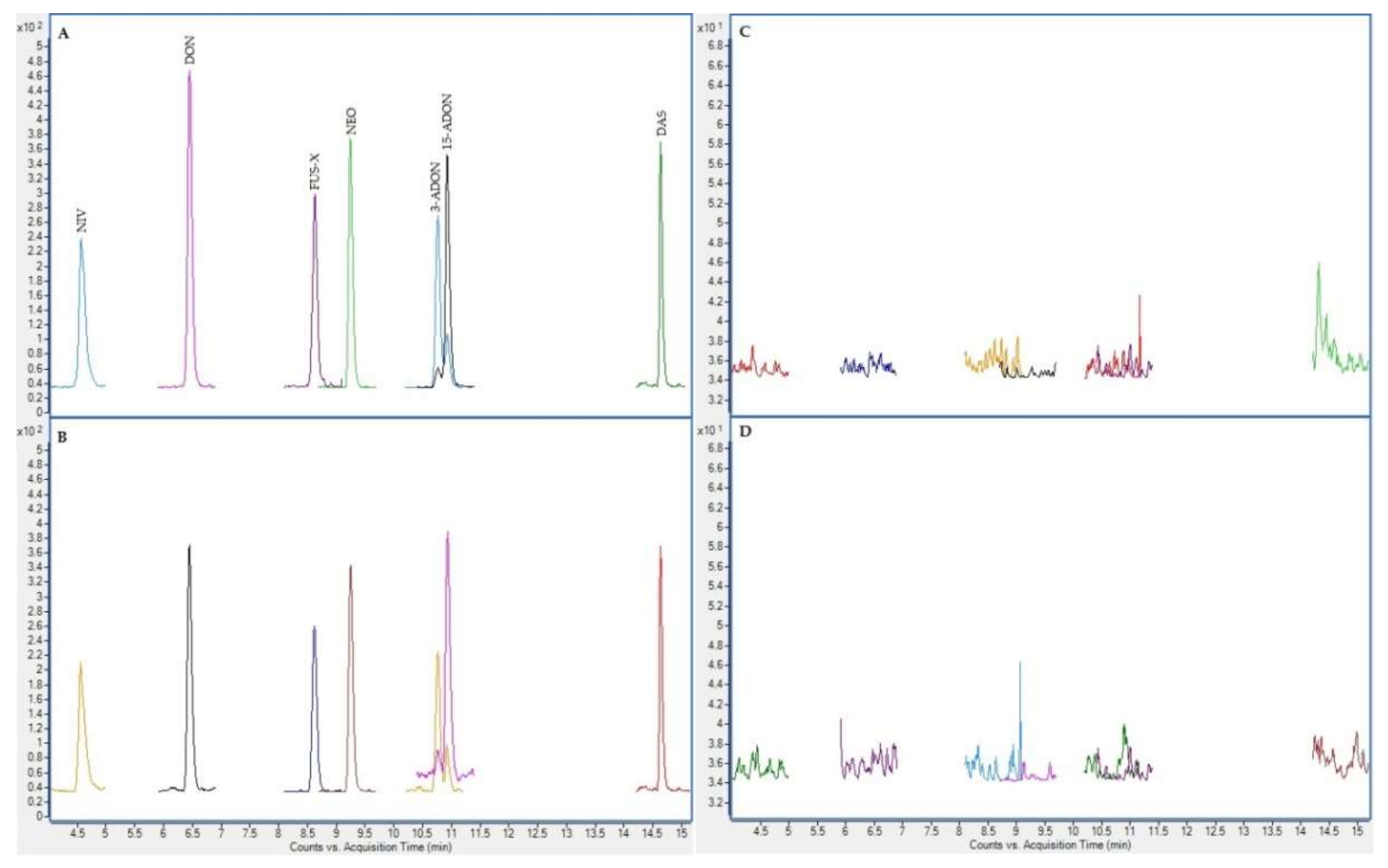
2.4. Chromatographic Results
2.5. Mycotoxins in Samples
2.5.1. Reanalysis
2.5.2. Results before Enzymatic treatment
2.5.3. Results after Enzymatic Treatment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Subject Recruitment
5.2. Plasma Sample Collection
5.3. Sample Analysis
5.4. Control of the Analytical Sequences
5.5. Analysis of the Plasma Samples
5.6. Reanalysis
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 15-ADON | 15-acetyldeoxynivalenol |
| 3-ADON | 3-acetyldeoxynivalenol |
| ACN | acetonitrile |
| AFB1 | aflatoxin B1 |
| AFB2 | aflatoxin B2 |
| AFG1 | aflatoxin G1 |
| AFG2 | aflatoxin G2 |
| AFM1 | aflatoxin M1 |
| AFs | aflatoxins |
| CI | confidence interval |
| DAS | diacetoxyscirpenol |
| DOM-1 | deepoxy-deoxynivalenol |
| DON | deoxynivalenol |
| EDI | estimated daily intake |
| EDTA | ethylendiaminetetraacetic acid |
| EFSA | European Food Safety Authority |
| EMEA | European Medicines Agency |
| ESI | electrospray ionization |
| FDA | Food and Drug Administration |
| FUS-X | fusarenon-X |
| HBM | human biomonitoring |
| IARC | International Agency for Research on Cancer |
| LC | liquid chromatography |
| LC-MS/MS | liquid chromatography- mass spectrometry |
| LOD | limit of detection |
| LOQ | limit of quantification |
| ME | matrix effect |
| NEO | neosolaniol |
| NIV | nivalenol |
| OTα | ochratoxin α |
| OTA | ochratoxin A |
| OTA-d5 | ochratoxin A-(phenyl-d5) |
| OTB | ochratoxin B |
| OTC | ochratoxin C |
| q | transition of qualification |
| Q | transition of quantification |
| QqQ | triple quadrupole |
| RE | relative error of the mean |
| RSD | relative standard deviation |
| S/N | signal-to-noise ratio |
| SRM | selected reaction monitoring |
| STER | sterigmatocystin |
| TWI | tolerable weekly intake |
| ZEA | zearalenone |
References
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viegas, S.; Viegas, C.; Oppliger, A. Occupational Exposure to Mycotoxins: Current Knowledge and Prospects. Ann. Work Expos. Heal. 2018, 62, 923–941. [Google Scholar] [CrossRef] [PubMed]
- Marín, S.; Cano-Sancho, G.; Sanchis, V.; Ramos, A.J. The role of mycotoxins in the human exposome: Application of mycotoxin biomarkers in exposome-health studies. Food Chem. Toxicol. 2018, 121, 504–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- World Health Organization Mycotoxins. Fact Sheets; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2019, 60, 2773–2789. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. The Fate of Mycotoxins During the Processing of Wheat for Human Consumption. Compr. Rev. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef] [Green Version]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Viegas, S.; Martins, C. The usefulness of human biomonitoring in the case of mycotoxins exposure assessment. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–6. ISBN 9780128096338. [Google Scholar]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Silins, I.; Högberg, J.; Silins, I.; Högberg, J. Combined Toxic Exposures and Human Health: Biomarkers of Exposure and Effect. Int. J. Environ. Res. Public Heal. 2011, 8, 629–647. [Google Scholar] [CrossRef] [Green Version]
- Warth, B.; Sulyok, M.; Krska, R. LC-MS/MS-based multibiomarker approaches for the assessment of human exposure to mycotoxins. Anal. Bioanal. Chem. 2013, 405, 5687–5695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurusankar, R.; Yenugadhati, N.; Krishnan, K.; Hays, S.; Haines, D.; Zidek, A.; Kuchta, S.; Kinniburgh, D.; Gabos, S.; Mattison, D.; et al. The role of human biological monitoring in health risk assessment. Int. J. Risk Assess. Manag. 2017, 20, 136–197. [Google Scholar] [CrossRef]
- WHO. Human Biomonitoring: Facts and Figures; WHO: Geneva, Switzerland, 2015; pp. 1–88. [Google Scholar]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Alvito, P.; Assunção, R. Burden of disease associated with dietary exposure to carcinogenic aflatoxins in Portugal using human biomonitoring approach. Food Res. Int. 2020, 134, 109210. [Google Scholar] [CrossRef]
- Vidal, A.; Marín, S.; Sanchis, V.; De Saeger, S.; De Boevre, M. Hydrolysers of modified mycotoxins in maize: α-Amylase and cellulase induce an underestimation of the total aflatoxin content. Food Chem. 2018, 248, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Aarøe Mørck, T.; Polcher, A.; Knudsen, L.E.; Joas, A. Review of the state of the art of human biomonitoring for chemical substances and its application to human exposure assessment for food safety. EFSA Support. Publ. 2015, 12, EN-724. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, M.; Humpf, H.U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Louro, H.; Heinälä, M.; Bessems, J.; Buekers, J.; Vermeire, T.; Woutersen, M.; Van Engelen, J.; Borges, T.; Rousselle, C.; Ougier, E.; et al. Human biomonitoring in health risk assessment in Europe: Current practices and recommendations for the future. Int. J. Hyg. Environ. Health 2019, 222, 727–737. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, D.; Kim, H.; Jahng, K.-Y. Quantitative determination of mycotoxins in urine by LC-MS/MS. Food Addit. Contam. Part A 2010, 27, 1674–1682. [Google Scholar] [CrossRef]
- Ali, N.; Muñoz, K.; Degen, G.H. Ochratoxin A and its metabolites in urines of German adults—An assessment of variables in biomarker analysis. Toxicol. Lett. 2017, 275, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Degen, G.H. Citrinin biomarkers: A review of recent data and application to human exposure assessment. Arch. Toxicol. 2019, 93, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Slobodchikova, I.; Vuckovic, D. Liquid chromatography—High resolution mass spectrometry method for monitoring of 17 mycotoxins in human plasma for exposure studies. J. Chromatogr. A 2018, 1548, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [Green Version]
- Arce-López, B.; Lizarraga, E.; Flores-Flores, M.; Irigoyen, Á.; González-Peñas, E. Development and validation of a methodology based on Captiva EMR-lipid clean-up and LC-MS/MS analysis for the simultaneous determination of mycotoxins in human plasma. Talanta 2020, 206, 120193. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Vea, M.; González-Peñas, E.; Lizarraga, E.; López De Cerain, A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in barley from a northern region of Spain. Food Chem. 2012, 132, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Remiro, R.; González-Peñas, E.; Lizarraga, E.; López de Cerain, A. Quantification of ochratoxin A and five analogs in Navarra red wines. Food Control 2012, 27, 139–145. [Google Scholar] [CrossRef]
- Murillo, M.; González-Peñas, E.; Amézqueta, S. Determination of patulin in commercial apple juice by micellar electrokinetic chromatography. Food Chem. Toxicol. 2008, 46, 57–64. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; González-Peñas, E. Short communication: Analysis of mycotoxins in Spanish milk. J. Dairy Sci. 2018, 101, 113–117. [Google Scholar] [CrossRef]
- Jimenez, A.M.; Lopez de Cerain, A.; Gonzalez-Peñas, E.; Bello, J.; Betbeder, A.M.; Creppy, E.E. Exposure to Ochratoxin a in Europe: Comparison with a Region of Northern Spain. J. Toxicol. Toxin Rev. 1998, 17, 479–491. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bioanalytical Method Validation Guidance; Food and Drug Administration: Silver Spring, MD, USA, 2018; Volume 1043.
