Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs
Abstract
1. Introduction
2. Results
2.1. Experiment of Depuration of Domoic Acid in Controlled Conditions
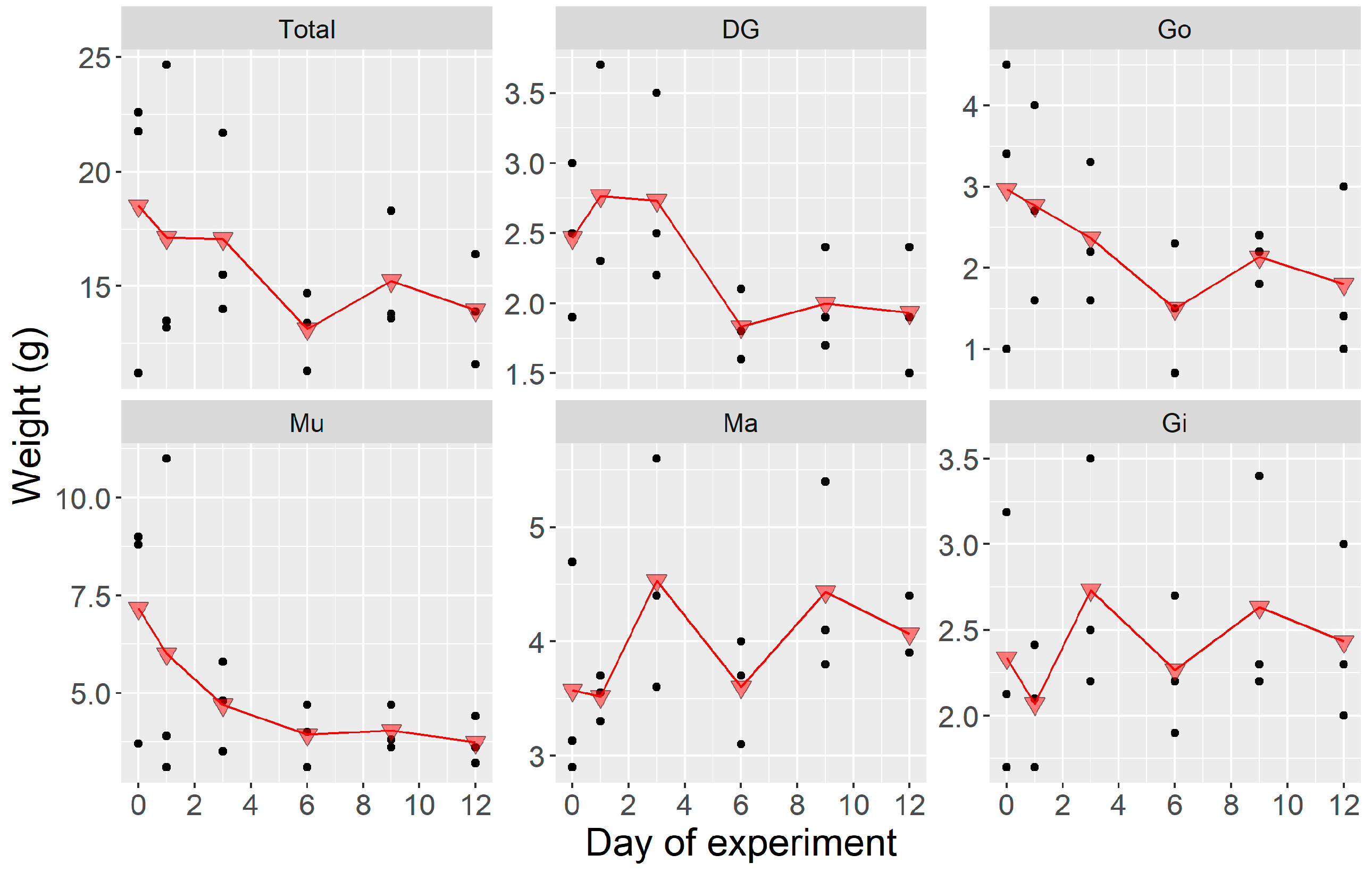
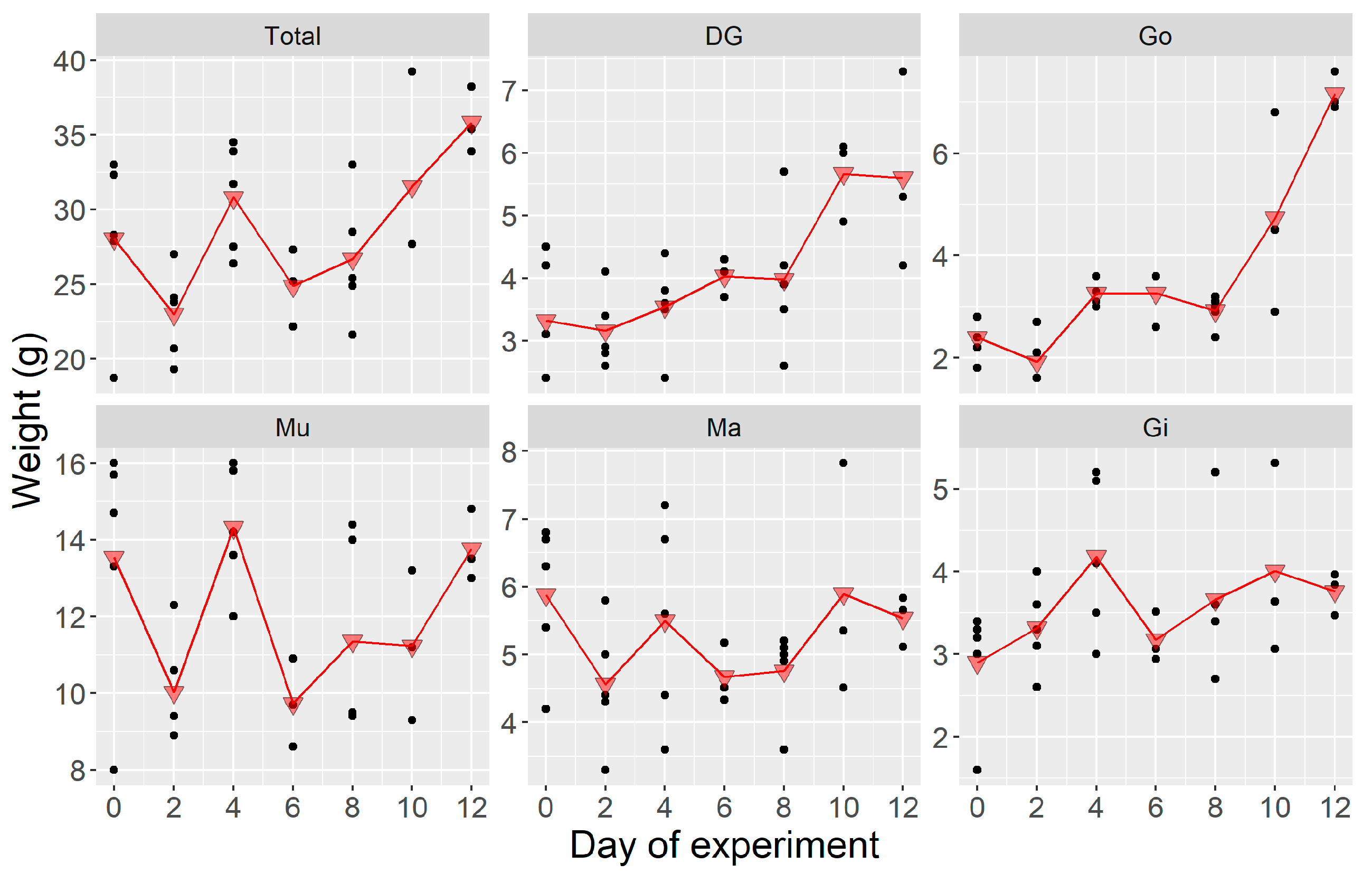
2.1.1. Weight
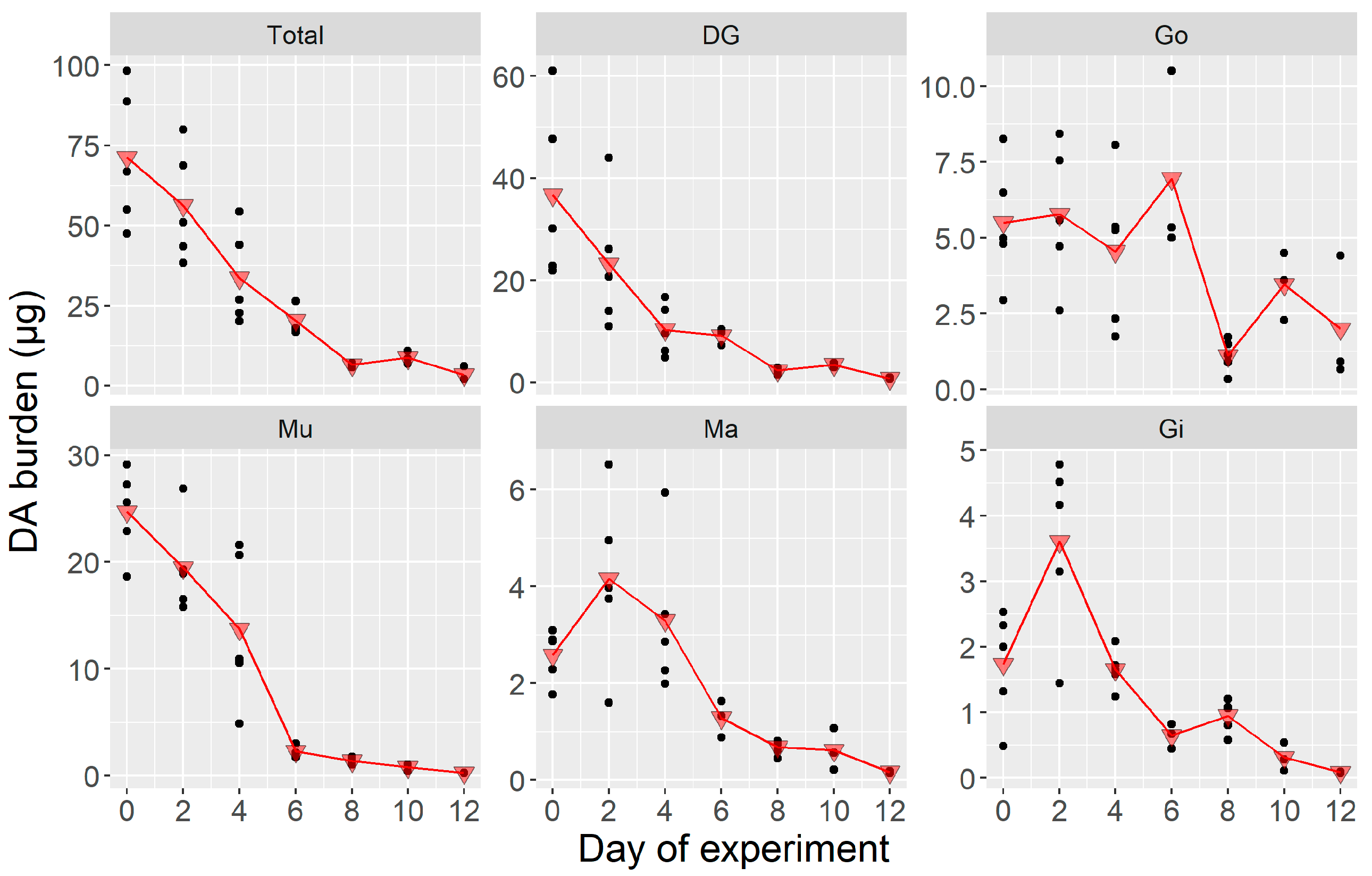
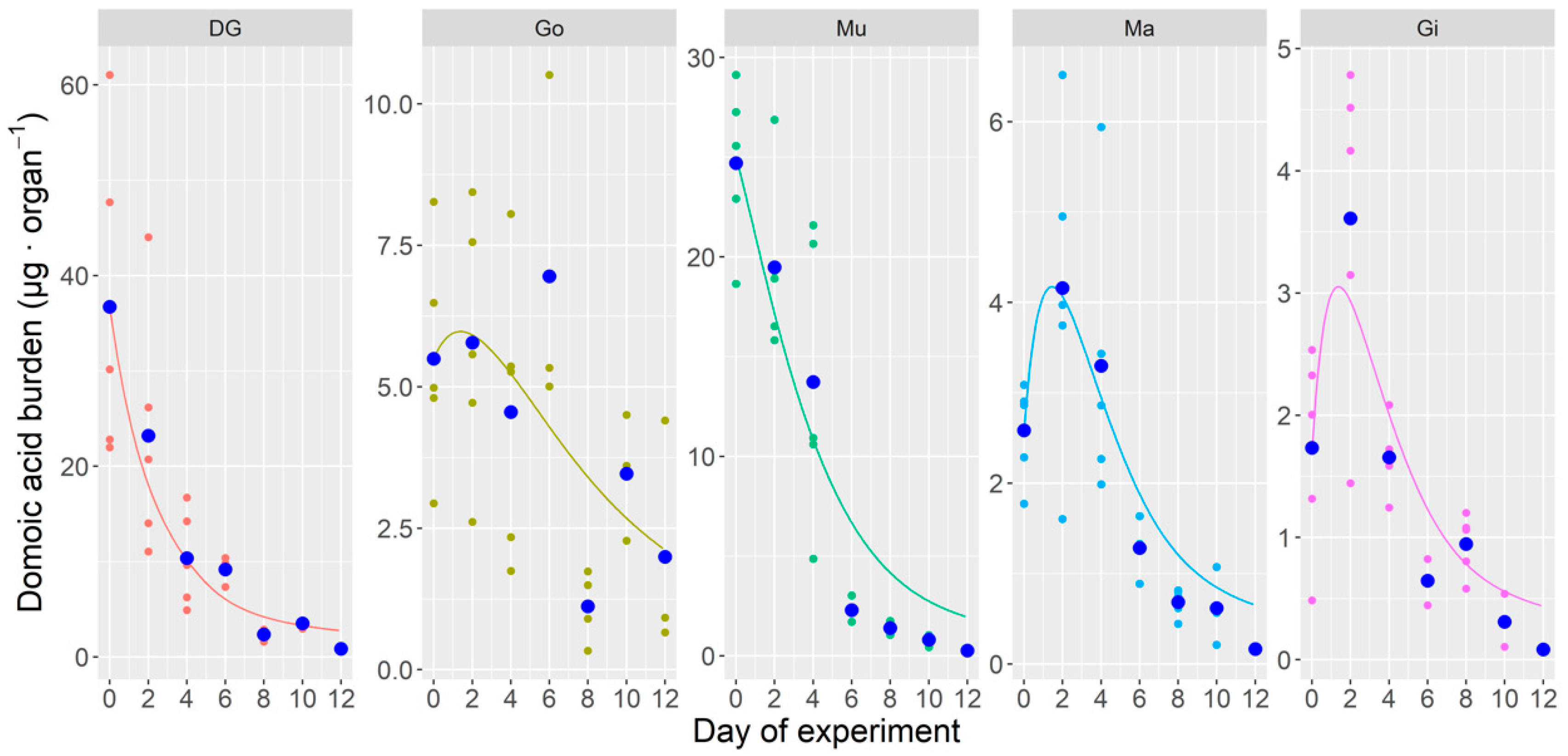
2.1.2. Toxin Burden
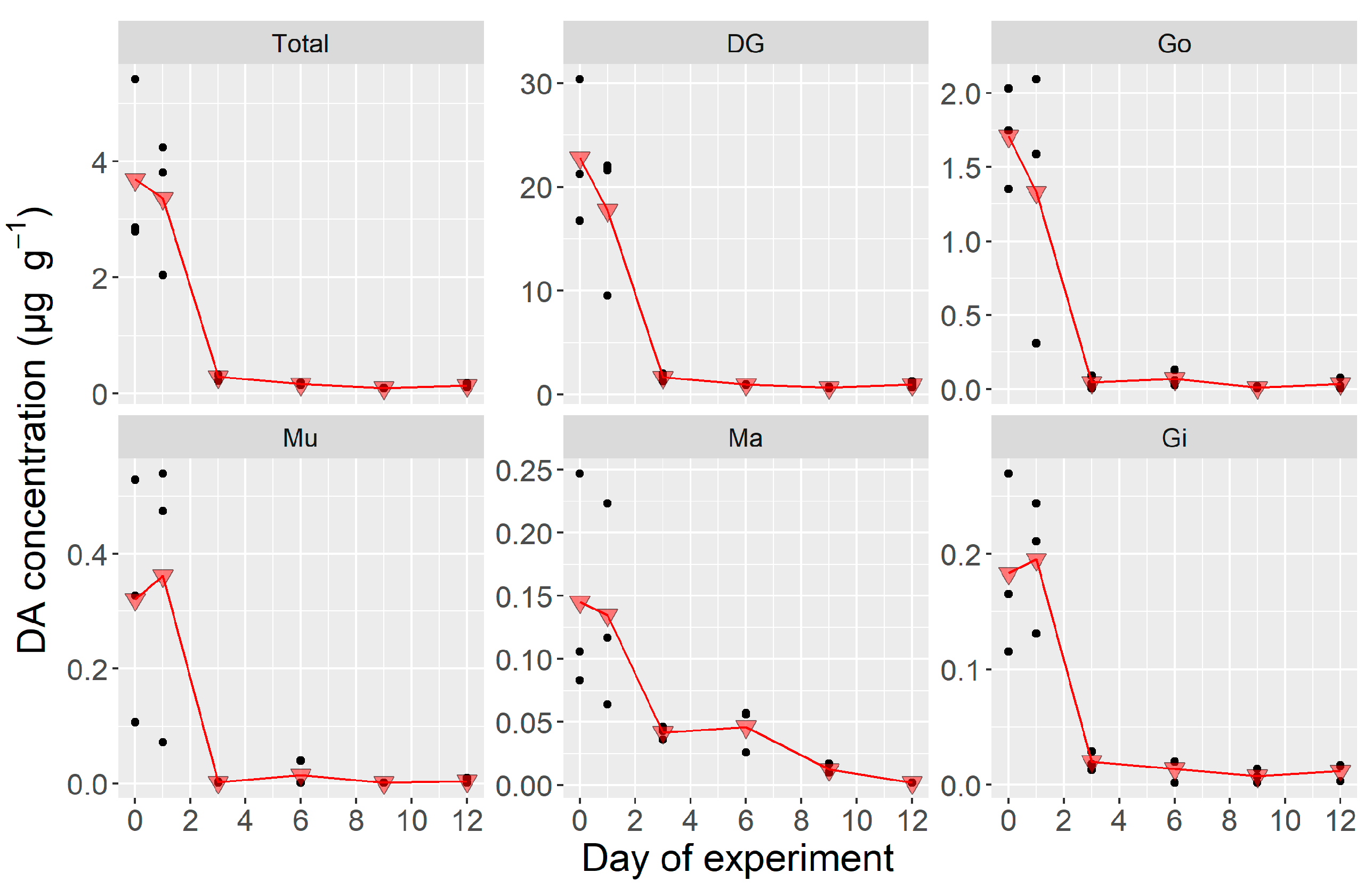
2.1.3. Whole Body and Tissue-Specific DA Concentrations During the Laboratory Experiment
2.2. Experiment of Domoic Acid Depuration in the Natural Environment
2.2.1. Weight
2.2.2. Toxin Burden
