Tetrodotoxin and Its Analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody Distribution and Secretions
Abstract
:1. Introduction
2. Results
2.1. TTXs Secretion by Cephalothrix cf. simula
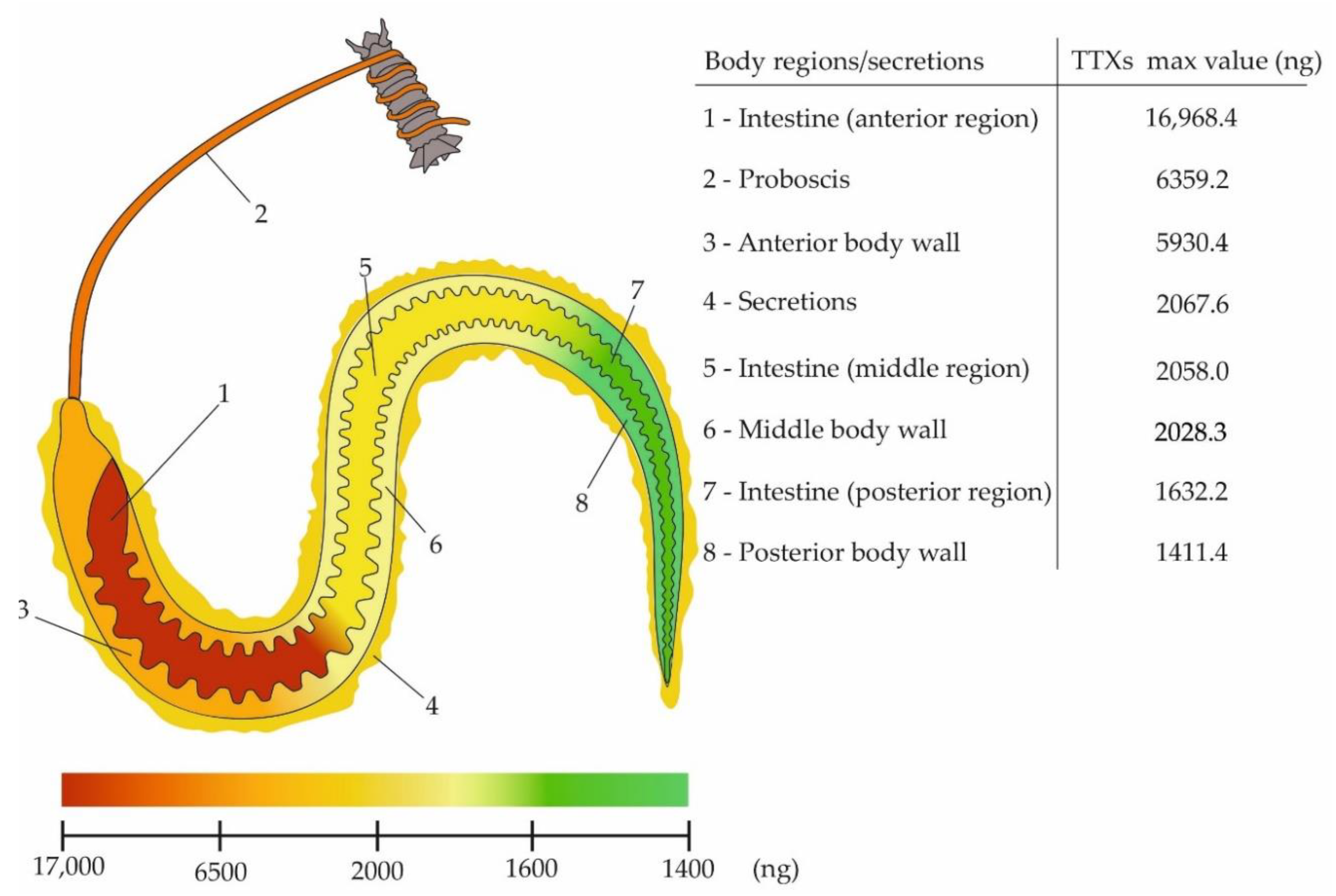
2.2. Distribution of TTXs in the Body of Cephalothrix cf. simula
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Collection
5.2. TTX and Its Analogues Extraction from the Cephalothrix cf. simula Body and Secretions
5.3. Analysis of TTX and Its Analogues in the Secretions and Body of Cephalothrix cf. simula
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, H.; Chernyshev, A.V.; Sun, S.C.; Sundberg, P.; Crandall, F.B. Checklist of nemertean genera and species published between 1995 and 2007. Species Divers. 2008, 13, 245–274. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Carroll, S.; McEvoy, E.G.; Gibson, R. The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria. J. Exp. Mar. Biol. Ecol. 2003, 288, 51–63. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Shida, Y.; Noguchi, T.; Miyazawa, K. Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 2000, 38, 763–773. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Ito, K.; Bessho, K.; Yamaguchi, C.; Tsunetsugu, S.; Shida, Y.; Kajihara, H.; Mawatari, S.F.; Noguchi, T.; et al. Paralytic toxicity in the ribbon worm Cephalothrix species (Nemertea) in Hiroshima Bay, Hiroshima Prefecture, Japan and the isolation of tetrodotoxin as a main component of its toxins. Toxicon 2003, 41, 747–753. [Google Scholar] [CrossRef]
- Asakawa, M.; Ito, K.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins 2013, 5, 376–395. [Google Scholar] [CrossRef]
- Vlasenko, A.E.; Velansky, P.V.; Chernyshev, A.V.; Kuznetsov, V.G.; Magarlamov, T.Y. Tetrodotoxin and its analogues profile in nemertean species from the Sea of Japan. Toxicon 2018, 156, 48–51. [Google Scholar] [CrossRef]
- Miyazawa, K.; Higashiyama, M.; Ito, K.; Noguchi, T.; Arakawa, O.; Shida, Y.; Hashimoto, K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 1988, 26, 867–874. [Google Scholar] [CrossRef]
- Campbell, M.E.; Schwartz, M. Immunohistological visualization of tetrodotoxin in Micrura verrili and Dushia atra (Phylum Nemertea). In Proceedings of the National Conferences for Undergraduate Research (NCUR), Salisbury, MD, USA, 10–12 April 2008. [Google Scholar]