- Al-Jaal, B.A.; Latiff, A.; Salama, S.; Barcaru, A.; Horvatovich, P.; Jaganjac, M. Determination of multiple mycotoxins in Qatari population serum samples by LC-MS/MS. World Mycotoxin J. 2020, 13, 57–65. [Google Scholar] [CrossRef]
- Tesfamariam, K.; De Boevre, M.; Kolsteren, P.; Belachew, T.; Mesfin, A.; De Saeger, S.; Lachat, C. Dietary mycotoxins exposure and child growth, immune system, morbidity, and mortality: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. The occurrence of ochratoxin A in human body fluids—Review. Toxin Rev. 2019, 38, 1–14. [Google Scholar] [CrossRef]
- Fan, K.; Xu, J.; Jiang, K.; Liu, X.; Meng, J.; Di Mavungu, J.D.; Guo, W.; Zhang, Z.; Jing, J.; Li, H.; et al. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ. Pollut. 2019, 248, 865–873. [Google Scholar] [CrossRef]
- Osteresch, B.; Viegas, S.; Cramer, B.; Humpf, H.-U. Multi-mycotoxin analysis using dried blood spots and dried serum spots. Anal. Bioanal. Chem. 2017, 409, 3369–3382. [Google Scholar] [CrossRef] [Green Version]
- Coronel, M.B.; Sanchis, V.; Ramos, A.J.; Marin, S. Ochratoxin A in adult population of Lleida, Spain: Presence in blood plasma and consumption in different regions and seasons. Food Chem. Toxicol. 2011, 49, 2697–2705. [Google Scholar] [CrossRef]
- Medina, Á.; Mateo, E.M.; Roig, R.J.; Blanquer, A.; Jiménez, M. Ochratoxin A levels in the plasma of healthy blood donors from Valencia and estimation of exposure degree: Comparison with previous national Spanish data. Food Addit. Contam. Part A 2010, 27, 1273–1284. [Google Scholar] [CrossRef]
- Coronel, M.B.; Sanchis, V.; Ramos, A.J.; Marin, S. Assessment of the exposure to ochratoxin A in the province of Lleida, Spain. Food Chem. Toxicol. 2009, 47, 2847–2852. [Google Scholar] [CrossRef]
- Pérez de Obanos, A.; López de Cerain, A.; Jiménez, A.; González Peñas, E.; Bello, J. Ocratoxina A en plasma humano: Nuevos datos de exposición en España. Rev. Toxicol. 2001, 18, 19–23. [Google Scholar]
- Burdaspal, P.A.; Legarda, T.M. Datos sobre presencia de ocratoxina A en plasma humano en España. Alimentaria 1998, 292, 103–109. [Google Scholar]
- Soto, J.B.; Ruiz, M.-J.; Manyes, L.; Juan-García, A. Blood, breast milk and urine: Potential biomarkers of exposure and estimated daily intake of ochratoxin A: A review. Food Addit. Contam. Part A 2015, 33, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Gareis, M.; Völkel, W.; Gottschalk, C. Overall internal exposure to mycotoxins and their occurrence in occupational and residential settings – An overview. Int. J. Hyg. Environ. Health 2016, 219, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Warensjö Lemming, E.; Montano Montes, A.; Schmidt, J.; Cramer, B.; Humpf, H.-U.; Moraeus, L.; Olsen, M. Mycotoxins in blood and urine of Swedish adolescents—Possible associations to food intake and other background characteristics. Mycotoxin Res. 2020, 36, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, K.; Cheng, X.; Guo, W.; Liu, X.; Zhang, Z.; Yao, Q.; Nie, D.; Yao, B.; Han, Z. Ochratoxin A in human blood plasma samples from apparently healthy volunteers in Nanjing, China. Mycotoxin Res. 2020, 36, 269–276. [Google Scholar] [CrossRef]
- Cao, X.; Li, X.; Li, J.; Niu, Y.; Shi, L.; Fang, Z.; Zhang, T.; Ding, H. Quantitative determination of carcinogenic mycotoxins in human and animal biological matrices and animal-derived foods using multi-mycotoxin and analyte-specific high performance liquid chromatography-tandem mass spectrometric methods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1073, 191–200. [Google Scholar] [CrossRef]
- Malir, F.; Louda, M.; Ostry, V.; Toman, J.; Ali, N.; Grosse, Y.; Malirova, E.; Pacovsky, J.; Pickova, D.; Brodak, M.; et al. Analyses of biomarkers of exposure to nephrotoxic mycotoxins in a cohort of patients with renal tumours. Mycotoxin Res. 2019, 35, 391–403. [Google Scholar] [CrossRef]
- De Santis, B.; Brera, C.; Mezzelani, A.; Soricelli, S.; Ciceri, F.; Moretti, G.; Debegnach, F.; Bonaglia, M.C.; Villa, L.; Molteni, M.; et al. Role of mycotoxins in the pathobiology of autism: A first evidence. Nutr. Neurosci. 2019, 22, 132–144. [Google Scholar] [CrossRef]
- De Santis, B.; Raggi, M.E.; Moretti, G.; Facchiano, F.; Mezzelani, A.; Villa, L.; Bonfanti, A.; Campioni, A.; Rossi, S.; Camposeo, S.; et al. Study on the Association among Mycotoxins and other Variables in Children with Autism. Toxins 2017, 9, 203. [Google Scholar] [CrossRef]
- Prati, G.M.; Cicognini, F.M.; Rossi, F.; Bertuzzi, T.; Pietri, A.; Casali, M.; Stasi, M.; Stasi, B.; Fornari, F. Ochratoxin A and Liver Damage: A Case-Control Study. EC Gastroenterol. Dig. Syst. 2016, 1, 66–75. [Google Scholar]
- Sueck, F.; Cramer, B.; Czeschinski, P.; Humpf, H.U. Human Study on the Kinetics of 2′R-Ochratoxin A in the Blood of Coffee Drinkers. Mol. Nutr. Food Res. 2019, 63, 1–9. [Google Scholar] [CrossRef]
- Osteresch, B.; Cramer, B.; Humpf, H.-U. Analysis of ochratoxin A in dried blood spots – Correlation between venous and finger-prick blood, the influence of hematocrit and spotted volume. J. Chromatogr. B 2016, 1020, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Cramer, B.; Osteresch, B.; Muñoz, K.A.; Hillmann, H.; Sibrowski, W.; Humpf, H.-U. Biomonitoring using dried blood spots: Detection of ochratoxin A and its degradation product 2′R-ochratoxin A in blood from coffee drinkers*. Mol. Nutr. Food Res. 2015, 59, 1837–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viegas, S.; Osteresch, B.; Almeida, A.; Cramer, B.; Humpf, H.-U.U.; Viegas, C. Enniatin B and ochratoxin A in the blood serum of workers from the waste management setting. Mycotoxin Res. 2018, 34, 85–90. [Google Scholar] [CrossRef]
- Ali, N.; Hossain, K.; Degen, G.H. Blood plasma biomarkers of citrinin and ochratoxin A exposure in young adults in Bangladesh. Mycotoxin Res. 2018, 34, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.S.J.; El-Nezami, H. Maternal-Fetal Cancer Risk Assessment of Ochratoxin A during Pregnancy. Toxins 2016, 8, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximun levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Araguás, C.; González-Peñas, E.; López de Cerain, A. Study on ochratoxin A in cereal-derived products from Spain. Food Chem. 2005, 92, 459–464. [Google Scholar] [CrossRef]
- López de Cerain, A.; González-Peñas, E.; Jiménez, A.M.; Bello, J. Contribution to the study of ochratoxin A in Spanish wines. Food Addit. Contam. 2002, 19, 1058–1064. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; Martínez, R.; González-Peñas, E.; Lizarraga, E.; López de Cerain, A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in breakfast cereals from spanish market. Food Control 2011, 22, 1949–1955. [Google Scholar] [CrossRef]
- Araguas, C.; Bello, J.; González-Peñas, E.; López de Cerain Salsamendi, A. Acerca de la posible contaminación por ocratoxina A en los alimentos I. Cereales cultivados en diversas zonas geográficas de la Comunidad Foral de Navarra. Aliment. Rev. Tecnol. e Hig. los Aliment. 2003, 343, 23–29. [Google Scholar]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 150. [Google Scholar]
- De Ruyck, K.; Huybrechts, I.; Yang, S.; Arcella, D.; Claeys, L.; Abbeddou, S.; De Keyzer, W.; De Vries, J.; Ocke, M.; Ruprich, J.; et al. Mycotoxin exposure assessments in a multi-center European validation study by 24-hour dietary recall and biological fluid sampling. Environ. Int. 2020, 137, 105539. [Google Scholar] [CrossRef] [PubMed]
- JECFA. Safety Evaluation of Certain Food Additives and Contaminants: Sixty-Eighth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) Food; World Health Organization—Technical Report Series; World Health Organization: Geneva, Switzerland, 2008; pp. 1–472. [Google Scholar]
- EFSA Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to ochratoxina A in food. EFSA J. 2006, 365, 1–56.