- Turner, A.D.; Fenwick, D.; Powell, A.; Dhanji-Rapkova, M.; Ford, C.; Hatfield, R.G.; Santos, A.; Martinez-Urtaza, J.; Bean, T.P.; Baker-Austin, C.; et al. New invasive nemertean species (Cephalothrix simula) in England with high levels of tetrodotoxin and a microbiome linked to toxin metabolism. Mar. Drugs 2018, 16, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanu, M.B.; Mahmud, Y.; Arakawa, O.; Takatani, T.; Kajihara, H.; Kawatsu, K.; Hamano, Y.; Asakawa, M.; Miyazawa, K.; Noguchi, T. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae). Toxicon 2004, 44, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L. Behavioral and chemical ecology of marine organisms with respect to tetrodotoxin. Mar. Drugs 2010, 8, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajihara, H. Resolving a 200-year-old taxonomic conundrum: Neotype designation for Cephalothrix linearis (Nemertea: Palaeonemertea) based on a topotype from Bergen, Norway. Fauna Nor. 2019, 39, 39–76. [Google Scholar] [CrossRef] [Green Version]
- Vlasenko, A.E.; Kuznetsov, V.G.; Petrova, I.Y.; Magarlamov, T.Y. Development of a polyclonal antibody-based indirect competitive ELISA for the determination of tetrodotoxins in marine ribbon worms (NEMERTEA) and its comparison with high performance liquid chromatography-tandem mass spectrometry. Toxicon 2020, 176, 30–33. [Google Scholar] [CrossRef]
- Göransson, U.; Jacobsson, E.; Strand, M.; Andersson, H.S. The toxins of nemertean worms. Toxins 2019, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.S.; Min, S.K.; Yeon, S.J.; Hwang, J.H.; Hong, J.S.; Shin, H.S. Assessment of neuronal cell-based cytotoxicity of neurotoxins from an estuarine nemertean in the Han river estuary. J. Microbiol. Biotechnol. 2017, 27, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Lopes, V.M.; Baptista, M.; Repolho, T.; Rosa, R.; Costa, P.R. Uptake, transfer and elimination kinetics of paralytic shellfish toxins in common octopus (Octopus vulgaris). Aquat. Toxicol. 2014, 146, 205–211. [Google Scholar] [CrossRef]
- Zhang, X.; Zong, J.; Chen, S.; Li, M.; Lu, Y.; Wang, R.; Xu, H. Accumulation and elimination of tetrodotoxin in the pufferfish Takifugu obscurus by dietary administration of the wild toxic gastropod Nassarius semiplicata. Toxins 2020, 12, 278. [Google Scholar] [CrossRef]
- Khor, S.; Wood, S.A.; Salvitti, L.; Taylor, D.I.; Adamson, J.; McNabb, P.; Cary, S.C. Investigating diet as the source of tetrodotoxin in Pleurobranchaea maculata. Mar. Drugs 2014, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Ogata, T.; Kodama, M. Accumulation of tetrodotoxin by marine snail Neptunea arthritica through tetrodotoxin-containing diet. Nippon Suisan Gakkaishi Jpn. Ed. 1991, 57, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R. The physiology of digestion in Nemertean worms. Bol. Zool. Biol. Mar. 1972, 29, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Nagashima, Y.; Kusuhara, H.; Sugiyama, Y.; Ishizaki, S.; Shimakura, K.; Shiomi, K. Involvement of carrier-mediated transport system in uptake of tetrodotoxin into liver tissue slices of puffer fish Takifugu rubripes. Toxicon 2007, 50, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, Y.; Ohta, A.; Yin, X.; Ishizaki, S.; Matsumoto, T.; Doi, H.; Ishibashi, T. Difference in uptake of tetrodotoxin and saxitoxins into liver tissue slices among pufferfish, boxfish and porcupinefish. Mar. Drugs 2018, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.F.; Arakawa, O.; Saito, T.; Noguchi, T.; Simidu, U.; Tsukamoto, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin-producing bacteria from the blue-ringed octopus Octopus maculosus. Mar. Biol. 1989, 100, 327–332. [Google Scholar] [CrossRef]
- Asakawa, M.; Matsumoto, T.; Umezaki, K.; Kaneko, K.; Yu, X.; Gomez-Delan, G.; Tomano, S.; Noguchi, T.; Ohtsuka, S. Toxicity and toxin composition of the greater blue-ringed octopus Hapalochlaena lunulata from ishigaki island, Okinawa prefecture, Japan. Toxins 2019, 11, 245. [Google Scholar] [CrossRef] [Green Version]
- Ritson-Williams, R.; Yotsu-Yamashita, M.; Paul, V.J. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. USA 2006, 103, 3176–3179. [Google Scholar] [CrossRef] [Green Version]
- Thuesen, E.V.; Kogure, K.; Hashimoto, K.; Nemoto, T. Poison arrowworms: A tetrodotoxin venom in the marine phylum Chaetognatha. J. Exp. Mar. Biol. Ecol. 1988, 116, 249–256. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Shokur, O.A.; Chernyshev, A.V. Distribution of tetrodotoxin in the ribbon worm Lineus alborostratus. Toxicon 2016, 112, 29–34. [Google Scholar] [CrossRef]
- McDermott, J.J.; Roe, P. Food, feeding behavior and feeding ecology of Nemerteans. Am. Zool. 1985, 25, 113–125. [Google Scholar] [CrossRef]
- Kodama, M.; Ogata, T.; Sato, S. External secretion of tetrodotoxin from puffer fishes stimulated by electric shock. Mar. Biol. 1985, 87, 199–202. [Google Scholar] [CrossRef]