- Yang, S.; Zhang, H.; De Saeger, S.; De Boevre, M.; Sun, F.; Zhang, S.; Cao, X.; Wang, Z. In vitro and in vivo metabolism of ochratoxin A: A comparative study using ultra-performance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, K.; Cramer, B.; Dopstadt, J.; Humpf, H.U.; Degen, G.H. Evidence of ochratoxin A conjugates in urine samples from infants and adults. Mycotoxin Res. 2017, 33, 39–47. [Google Scholar] [CrossRef]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans significantly metabolize and excrete the mycotoxin deoxynivalenol and its modified form deoxynivalenol-3-glucoside within 24 hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; World Health Organization: Geneva, Switzerland, 1987; Volume 46. [Google Scholar]
- Díaz Nieto, C.H.; Granero, A.M.; Zon, M.A.; Fernández, H. Sterigmatocystin: A mycotoxin to be seriously considered. Food Chem. Toxicol. 2018, 118, 460–470. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002/657/EC). Off. J. Eur. Commun. 2002, 221, 8–36. [Google Scholar]
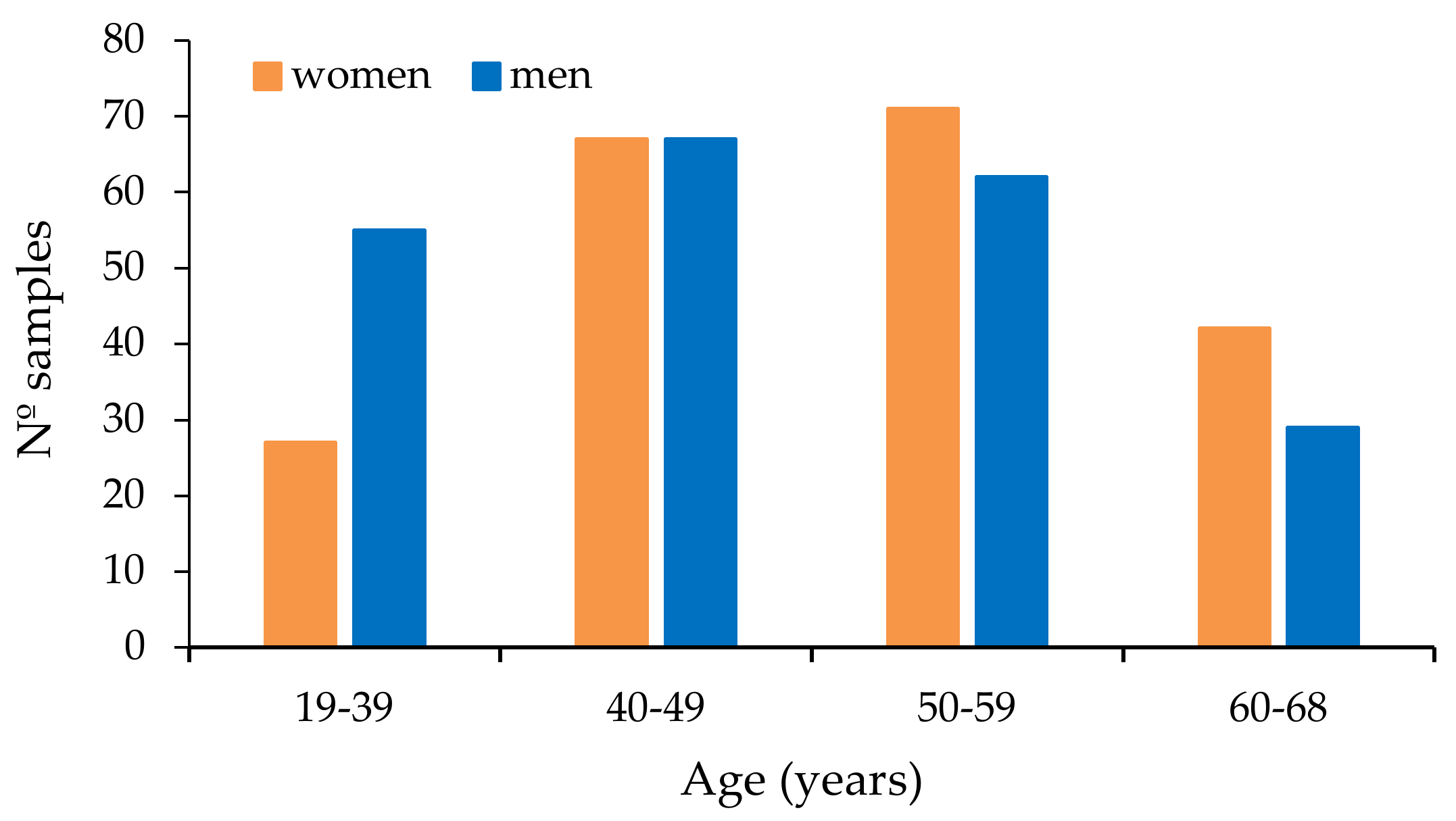



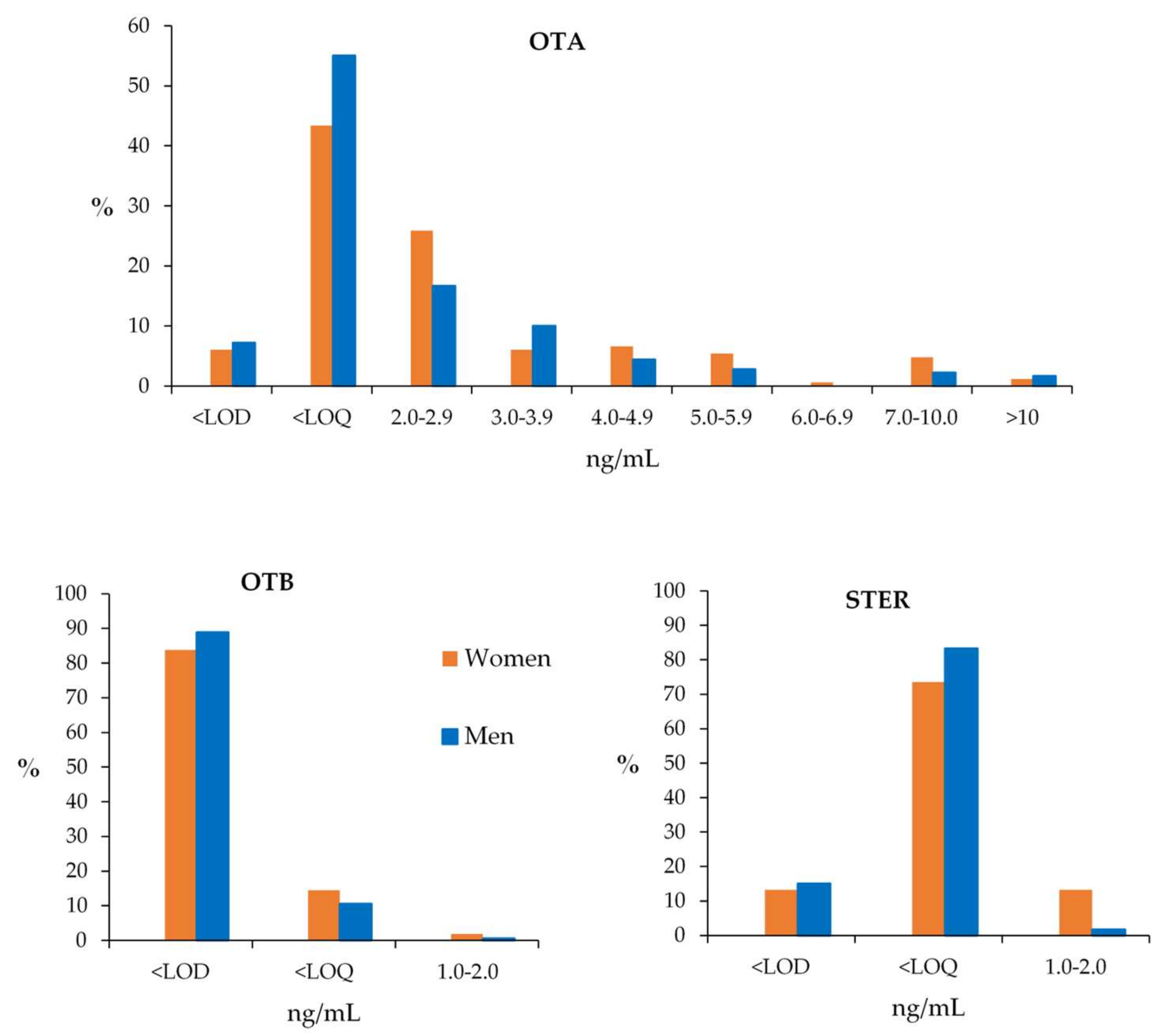

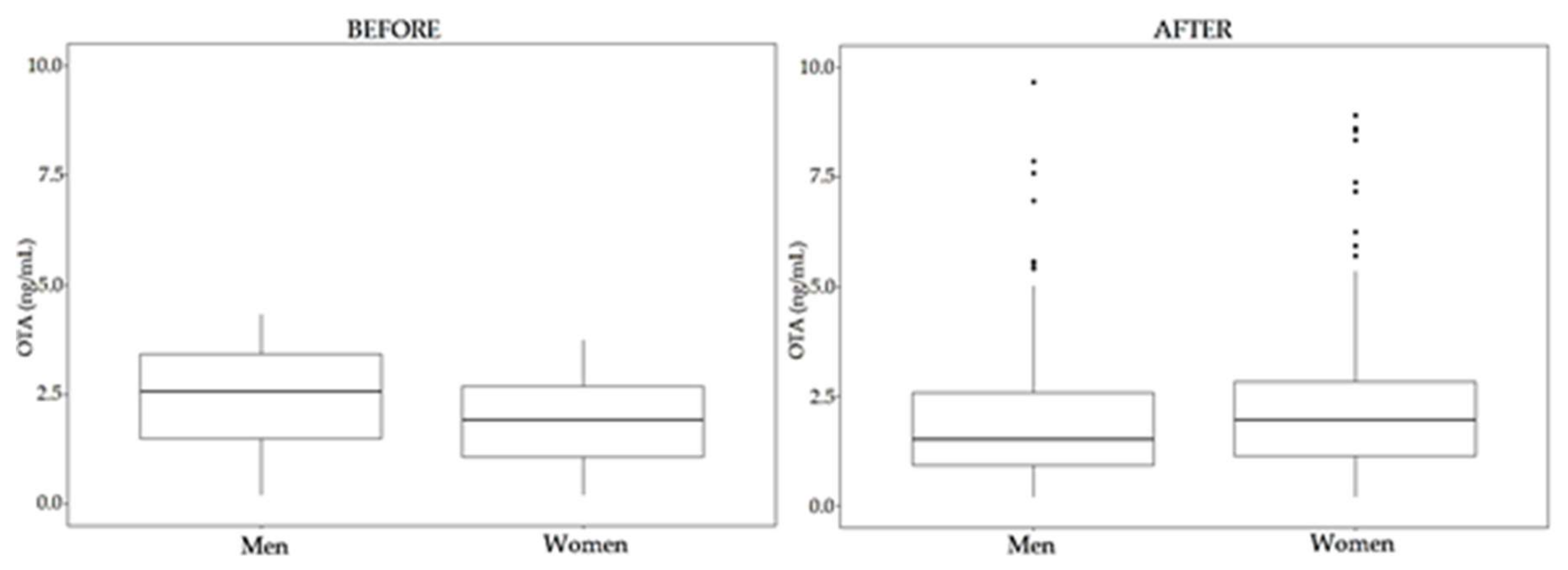
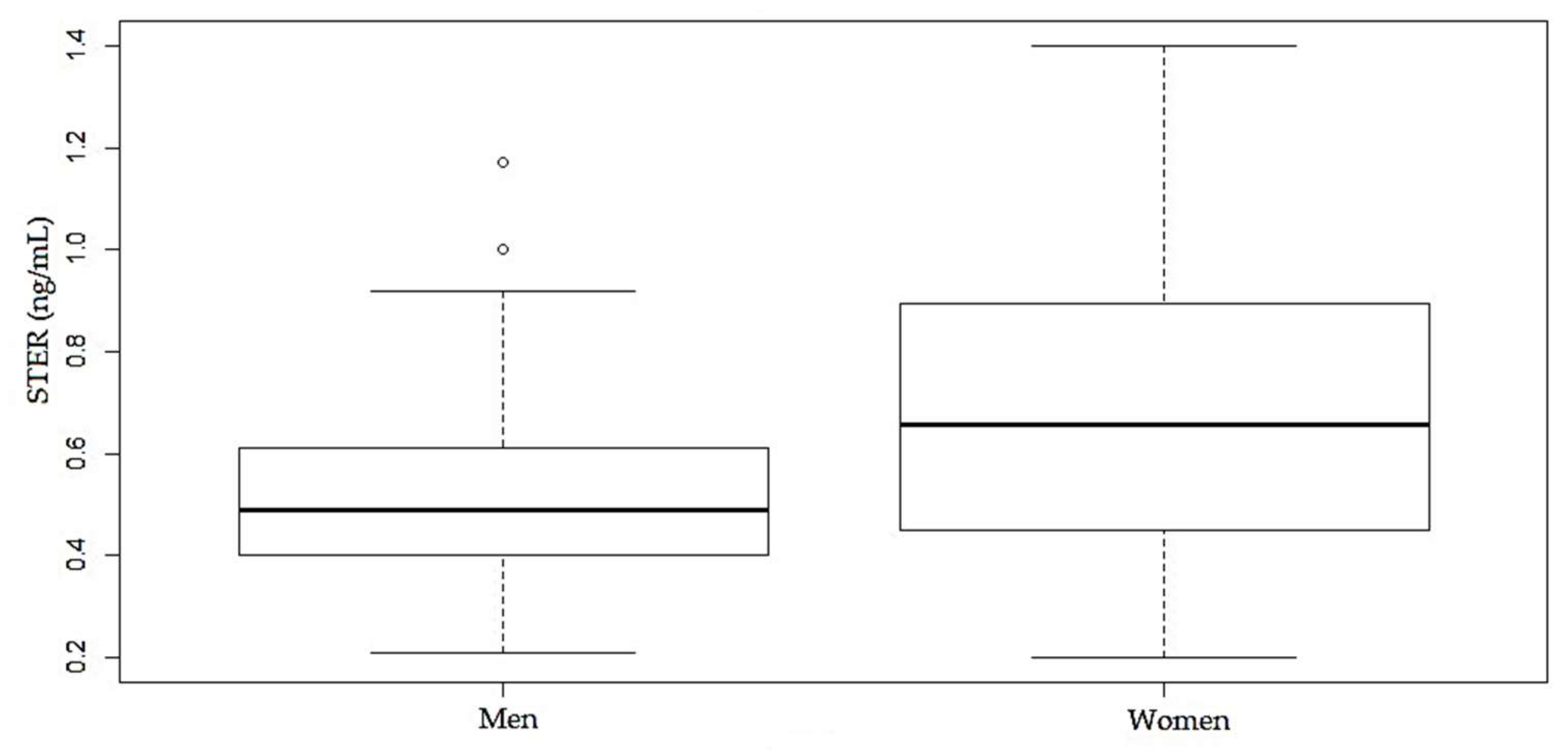
| OTA | OTB | STER | |||
|---|---|---|---|---|---|
| BE a | AE b | BE | AE | AE | |
| Calibrators | 77.2 ± 7.2 | 66.6 ± 1.9 | 43.3 ± 1.5 | 42.2 ± 0.9 | 86.6 ± 1.7 |
| Samples | 75.9 ± 10.1 | 67.3 ± 3.6 | 43.5 ± 4.7 | 42.6 ± 2.3 | 85.0 ± 4.3 |
| OTA | OTB | STER | |||
|---|---|---|---|---|---|
| BE a | AE b | BE | AE | AE | |
| Calibrators | 15.56 ± 0.28 | 15.30 ± 0.06 | 11.31 ± 0.27 | 11.13 ± 0.06 | 15.36 ± 0.06 |
| Samples | 15.64 ± 0.27 | 15.45 ± 0.06 | 11.27 ± 0.21 | 11.12 ± 0.06 | 15.38 ± 0.07 |
| Sample | OTA (ng/mL) | OTB (ng/mL) | Sample | OTA (ng/mL) | OTB (ng/mL) |
|---|---|---|---|---|---|
| 29 | 2.9 | 38 | 4.4 | ||
| 30 | 2.4 | 39 | 3.1 | ||
| 31 | 1.9a | 348 | 4.8 | 0.5 | |
| 32 | 2.0 | 406 | 4.5 | ||
| 33 | 1.3 | 407 | 5.5 | ||
| 34 | 0.7 | 408 | 4.0 | ||
| 35 | 1.5 | 409 | 10.5 | ||
| 36 | 1.2 | 410 | 3.7 | ||
| 37 | 1.5 | 411 | 4.4 |
| Sample | Age | Before Enzymatic Treatment | After Enzymatic Treatment | Sample | Age | Before Enzymatic Treatment | After Enzymatic Treatment | Sample | Age | Before Enzymatic Treatment | After Enzymatic Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OTA | OTB | OTA | OTB | STER | OTA | OTB | OTA | OTB | STER | OTA | OTB | OTA | OTB | STER | ||||||
| 40 | 43 | 3.2 | 5.3 | 1.1 | 63 | 62 | 2.6 | 5.0 | 0.6 | 0.9 | 90 | 49 | 4.9 | 5.9 | 0.7 | 0.9 | ||||
| 41 | 65 | 2.8 | 5.0 | 0.9a | 65 | 44 | 2.0 | 2.4 | 0.9 | 91 | 35 | 1.6 | n.e. | n.e. | n.e. | |||||
| 42 | 49 | 1.3 | 2.6 | 1.1 | 66 | 39 | 1.5 | 2.6 | 1.0 | 93 | 58 | 2.4 | 2.8 | 1.1 | ||||||
| 43 | 49 | 1.8 | 1.0 | 68 | 43 | 2.4 | n.e. | n.e. | n.e. | 94 | 46 | 2.6 | 2.8 | 0.9 | ||||||
| 44 | 43 | 4.7 | 7.2 | 0.9 | 69 | 50 | 4.4 | 8.6 | 0.8 | 0.9 | 95 | 51 | 1.8 | 2.2 | 0.9 | |||||
| 45 | 63 | 0.6 | 2.3 | 0.9 | 70 | 58 | 1.4 | 1.8 | 0.8 | 96 | 68 | 1.6 | 2.3 | 0.9 | ||||||
| 47 | 66 | 45.7 | 1.7 | n.e. | n.e. | n.e. | 73 | 41 | 1.9 | 2.0 | 0.9 | 97 | 58 | 2.4 | 2.2 | 1.0 | ||||
| 48 | 50 | 1.3 | 2.7 | 0.9 | 74 | 60 | 2.1 | 3.7 | 0.9 | 98 | 42 | 2.6 | 4.7 | 0.9 | ||||||
| 49 | 60 | 0.7 | 2.4 | 0.9 | 75 | 41 | 3.1 | 5.3 | 0.7 | 0.9 | 99 | 49 | 1.5 | 1.8 | 0.8 | |||||
| 50 | 50 | 2.0 | 2.1 | 0.9 | 76 | 53 | 1.6 | n.e. | n.e. | n.e. | 101 | 66 | 4.8 | n.e. | n.e. | n.e. | ||||
| 51 | 39 | 0.5 | 2.2 | 0.9 | 79 | 44 | 0.7 | 2.4 | 0.8 | 102 | 46 | 2.2 | 2.5 | 0.8 | ||||||
| 52 | 46 | 1.3 | 2.4 | 0.8 | 80 | 39 | 2.5 | 3.0 | 1.0 | 103 | 52 | 5.4 | 8.6 | 0.8 | 0.8 | |||||
| 53 | 56 | 1.0 | 4.5 | 1.4 | 81 | 37 | 3.6 | 4.1 | 0.6 | 0.7 | 104 | 45 | 2.4 | 2.9 | 0.9 | |||||
| 54 | 55 | 2.6 | 4.3 | 0.8 | 82 | 62 | 1.9 | 2.3 | 0.8 | 107 | 52 | 2.1 | n.e. | n.e. | n.e. | |||||
| 55 | 47 | 1.2 | 3.4 | 1.1 | 83 | 30 | 2.0 | 2.7 | 0.7 | 108 | 53 | 1.8 | 3.7 | 0.5 | 0.8 | |||||
| 56 | 32 | 2.5 | 4.6 | 1.0 | 85 | 51 | 2.8 | 2.8 | 0.9 | 110 | 48 | 6.6 | 0.5 | 8.9 | 0.6 | 0.9 | ||||
| 58 | 55 | 1.0 | n.e. | n.e. | n.e. | 86 | 52 | 2.6 | 4.0 | 1.0 | 111 | 56 | 3.5 | 0.4 | 2.3 | 0.9 | ||||
| 59 | 47 | 1.4 | 2.6 | 1.0 | 87 | 57 | 3.8 | 4.1 | 0.9 | 112 | 46 | 4.5 | 4.1 | 0.9 | ||||||
| 60 | 32 | 0.6 | 2.6 | 1.0 | 88 | 67 | 3.5 | 4.7 | 1.0 | 114 | 60 | 3.6 | 0.4 | 2.3 | 1.0 | |||||
| 62 | 46 | 2.8 | 0.8 | 89 | 42 | 2.3 | 2.7 | 0.9 | 115 | 51 | 3.8 | 0.4 | 2.3 | 0.5 | 1.0 | |||||
| 116 | 53 | 3.6 | 0.4 | 3.9 | 0.5 | 0.7 | 201 | 48 | 2.5 | 0.9 | n.e. | n.e. | n.e. | 228 | 61 | 3.4 | 2.0 | 0.3 | ||
| 117 | 48 | 3.3 | 2.1 | 0.6 | 1.4 | 202 | 63 | 2.0 | 1.0 | n.e. | n.e. | n.e. | 229 | 55 | 1.5 | 1.2 | 0.4 | |||
| 118 | 37 | 5.9 | 0.6 | 6.2 | 0.9 | 203 | 55 | 2.6 | 0.7 | n.e. | n.e. | n.e. | 230 | 40 | 0.9 | 0.9 | 0.5 | |||
| 119 | 61 | 8.3 | 0.7 | 8.6 | 0.6 | 1.0 | 204 | 45 | 2.3 | 0.7 | n.e. | n.e. | n.e. | 231 | 64 | 0.9 | 0.5 | 0.3 | ||
| 120 | 53 | 2.8 | n.e. | n.e. | n.e. | 205 | 52 | 1.9 | n.e. | n.e. | n.e. | 232 | 56 | 14.7 | 0.7 | 13.4 | 1.1 | |||
| 121 | 50 | 5.3 | 5.9 | 0.5 | 0.9 | 206 | 39 | 3.3 | 0.7 | n.e. | n.e. | n.e. | 233 | 49 | 2.0 | 2.0 | 0.3 | |||
| 122 | 45 | 2.9 | 1.9 | 0.9 | 207 | 64 | 3.0 | n.e. | n.e. | n.e. | 234 | 51 | 1.3 | 0.9 | 0.3 | |||||
| 123 | 45 | 3.3 | 1.9 | 0.8 | 208 | 42 | 2.2 | n.e. | n.e. | n.e. | 235 | 61 | 1.1 | 0.9 | 0.5 | |||||
| 124 | 44 | 2.9 | 1.9 | 0.9 | 210 | 61 | 2.5 | n.e. | n.e. | n.e. | 236 | 48 | 1.1 | 1.2 | ||||||
| 125 | 47 | 3.1 | 1.9 | 0.8 | 211 | 54 | 0.8 | 0.7 | 0.5 | 237 | 56 | 1.4 | 1.1 | 1.1 | 1.3 | |||||
| 126 | 49 | 5.1 | 0.5 | 7.4 | 0.7 | 1.0 | 212 | 68 | 2.0 | 0.9 | 0.2 | 238 | 57 | 1.5 | 0.5 | 1.7 | 0.6 | |||
| 127 | 66 | 2.8 | 2.0 | 0.7 | 213 | 62 | 11.4 | 0.7 | 17.3 | 1.3 | 0.2 | 239 | 52 | 1.4 | 1.3 | 0.6 | ||||
| 128 | 41 | 3.7 | 2.9 | 0.9 | 214 | 53 | 2.1 | 1.6 | 0.4 | 240 | 59 | 10.1 | 0.7 | n.e. | n.e. | n.e. | ||||
| 129 | 49 | 3.6 | 2.5 | 0.9 | 215 | 39 | 1.6 | n.e. | n.e. | n.e. | 241 | 58 | 1.6 | 1.6 | 0.4 | |||||
| 195 | 58 | 3.5 | n.e. | n.e. | n.e. | 216 | 51 | 2.7 | 1.8 | 0.4 | 242 | 56 | 1.0 | 1.6 | 0.6 | |||||
| 196 | 64 | 3.8 | 0.8a | n.e. | n.e. | n.e. | 217 | 49 | 1.3 | 1.6 | 0.4 | 243 | 37 | 1.4 | 1.6 | 0.7 | ||||
| 197 | 55 | 2.8 | 0.8 | n.e. | n.e. | n.e. | 218 | 58 | 1.8 | n.e. | n.e. | n.e. | 245 | 57 | 3.5 | 2.8 | 0.4 | |||
| 198 | 54 | 2.9 | 0.8 | n.e. | n.e. | n.e. | 225 | 42 | 1.2 | 0.8 | 0.3 | 247 | 58 | 0.9 | 1.1 | 0.7 | 0.7 | |||
| 199 | 66 | 4.6 | 0.9 | n.e. | n.e. | n.e. | 226 | 61 | 0.8 | 0.9 | 0.4 | 258 | 60 | 0.8 | 0.7 | 0.4 | ||||
| 200 | 57 | 3.9 | 0.9 | n.e. | n.e. | n.e. | 227 | 63 | 1.3 | 1.4 | 0.5 | 264 | 54 | 0.7 | ||||||
| 265 | 47 | 0.8 | 1.2 | 0.4 | 299 | 37 | 1.1 | 1.8 | 0.4 | 327 | 57 | 7.4 | 0.5 | 8.6 | 0.5 | |||||
| 270 | 60 | 0.5 | 0.7 | 0.4 | 300 | 47 | 3.1 | n.e. | n.e. | n.e. | 328 | 35 | 2.4 | 3.3 | 0.5 | |||||
| 276 | 43 | 0.9 | n.e. | n.e. | n.e. | 301 | 32 | 0.6 | 1.0 | 0.5 | 335 | 62 | 1.6 | n.e. | n.e. | n.e. | ||||
| 277 | 50 | 0.8 | 0.7 | 303 | 49 | 0.8 | 1.1 | 0.4 | 336 | 65 | 1.9 | 2.2 | 0.6 | |||||||
| 278 | 43 | 0.4 | 1.5 | 0.5 | 304 | 63 | 0.8 | 0.5 | 337 | 61 | 0.5 | 1.0 | 0.6 | |||||||
| 279 | 51 | 2.5 | 5.4 | 305 | 57 | 0.5 | 0.7 | 0.3 | 347 | 50 | 3.5 | 2.0 | 0.7 | |||||||
| 280 | 60 | 0.9 | 0.6 | 306 | 54 | 2.0 | 3.1 | 0.4 | 352 | 50 | 6.9 | 2.7 | ||||||||
| 281 | 51 | 0.5 | 0.3 | 307 | 56 | 0.7 | 1.8 | 353 | 53 | 3.1 | 0.5 | |||||||||
| 282 | 40 | 1.7 | 1.7 | 0.3 | 308 | 30 | 1.1 | 1.5 | 0.6 | 364 | 47 | 3.2 | 1.6 | |||||||
| 283 | 42 | 309 | 56 | 2.6 | 2.3 | 0.5 | 369 | 46 | 4.3 | 0.5 | ||||||||||
| 284 | 66 | 0.6 | 0.6 | 0.3 | 310 | 65 | 2.4 | 3.9 | 378 | 55 | 4.7 | 0.7 | 0.4 | |||||||
| 285 | 40 | 0.8 | 3.0 | 0.7 | 315 | 62 | 0.5 | 0.9 | 0.4 | 379 | 35 | 5.1 | 1.8 | 0.5 | ||||||
| 286 | 57 | 4.0 | 5.4 | 0.6 | 317 | 49 | 1.7 | 1.9 | 0.5 | 380 | 60 | 4.2 | n.e. | n.e. | n.e. | |||||
| 287 | 40 | 0.4 | 0.3 | 318 | 58 | 0.8 | 0.7 | 0.4 | 381 | 48 | 4.0 | 0.5 | ||||||||
| 290 | 34 | 1.2 | 1.2 | 0.7 | 320 | 67 | 15.6 | 0.6 | 8.4 | 382 | 48 | 5.5 | 2.2 | 0.6 | ||||||
| 291 | 63 | 2.6 | 4.5 | 0.5 | 322 | 40 | 2.6 | 1.5 | 0.4 | 383 | 50 | 4.1 | 0.5 | |||||||