- Itoi, S.; Yoshikawa, S.; Asahina, K.; Suzuki, M.; Ishizuka, K.; Takimoto, N.; Mitsuoka, R.; Yokoyama, N.; Detake, A.; Takayanagi, C.; et al. Larval pufferfish protected by maternal tetrodotoxin. Toxicon 2014, 78, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuñez-Vázquez, E.J.; Yotsu-Yamashita, M.; Sierra-Beltrán, A.P.; Yasumoto, T.; Ochoa, J.L. Toxicities and distribution of tetrodotoxin in the tissues of puffer fish found in the coast of the Baja California Peninsula, Mexico. Toxicon 2000, 38, 729–734. [Google Scholar] [CrossRef]
- Miyazawa, K.; Jeon, J.K.; Noguchi, T.; Ito, K.; Hashimoto, K. Distribution of tetrodotoxin in the tissues of the flatworm Planocera multitentaculata (Platyhelminthes). Toxicon 1987, 25, 975–980. [Google Scholar] [CrossRef]
- Kajihara, H.; Sun, S.C.; Chernyshev, A.V.; Chen, H.X.; Ito, K.; Asakawa, M.; Maslakova, S.A.; Norenburg, J.L.; Strand, M.; Sundberg, P.; et al. Taxonomic identity of a tetrodotoxin-accumulating ribbon-worm Cephalothrix simula (Nemertea: Palaeonemertea): A species artificially introduced from the pacific to Europe. Zool. Sci. 2013, 30, 985–997. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef]
- Bane, V.; Brosnan, B.; Barnes, P.; Lehane, M.; Furey, A. High-resolution mass spectrometry analysis of tetrodotoxin (TTX) and its analogues in puffer fish and shellfish. Food Addit. Contam. Part A 2016, 33, 1468–1489. [Google Scholar] [CrossRef]
- Vale, P. Complex profiles of hydrophobic paralytic shellfish poisoning compounds in Gymnodinium catenatum identified by liquid chromatography with fluorescence detection and mass spectrometry. J. Chromatogr. A 2008, 1195, 85–93. [Google Scholar] [CrossRef]
- Kudo, Y.; Yasumoto, T.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Isolation and structural determination of the first 8-epi-type tetrodotoxin analogs from the newt, Cynops ensicauda popei, and comparison of tetrodotoxin analogs profiles of this newt and the puffer fish, Fugu poecilonotus. Mar. Drugs 2012, 10, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Puilingi, C.G.; Kudo, Y.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Tetrodotoxin and its analogues in the pufferfish Arothron hispidus and A. nigropunctatus from the Solomon Islands: A comparison of their toxin profiles with the same species from Okinawa, Japan. Toxins 2015, 7, 3436–3454. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.D.; Boundy, M.J.; Rapkova, M.D. Development and single-laboratory validation of a liquid chromatography tandem mass spectrometry method for quantitation of tetrodotoxin in mussels and oysters. J. AOAC Int. 2017, 100, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Liu, H.X.; Jin, Y.B.; Li, S.F.; Bi, X.; Chung, S.; Zhang, S.S.; Jiang, Y.Y. Separation, identification and quantification of tetrodotoxin and its analogs by LC-MS without calibration of individual analogs. Toxicon 2011, 57, 938–943. [Google Scholar] [CrossRef] [PubMed]