| 293 | 50 | 3.0 | 4.0 | 323 | 46 | 1.8 | 2.1 | 0.5 | 384 | 54 | 9.4 | 5.7 | 0.9 | 0.7 | ||||||
| 294 | 38 | 1.7 | 1.6 | 0.6 | 324 | 33 | 1.9 | 1.5 | 0.4 | 385 | 21 | 4.6 | 0.5 | |||||||
| 296 | 64 | 2.7 | 0.7 | 325 | 52 | 0.8 | 1.1 | 0.6 | 386 | 37 | 3.9 | 0.5 | ||||||||
| 297 | 59 | 1.8 | 2.8 | 0.9 | 326 | 42 | 0.7 | 0.9 | 0.4 | 397 | 54 | 3.7 | 1.8 | 0.6 | ||||||
| 399 | 49 | 4.3 | 0.8 | 0.5 | 447 | 46 | 2.6 | 0.7 | 475 | 52 | 1.9 | n.e. | n.e. | n.e. | ||||||
| 400 | 45 | 5.0 | n.e. | n.e. | n.e. | 448 | 55 | 2.4 | 1.7 | 0.8 | 476 | 38 | 0.9 | 0.4 | 0.4 | |||||
| 401 | 61 | 3.7 | 0.4 | 449 | 52 | 4.3 | 3.2 | 1.0 | 483 | 42 | 1.6 | 1.7 | 0.4 | |||||||
| 402 | 66 | 3.7 | 0.6 | 0.5 | 452 | 41 | 2.8 | n.e. | n.e. | n.e. | 484 | 47 | 2.3 | n.e. | n.e. | n.e. | ||||
| 404 | 39 | 4.5 | 2.6 | 1.0 | 453 | 40 | 2.9 | n.e. | n.e. | n.e. | 495 | 59 | 3.7 | n.e. | n.e. | n.e. | ||||
| 405 | 41 | 4.1 | 1.9 | 0.8 | 454 | 39 | 2.7 | n.e. | n.e. | n.e. | 496 | 47 | 2.3 | n.e. | n.e. | n.e. | ||||
| 421 | 57 | 4.3 | 0.7 | 455 | 37 | 6.4 | n.e. | n.e. | n.e. | 518 | 31 | 2.3 | n.e. | n.e. | n.e. | |||||
| 422 | 48 | 3.0 | n.e. | n.e. | n.e. | 468 | 41 | 2.7 | 1.1 | 0.3 | 519 | 67 | 2.3 | 0.8 | 0.4 | |||||
| 446 | 43 | 2.9 | n.e. | n.e. | n.e. | 474 | 52 | 1.9 | 1.6 | 522 | 58 | 3.5 | n.e. | n.e. | n.e. | |||||
| Sample | Age | Before Enzymatic Treatment | After Enzymatic Treatment | Sample | Age | Before Enzymatic Treatment | After Enzymatic Treatment | Sample | Age | Before Enzymatic Treatment | After Enzymatic Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OTA | OTB | OTA | OTB | STER | OTA | OTB | OTA | OTB | STER | OTA | OTB | OTA | OTB | STER | ||||||
| 46 | 48 | 1.7a | n.e. | n.e. | n.e. | 249 | 65 | 1.5 | 1.1 | 0.3 | 273 | 57 | 1.3 | 1.8 | 0.4 | |||||
| 57 | 56 | 1.4 | n.e. | n.e. | n.e. | 250 | 48 | 1.4 | n.e. | n.e. | n.e. | 274 | 32 | 0.4 | 0.4 | 0.4 | ||||
| 61 | 32 | 2.7 | 0.9 | 251 | 59 | 0.9 | 1.1 | 275 | 49 | 1.9 | 1.6 | 0.4 | ||||||||
| 64 | 39 | 2.8 | 5.0 | 0.8 | 0.8 | 252 | 55 | 0.7 | 288 | 53 | 1.2 | n.e. | n.e. | n.e. | ||||||
| 67 | 67 | 0.7 | n.e. | n.e. | n.e. | 253 | 57 | 1.7 | 1.7 | 0.4 | 289 | 53 | 0.9 | 2.4 | ||||||
| 71 | 57 | 4.6 | 3.6 | 0.5 | 0.7 | 254 | 52 | 0.8 | 292 | 54 | 0.6 | 0.9 | 0.4 | |||||||
| 72 | 51 | 3.0 | 4.9 | 0.8 | 1.2 | 255 | 51 | 4.5 | 0.4 | 4.8 | 0.6 | 295 | 65 | 0.8 | 0.5 | |||||
| 77 | 48 | 2.2 | n.e. | n.e. | n.e. | 256 | 48 | 0.8 | 0.5 | 0.3 | 298 | 19 | 4.3 | 4.6 | ||||||
| 78 | 53 | 5.0 | 7.9 | 0.6 | 0.9 | 257 | 46 | 1.1 | 2.1 | 0.7 | 0.5 | 302 | 47 | 0.8 | 2.2 | |||||
| 84 | 35 | 1.7 | 3.8 | 0.6 | 0.9 | 259 | 47 | 1.2 | 1.2 | 0.4 | 311 | 46 | 1.9 | 2.0 | 0.4 | |||||
| 92 | 34 | 2.2 | n.e. | n.e. | n.e. | 260 | 53 | 4.7 | 5.6 | 312 | 42 | 0.9 | 1.1 | 0.6 | ||||||
| 100 | 55 | 2.7 | n.e. | n.e. | n.e. | 261 | 45 | 2.1 | 2.0 | 0.5 | 313 | 48 | 5.6 | 5.4 | ||||||
| 105 | 51 | 1.9 | n.e. | n.e. | n.e. | 262 | 60 | 0.5 | 0.8 | 0.5 | 314 | 56 | 1.0 | 1.1 | 0.4 | |||||
| 106 | 56 | 1.8 | 2.2 | 0.8 | 263 | 63 | 1.5 | 2.7 | 0.8 | 0.6 | 316 | 68 | 1.0 | 1.4 | 0.4 | |||||
| 109 | 48 | 1.6 | 2.5 | 0.8 | 266 | 41 | 1.6 | 7.0 | 0.9 | 0.6 | 319 | 41 | 1.1 | 1.3 | 0.4 | |||||
| 113 | 52 | 3.4 | n.e. | n.e. | n.e. | 267 | 27 | 0.6 | 2.6 | 0.4 | 321 | 63 | 1.3 | 1.7 | 0.6 | |||||
| 209 | 46 | 2.6 | n.e. | n.e. | n.e. | 268 | 47 | 1.7 | 7.6 | 329 | 39 | 0.9 | 2.2 | |||||||
| 244 | 45 | 2.0 | 1.4 | 0.3 | 269 | 34 | 1.0 | 1.3 | 0.3 | 330 | 57 | 3.0 | 3.1 | 0.4 | ||||||
| 246 | 52 | 1.3 | 1.5 | 271 | 51 | 0.7 | 1.1 | 0.3 | 331 | 42 | 1.0 | 1.4 | 0.5 | |||||||
| 248 | 58 | 5.7 | 9.7 | 0.5 | 0.3 | 272 | 53 | 0.7 | 332 | 61 | 17.7 | 0.5 | 20.5 | 0.6 | ||||||
| 333 | 44 | 1.4 | n.e. | n.e. | n.e. | 363 | 56 | 3.5 | 1.3 | 0.6 | 418 | 45 | 5.4 | 3.6 | 0.9 | |||||
| 334 | 40 | 1.0 | 1.4 | 0.4 | 365 | 55 | 3.2 | 1.6 | 0.7 | 419 | 35 | 4.2 | 1.4 | 0.5 | ||||||
| 338 | 65 | 1.1 | 1.1 | 0.5 | 366 | 57 | 3.9 | 1.3 | 0.7 | 420 | 65 | 7.1 | 1.3 | 0.6 | ||||||
| 340 | 61 | 3.4 | 0.5 | 0.7 | 0.4 | 367 | 50 | 4.1 | 1.6 | 0.6 | 423 | 66 | 3.4 | 0.8 | 0.5 | |||||