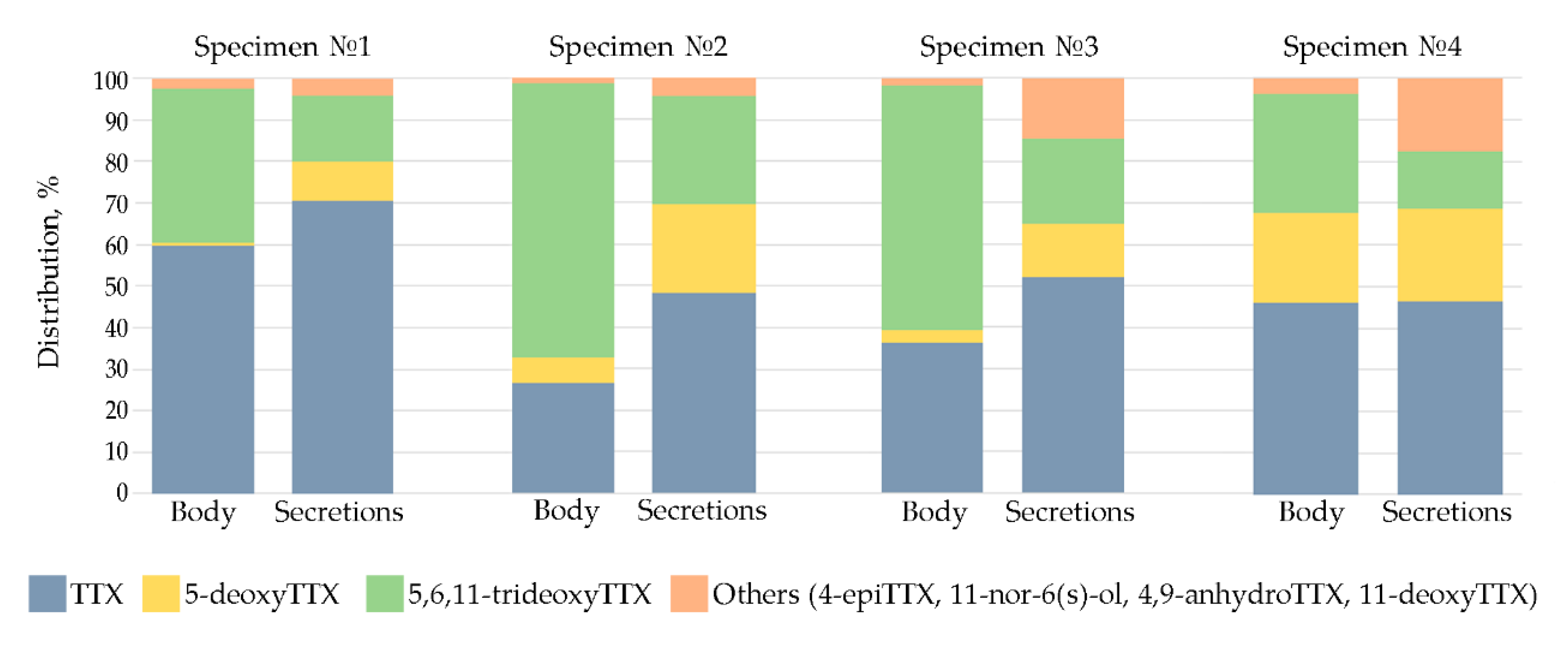
| No. of Specimen | Weight (g) | Sample | TTX and Its Analogues (TTXs) (ng) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TTX | 4-epiTTX | 11-norTTX-6(s)-ol | 4,9-anhydroTTX | 11-deoxyTTX | 5-deoxyTTX | 5,6,11-trideoxyTTX | Sum of TTXs | Total Amount of TTXs | |||
| 1 | 0.20 | body | 254,758.0 | 10,641.2 | 13.2 | 282.0 | 338.5 | 3340.2 | 158,354.4 | 427,727.5 | 428,596.9 |
| secretions | 613.0 | 29.7 | ND | 4.2 | 1.0 | 83.2 | 138.3 | 869.4 | |||
| 2 | 0.17 | body | 51,323.0 | 2137.1 | 6.2 | 91.9 | 94.0 | 11,573.8 | 127,065.6 | 192,291.6 | 193,059.9 |
| secretions | 369.2 | 25.0 | 2.2 | 5.0 | 1.0 | 165.7 | 200.2 | 768.3 | |||
| 3 | 0.25 | body | 53,233.0 | 1500.4 | ND | 329.1 | 542.4 | 4311.5 | 87,096.5 | 147,013.0 | 149,080.5 |
| secretions | 1076.1 | 240.2 | ND | 58.7 | 5.1 | 265.0 | 422.5 | 2067.6 | |||
| 4 | 0.30 | body | 45,345.0 | 1813.3 | ND | 467.5 | 1334.7 | 20,870.9 | 28,268.8 | 98,100.2 | 98,767.5 |
| secretions | 308.6 | 69.6 | ND | 44.0 | 4.5 | 148.5 | 92.1 | 667.3 | |||
| No. of Specimen | Weight (g) | Organ | Region | TTX and Its Analogues (TTXs) (ng) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTX | 4-epiTTX | 11-norTTX-6(s)-ol | 11-norTTX-6(r)-ol | 4,9-anhydroTTX | 11-deoxyTTX | 5-deoxyTTX | 5,6,11-trideoxyTTX | 11-oxoTTX | 4,9-anhydro-8-epi-5,6,11-trideoxyTTX | 1-hydroxy-8-epi-5,6,11-trideoxyTTX | Sum of TTXs | Sum of TTXs in the Organ | Total Amount of TTXs | ||||
| 5 | 0.17 | Intestine | Anterior | 9608.3 | 539.8 | 9.4 | 42.8 | 47.4 | 86.6 | 3131.7 | 3487.5 | <LoQ | 9.9 | 5.0 | 16,968.4 | 19,954.5 | 29,012.6 |
| Middle | 561.3 | 53.6 | 0.7 | 4.6 | 5.9 | 37.1 | 380.6 | 308.5 | <LoQ | 1.0 | 0.6 | 1354.9 | |||||
| Posterior | 582.8 | 93.0 | 0.7 | 2.4 | 8.6 | 6.1 | 621.7 | 315.6 | <LoQ | 0.6 | 0.7 | 1632.2 | |||||
| Body wall | Anterior | 714.5 | 42.5 | 0.7 | 3.5 | 4.0 | 6.6 | 257.5 | 204.1 | <LoQ | 0.9 | 0.4 | 1234.7 | 2698.9 | |||
| Middle | 491.9 | 44.0 | 0.4 | 3.2 | 4.7 | 12.4 | 337.4 | 239.5 | <LoQ | 0.9 | 0.6 | 1135.0 | |||||
| Posterior | 122.3 | 15.5 | 0.1 | 0.3 | 1.9 | 1.7 | 138.5 | 48.3 | <LoQ | 0.3 | 0.3 | 329.2 | |||||
| Proboscis | 2534.6 | 493.4 | 48.2 | 31.4 | 35.9 | 34.9 | 1992.4 | 1177.9 | 2.0 | 4.3 | 4.2 | 6359.2 | 6359.2 | ||||