| 341 | 38 | 3.5 | 0.5 | 0.5 | 0.5 | 368 | 47 | 3.9 | 0.7 | 0.8 | 424 | 62 | 4.1 | 0.9 | 0.6 | |||||
| 344 | 48 | 3.2 | 0.5 | 387 | 31 | 4.3 | 1.1 | 0.6 | 425 | 51 | 2.6 | n.e. | n.e. | n.e. | ||||||
| 345 | 60 | 3.8 | 1.4 | 0.5 | 389 | 54 | 4.1 | 1.2 | 0.7 | 426 | 34 | 2.7 | 0.5 | 0.6 | ||||||
| 346 | 47 | 4.0 | 3.0 | 1.0 | 390 | 35 | 4.4 | n.e. | n.e. | n.e. | 427 | 38 | 2.6 | 0.4 | 0.6 | |||||
| 349 | 53 | 3.6 | 0.5 | 0.5 | 391 | 40 | 4.1 | 1.3 | 0.6 | 428 | 53 | 3.1 | 0.9 | 0.7 | ||||||
| 350 | 36 | 4.0 | 0.5 | 1.4 | 0.4 | 393 | 28 | 4.2 | 1.6 | 0.7 | 429 | 47 | 2.7 | 2.0 | 0.6 | |||||
| 351 | 43 | 4.2 | 1.6 | 0.5 | 394 | 39 | 5.3 | 3.9 | 0.4 | 0.8 | 430 | 37 | 5.7 | 3.3 | 0.5 | |||||
| 354 | 48 | 3.0 | 1.9 | 0.2 | 395 | 29 | 4.2 | 1.6 | 0.6 | 431 | 45 | 2.7 | 0.6 | |||||||
| 355 | 45 | 3.0 | 0.4 | 396 | 19 | 5.7 | 3.5 | 0.8 | 432 | 31 | 2.8 | 0.6 | ||||||||
| 356 | 53 | 3.9 | 1.1 | 0.5 | 398 | 58 | 3.8 | 0.9 | 0.6 | 433 | 63 | 3.1 | 1.1 | 0.7 | ||||||
| 357 | 47 | 3.3 | 0.4 | 412 | 52 | 4.3 | 1.9 | 0.6 | 434 | 27 | 2.2 | 0.6 | ||||||||
| 358 | 49 | 5.7 | 4.0 | 0.7 | 413 | 50 | 6.2 | 5.4 | 0.7 | 435 | 34 | 2.7 | 2.7 | 0.8 | ||||||
| 359 | 63 | 3.4 | 0.5a | 414 | 38 | 4.5 | 1.5 | 0.6 | 436 | 37 | 3.9 | 1.4 | 0.6 | |||||||
| 360 | 37 | 4.0 | n.e. | n.e. | n.e. | 415 | 65 | 4.1 | 2.7 | 1.0 | 437 | 29 | 4.0 | 2.3 | 0.6 | |||||
| 361 | 51 | 3.2 | 0.4 | 416 | 47 | 4.6 | 1.5 | 0.5 | 438 | 47 | 2.5 | 2.6 | 0.6 | |||||||
| 362 | 39 | 3.8 | 1.0 | 0.5 | 417 | 50 | 3.5 | 0.8 | 0.6 | 439 | 59 | 2.8 | 0.4 | 0.6 | ||||||
| 440 | 23 | 2.5 | 0.6 | 469 | 40 | 3.5 | 2.4 | 0.6 | 494 | 43 | 2.9 | 2.2 | 0.5 | |||||||
| 441 | 35 | 3.4 | 0.7 | 0.6 | 470 | 28 | 2.4 | 0.8 | n.e. | n.e. | n.e. | 497 | 32 | 4.4 | 0.5 | 3.8 | 0.5 | |||
| 442 | 40 | 3.7 | 1.8 | 0.5 | 471 | 40 | 1.6 | 0.6 | 2.0 | 0.5 | 498 | 38 | 3.1 | 1.8 | ||||||
| 443 | 49 | 2.7 | 2.7 | 0.6 | 472 | 59 | 1.2 | 0.9 | 0.6 | 499 | 42 | 1.9 | 0.4 | 0.3 | ||||||
| 444 | 67 | 2.1 | 0.5 | 473 | 46 | 1.8 | 0.7 | 500 | 66 | 2.1 | n.e. | n.e. | n.e. | |||||||
| 445 | 66 | 2.2 | n.e. | n.e. | n.e. | 477 | 57 | 0.7 | 0.8 | 501 | 39 | 2.3 | 1.4 | 0.5 | ||||||
| 450 | 27 | 4.3 | n.e. | n.e. | n.e. | 478 | 41 | 0.8 | 0.8 | 0.5 | 502 | 36 | 3.5 | 1.8 | 0.3 | |||||
| 451 | 48 | 2.8 | n.e. | n.e. | n.e. | 479 | 55 | 3.1 | 3.3 | 0.7 | 503 | 65 | 2.3 | 1.0 | 0.4 | |||||
| 456 | 28 | 3.4 | n.e. | n.e. | n.e. | 480 | 50 | 1.7 | 2.2 | 0.3 | 504 | 39 | 2.1 | n.e. | n.e. | n.e. | ||||
| 457 | 24 | 3.7 | n.e. | n.e. | n.e. | 481 | 22 | 1.5 | 1.1 | 1.8 | 0.6 | 505 | 48 | 3.6 | 3.0 | 0.4 | ||||
| 458 | 48 | 2.7 | n.e. | n.e. | n.e. | 482 | 61 | 1.2 | 0.6 | 1.4 | 0.6 | 0.7 | 506 | 54 | 2.5 | 1.7 | 0.5 | |||
| 459 | 37 | 5.1 | n.e. | n.e. | n.e. | 485 | 33 | 2.6 | 3.8 | 0.7 | 0.2 | 507 | 50 | 2.5 | 1.4 | 0.4 | ||||
| 460 | 46 | 2.3 | n.e. | n.e. | n.e. | 486 | 39 | 1.2 | 0.7 | 0.2 | 508 | 46 | 5.5 | 4.4 | ||||||
| 461 | 44 | 2.9 | n.e. | n.e. | n.e. | 487 | 50 | 1.5 | 0.6 | 0.3 | 509 | 53 | 2.9 | 1.5 | 0.4 | |||||
| 462 | 29 | 2.6 | n.e. | n.e. | n.e. | 488 | 19 | 1.2 | 0.8 | 0.7 | 510 | 38 | 2.2 | n.e. | n.e. | n.e. | ||||
| 463 | 56 | 5.2 | 3.4 | 0.6 | 489 | 21 | 1.2 | 0.7 | 0.4 | 511 | 25 | 2.9 | 2.2 | 0.4 | ||||||
| 464 | 49 | 3.5 | 2.3 | 0.6 | 490 | 54 | 1.3 | 0.5 | 0.3 | 512 | 56 | 19.9 | 0.9 | 23.3 | 1.1 | |||||
| 465 | 58 | 3.0 | 1.4 | 0.6 | 491 | 42 | 2.7 | n.e. | n.e. | n.e. | 513 | 61 | 2.2 | 1.3 | 0.4 | |||||
| 466 | 65 | 4.9 | 4.1 | 0.6 | 492 | 51 | 1.2 | 0.7 | 0.3 | 514 | 63 | 3.6 | 2.6 | 0.5 | ||||||
| 467 | 50 | 4.2 | 5.5 | 0.6 | 493 | 44 | 7.5 | 10.6 | 0.8 | 0.5 | 515 | 35 | 2.7 | 3.8 | ||||||
| 516 | 46 | 3.5 | 2.7 | 0.6 | 530 | 56 | 5.8 | 0.5 | n.e. | n.e. | n.e. | 541 | 47 | 4.2 | 0.5 | 2.9 | 0.5 | |||
| 517 | 44 | 8.2 | n.e. | n.e. | n.e. | 531 | 46 | 2.5 | 0.9 | 0.3 | 542 | 52 | 2.4 | 0.9 | 0.4 | |||||
| 520 | 57 | 2.7 | 2.3 | 532 | 39 | 3.8 | 2.0 | 0.4 | 543 | 63 | 2.8 | 1.5 | 0.4 | |||||||
| 521 | 36 | 3.3 | 1.9 | 0.3 | 533 | 24 | 2.5 | 1.0 | 0.4 | 544 | 50 | 4.4 | 3.8 | 0.6 | ||||||
| 523 | 64 | 2.4 | 1.1 | 0.3 | 534 | 44 | 3.9 | 0.5 | 1.9 | 0.3 | 545 | 62 | 2.1 | 1.2 | ||||||
| 524 | 66 | 2.7 | 2.4 | 0.6 | 535 | 45 | 2.6 | n.e. | n.e. | n.e. | 546 | 23 | 1.9 | 0.5 | 0.3 | |||||
| 525 | 45 | 5.8 | 0.7 | 3.1 | 0.6 | 536 | 50 | 3.8 | 1.4 | 0.3 | 547 | 48 | 2.5 | 1.2 | 0.4 | |||||
| 526 | 52 | 6.0 | 3.3 | 0.4 | 537 | 46 | 2.9 | 1.6 | 0.6 | 548 | 46 | 4.0 | 2.4 | 0.4 | ||||||
| 527 | 47 | 6.2 | 4.5 | 0.6 | 538 | 50 | 3.2 | 1.1 | 0.3 | 549 | 39 | 2.2 | 1.1 | 0.2 | ||||||
| 528 | 40 | 2.8 | 0.5 | 1.8 | 0.3 | 539 | 49 | 5.5 | 0.6 | 3.7 | 0.4 | 550 | 42 | 1.9 | 0.9 | 0.4 | ||||
| 529 | 57 | 2.8 | 1.5 | 0.4 | 540 | 58 | 4.1 | 4.0 | 0.4 | 551 | 40 | 5.6 | 0.6 | 0.9 | ||||||
| Age (Years) | Gender | n | % Positives | Mean ± SD | Median | Max Value | p-Value (Age Groups) |
|---|---|---|---|---|---|---|---|
| 19–39 | Women | 27 | 100.0 | 2.49 ± 1.64 | 2.00 | 6.4 | |
| Men | 55 | 98.2 | 2.99 ± 1.36 | 2.82 | 5.7 | ||
| Total | 82 | 98.8 | 2.82 ± 1.46 | 2.64 | 6.4 | ||
| 40–59 | Women | 138 | 94.9 | 2.69 ± 1.97 | 2.42 | 14.7 | |
| Men | 129 | 100.0 | 3.08 ± 2.17 | 2.84 | 19.9 | ||
| Total | 267 | 97.4 | 2.88 ± 2.08 | 2.64 | 19.9 | ||
| 60–68 | Women | 42 | 92.9 | 2.77 ± 2.98 | 2.09 | 45.7 | |
| Men | 29 | 96.6 | 3.05 ± 3.17 | 2.34 | 17.7 | ||
| Total | 71 | 94.4 | 2.89 ± 3.04 | 2.28 | 45.7 | ||
| 19–68 | Women | 207 | 95.2 | 2.68 ± 2.16 | 2.36 | 45.7 | 0.581 b |
| Men | 213 | 99.0 | 3.05 ± 2.15 | 2.81 | 19.9 | 0.403 | |
| n.i. a | 18 | 100 | 3.35 ± 2.31 | 3.02 | 10.5 | - | |
| Total | 438 | 97.3 | 2.87 ± 2.16 | 2.60 | 45.7 | 0.208 b |
| Age (Years) | Gender | n | % Positives | Mean ± SD | Median | Max Value | p-Value (Age Groups) |
|---|---|---|---|---|---|---|---|
| 19–39 | Women | 27 | 7.4 | 0.64 ± 0.11 | 0.64 | 0.7 | |
| Men | 55 | 9.1 | 0.58 ± 0.29 | 0.50 | 1.1 | ||
| Total | 82 | 8.5 | 0.60 ± 0.25 | 0.54 | 1.1 | ||
| 40–59 | Women | 138 | 10.9 | 0.45 ± 0.25 | 0.44 | 0.9 | |
| Men | 129 | 7.8 | 0.43 ± 0.22 | 0.46 | 0.9 | ||
| Total | 267 | 9.4 | 0.45 ± 0.23 | 0.45 | 0.9 | ||
| 60–68 | Women | 42 | 19.0 | 0.71 ± 0.43 | 0.69 | 1.7 | |
| Men | 29 | 10.3 | 0.53 ± 0.07 | 0.53 | 0.6 | ||
| Total | 71 | 15.5 | 0.67 ± 0.38 | 0.60 | 1.7 | ||
| 19–68 | Women | 207 | 12.1 | 0.53 ± 0.32 | 0.50 | 1.7 | 0.163 |
| Men | 213 | 8.5 | 0.48 ± 0.23 | 0.48 | 1.1 | 0.467 | |
| n.i. a | 18 | 5.6 | 0.49 | - | 0.5 | - | |
| Total | 438 | 10.0 | 0.51 ± 0.29 | 0.49 | 1.7 | 0.051 |
| Age (Years) | Gender | n | % Positives | Mean ± SD | Median | Max Value | p-Value (Age Groups) |
|---|---|---|---|---|---|---|---|
| 19–39 | Women | 21 | 90.5 | 2.22 ± 1.48 | 1.76 | 6.20 | |
| Men | 44 | 93.2 | 1.84 ± 1.28 | 1.53 | 4.60 | ||
| Total | 65 | 92.3 | 1.96 ± 1.35 | 1.60 | 6.20 | ||
| 40–59 | Women | 112 | 93.8 | 2.60 ± 2.17 | 1.98 | 13.4 | |
| Men | 110 | 92.7 | 2.40 ± 2.77 | 1.62 | 23.3 | ||
| Total | 222 | 93.2 | 2.50 ± 2.48 | 1.86 | 23.3 | ||
| 60–68 | Women | 33 | 97.0 | 2.78 ± 3.35 | 2.01 | 17.3 | |
| Men | 26 | 92.3 | 2.15 ± 3.84 | 1.23 | 20.5 | ||
| Total | 59 | 94.9 | 2.50 ± 3.56 | 1.36 | 20.5 | ||
| 19–68 | Women | 166 | 94.0 | 2.58 ± 2.37 | 1.99 | 17.3 | 0.651 |
| Men | 180 | 93.3 | 2.23 ± 2.68 | 1.53 | 23.3 | 0.220 | |
| Total | 346 | 93.6 | 2.40 ± 2.54 | 1.75 | 23.3 | 0.143 |
| Age (Years) | Gender | n | % Positives | Mean ± SD | Median | Max Value | p-Value (Age Groups) |
|---|---|---|---|---|---|---|---|
| 19–39 | Women | 21 | 4.8 | 0.57 | - | 0.6 | |
| Men | 44 | 11.4 | 0.62 ± 0.15 | 0.65 | 0.8 | ||
| Total | 65 | 9.2 | 0.61 ± 0.14 | 0.61 | 0.8 | ||
| 40–59 | Women | 112 | 18.8 | 0.71 ± 0.18 | 0.66 | 1.1 | |
| Men | 110 | 10.9 | 0.70 ± 0.18 | 0.63 | 1.1 | ||
| Total | 222 | 14.9 | 0.71 ± 0.18 | 0.65 | 1.1 | ||
| 60–68 | Women | 33 | 15.2 | 0.82 ± 0.27 | 0.69 | 1.3 | |
| Men | 26 | 11.5 | 0.66 ± 0.12 | 0.59 | 0.8 | ||
| Total | 59 | 13.6 | 0.76 ± 0.23 | 0.67 | 1.3 | ||
| 19–68 | Women | 166 | 16.3 | 0.73 ± 0.20 | 0.66 | 1.3 | 0.434 |
| Men | 180 | 11.1 | 0.67 ± 0.16 | 0.63 | 1.1 | 0.903 | |
| Total | 346 | 13.6 | 0.70 ± 0.18 | 0.65 | 1.3 | 0.498 |
| Age (Years) | Gender | n | % Positives | Mean ± SD | Median | Max Value | p-Value (Age Groups) |
|---|---|---|---|---|---|---|---|
| 19–39 | Women | 21 | 100 | 0.68 ± 0.22 | 0.65 | 1.0 | |