| 6 | 0.07 | Intestine | Anterior | 1342.1 | 15.2 | 0.4 | 0.8 | 3.2 | 12.5 | 194.1 | 627.3 | ND | 1.5 | 0.2 | 2197.3 | 4395.2 | 15,190.3 |
| Middle | 873.9 | 18.4 | ND | 0.7 | 1.6 | 9.6 | 142.8 | 563.9 | ND | 1.1 | 0.3 | 1612.3 | |||||
| Posterior | 301.8 | 9.1 | 0.1 | 0.3 | 1.0 | 9.0 | 83.8 | 179.9 | ND | 0.4 | 0.2 | 585.6 | |||||
| Body wall | Anterior | 4134.5 | 47.7 | 1.0 | ND | 6.4 | 6.0 | 358.3 | 1372.4 | ND | 4.1 | ND | 5930.4 | 9370.1 | |||
| Middle | 1112.0 | 10.7 | 0.2 | 0.8 | 2.0 | 3.0 | 173.1 | 724.0 | ND | 2.2 | 0.3 | 2028.3 | |||||
| Posterior | 630.7 | 10.0 | ND | 0.7 | 2.3 | 11.6 | 189.0 | 564.8 | ND | 1.5 | 0.8 | 1411.4 | |||||
| Proboscis | 900.0 | 9.4 | 2.9 | 1.5 | 1.6 | 3.2 | 115.2 | 389.8 | ND | 1.2 | 0.2 | 1425.0 | 1425.0 | ||||
| 7 | 0.10 | Intestine | Anterior | 2836.2 | 42.0 | 1.1 | 3.7 | 9.6 | 19.5 | 1013.6 | 978.6 | ND | 3.2 | 0.5 | 4908.0 | 7453.9 | 13,065.2 |
| Middle | 1038.7 | 19.4 | 0.8 | 1.7 | 6.0 | 9.2 | 490.9 | 489.2 | ND | 1.7 | 0.4 | 2058.0 | |||||
| Posterior | 186.7 | 8.5 | 0.1 | 0.5 | 1.8 | 2.0 | 175.7 | 111.8 | ND | 0.5 | 0.3 | 487.9 | |||||
| Body wall | Anterior | 2906.6 | 24.7 | 1.8 | 2.6 | 5.9 | 3.5 | 438.1 | 463.9 | ND | 1.8 | 0.3 | 3849.2 | 4730.8 | |||
| Middle | 331.2 | 8.9 | 0.1 | 0.4 | 1.3 | 0.5 | 131.1 | 122.9 | ND | 0.5 | 0.1 | 597.0 | |||||
| Posterior | 113.5 | 4.6 | 0.1 | 0.3 | 1.0 | 0.4 | 98.2 | 66.0 | ND | 0.3 | 0.2 | 284.6 | |||||
| Proboscis | 516.1 | 7.5 | 1.9 | 0.8 | 1.2 | 1.3 | 89.2 | 261.6 | ND | 0.9 | ND | 880.5 | 880.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasenko, A.E.; Magarlamov, T.Y. Tetrodotoxin and Its Analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody Distribution and Secretions. Toxins 2020, 12, 745. https://doi.org/10.3390/toxins12120745
Vlasenko AE, Magarlamov TY. Tetrodotoxin and Its Analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody Distribution and Secretions. Toxins. 2020; 12(12):745. https://doi.org/10.3390/toxins12120745
Chicago/Turabian StyleVlasenko, Anna E., and Timur Yu. Magarlamov. 2020. "Tetrodotoxin and Its Analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody Distribution and Secretions" Toxins 12, no. 12: 745. https://doi.org/10.3390/toxins12120745
APA StyleVlasenko, A. E., & Magarlamov, T. Y. (2020). Tetrodotoxin and Its Analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody Distribution and Secretions. Toxins, 12(12), 745. https://doi.org/10.3390/toxins12120745