| Men | 44 | 88.6 | 0.53 ± 0.18 | 0.54 | 0.9 | ||
| Total | 65 | 92.3 | 0.58 ± 0.21 | 0.58 | 1.0 | ||
| 40–59 | Women | 112 | 83.9 | 0.70 ± 0.27 | 0.70 | 1.4 | |
| Men | 110 | 81.8 | 0.51 ± 0.18 | 0.49 | 1.2 | ||
| Total | 222 | 82.9 | 0.61 ± 0.25 | 0.56 | 1.4 | ||
| 60–68 | Women | 33 | 87.9 | 0.58 ± 0.26 | 0.54 | 1.0 | |
| Men | 26 | 92.3 | 0.52 ± 0.15 | 0.50 | 1.0 | ||
| Total | 59 | 89.8 | 0.55 ± 0.22 | 0.50 | 1.0 | ||
| 19–68 | Women | 166 | 86.7 | 0.68 ± 0.26 | 0.66 | 1.4 | 0.148 |
| Men | 180 | 85.0 | 0.51 ± 0.17 | 0.49 | 1.2 | 0.645 | |
| Total | 346 | 85.8 | 0.59 ± 0.23 | 0.55 | 1.4 | 0.480 |
| Region | Matrix | Total Samples | Positive (%) | LOD (ng/mL) | Mean or Median * and (Range) (ng/mL) | Year/Reference |
|---|---|---|---|---|---|---|
| Navarra | Plasma | 438 | 97.3 | 0.4 | 2.99 (<LOD–45.7) | This study |
| Lleida | Blood | 325 | 100 | 0.018 | 0.050 * (0.06–10.92) | 2011 [40] |
| Valencia | Serum | 168 | 100 | 0.01 | 1.09 (0.15–5.71) | 2010 [41] |
| Lleida | Blood | 279 | 98.6 | 0.075 | 0.86 (0.11–8.68) | 2009 [42] |
| Granada | Plasma | 83 | 72 | 0.21 | 0.63 (<0.22–6.96) | 2001 [43] |
| Madrid | Plasma | 168 | 100 | 0.02 | 1.192 (0.12–5.58) | 1998 [44] |
| Navarra | Plasma | 75 | 53.3 | 0.52 | 0.71 (<LOD–4.0) | 1998 [33] |
| Country | Matrix | Total Samples | Positive (%) | LOD (ng/mL) | Mean and/or (Range) (ng/mL) | Year/Reference |
|---|---|---|---|---|---|---|
| Sweden | Serum | 1096 | 100 | 0.014 | 0.055 (0.05–0.658) | 2020 [47] |
| China | Plasma | 147 | 80.7 | 0.04 | 0.29 (0.04–6.59) | 2020 [48] |
| Plasma | 260 | 27.7 | 0.04 | 1.21 (0.312–9.18) | 2019 [38] | |
| Plasma | 30 | 0 | 0.15 | < LOD | 2018 [49] | |
| Czech Republic | Serum | 50 | 48 | 0.04 | 0.14 (< LOD–0.83) | 2019 [50] |
| Italy | Serum | 58 | 69.2 | 0.005 | (0.01–2.60) | 2019 [51] |
| Serum | 53 | 76.4 | 0.08 | (< LOD–0.79) | 2017 [52] | |
| Serum | 62 | 54.8 | 0.025 | 0.026 | 2016 [53] | |
| Germany | Blood | 16 | 100 | n.i. | 0.157 (0.079–0.262) | 2019 [54] |
| Blood | 50 | 100 | 0.012 | 0.204 | 2017 [39] | |
| Blood | 50 | 100 | 0.006 | 0.211 (0.071–0.383) | 2016 [55] | |
| Blood | 50 | 100 | 0.005 | 0.21 (0.071–0.383) | 2015 [56] | |
| Portugal | Serum | 42 | 100 | 0.012 | 0.76 (0.36–4.99) | 2018 [57] |
| Bangladesh | Plasma | 104 | 100 | 0.05 | 0.72 (< LOD–6.63) | 2018 [58] |
| Egypt | Serum | 98 | 81.6 | 0.2 | 0.33 (0.20–1.53) | 2016 [59] |
| Matrix | Total samples | Positive (%) | LOD (ng/mL) | Mean and/or (range) (ng/mL) | Year/Reference |
|---|---|---|---|---|---|
| Cow milk | 7 | 0 | 0.2 | n.d. | 2018 [32] |
| Wine | 51 | 100 | 0.0032 | 0.016 (0.005–0.14) | 2012 [30] |
| Beer | 31 | 77 | 0.012 | 0.044 (<LOD–0.205) | 2005 [61] |
| Wine | 40 | 50 | 0.005 | (LOD–0.316) | 2002 [62] |
| (μg/kg) | (μg/kg) | ||||
| Cereals (barley) | 123 | 58 | 0.013 | 0.10 (<LOD–3.53) | 2012 [29] |
| Breakfast cereals | 46 | 39 | 0.062 | 0.29 (<LOD–1.12) | 2011 [63] |
| Breakfast cereals | 21 | 90 | 0.066 | 0.265 (<LOD–0.975) | 2005 [61] |
| Cereals (baby food) | 20 | 70 | 0.035 | 0.187 (<LOD–0.740) | 2005 [61] |
| Cereals (wheat, barley, corn) | 115 | 58 | 0.066 | 0.219 (<LOD–7.61) | 2003 [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arce-López, B.; Lizarraga, E.; Irigoyen, Á.; González-Peñas, E. Presence of 19 Mycotoxins in Human Plasma in a Region of Northern Spain. Toxins 2020, 12, 750. https://doi.org/10.3390/toxins12120750
Arce-López B, Lizarraga E, Irigoyen Á, González-Peñas E. Presence of 19 Mycotoxins in Human Plasma in a Region of Northern Spain. Toxins. 2020; 12(12):750. https://doi.org/10.3390/toxins12120750
Chicago/Turabian StyleArce-López, Beatriz, Elena Lizarraga, Ángel Irigoyen, and Elena González-Peñas. 2020. "Presence of 19 Mycotoxins in Human Plasma in a Region of Northern Spain" Toxins 12, no. 12: 750. https://doi.org/10.3390/toxins12120750
APA StyleArce-López, B., Lizarraga, E., Irigoyen, Á., & González-Peñas, E. (2020). Presence of 19 Mycotoxins in Human Plasma in a Region of Northern Spain. Toxins, 12(12), 750. https://doi.org/10.3390/toxins12120750