2.2.3. Whole Body and Tissue-Specific DA Concentrations During the Field Experiment
2.3. Model Fitting
2.3.1. Depuration of Domoic Acid in the Natural Environment
2.3.2. Depuration of Domoic Acid in Laboratory
3. Discussion
3.1. Physiological Conditions in the Two Experiments
3.2. Domoic Acid Depuration
3.3. Domoic Acid Depuration Kinetics
4. Conclusions
5. Materials and Methods
5.1. Depuration of Domoic Acid in Controlled Conditions
5.2. Depuration of Domoic Acid in Natural Environment
5.3. Scallop Dissection and Domoic Acid Analysis
5.4. Modelling
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perl, T.M.; Bedard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.; McNutt, L.A.; Remis, R.S. Amnesic shellfish poisoning: A new clinical syndrome due to domoic acid. Can. Dis. Wkly. Rep. 1990, 16, 7–8. [Google Scholar] [PubMed]
- Bates, S.; Bird, C.; Defreitas, A.; Foxall, R.; Gilgan, M.; Hanic, L.; Johnson, G.; McCulloch, A.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Levin, M.; Leibrecht, H.; Ryan, J.; Van Dolah, F.; De Guise, S. Immunomodulatory Effects of Domoic Acid Differ Between In vivo and In vitro Exposure in Mice. Mar. Drugs 2008, 6, 636–659. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Lelong, A.; Hegaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Uribe, E.; Quijano-Scheggia, S.; Lopez-Rivera, A.; Mariño, C.; Blanco, J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar] [CrossRef]
- Suárez Isla, B.A.; López, A.; Hernández, C.; Clement, A.; Guzmán, L. Impacto económico de las floraciones de microalgas nocivas en Chile y datos recientes sobre la ocurrencia de veneno amnésico de los mariscos. In Floraciones Algales Nocivas en el ConoSur Americano; Sar, E.A., Ferrario, M.E., Reguera, B., Eds.; Instituto Español de Oceanografía: Madrid, Spain, 2002; pp. 259–268. [Google Scholar]
- López-Rivera, A.; Pinto, M.; Insinilla, A.; Isla, B.S.; Uribe, E.; Álvarez, G.; Lehane, M.; Furey, A.; James, K.J. The occurrence of domoic acid linked to a toxic diatom bloom in a new potential vector: The tunicate Pyura chilensis (piure). Toxicon 2009, 54, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Álvarez, G.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Cranford, P.J. Chapter 13 Scallops and marine contaminants. In Scallops: Biology, Ecology and Aquaculture; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, Netherlands, 2006; pp. 745–764. [Google Scholar]
- Blanco, J.; Bermúdez de la Puente, M.; Arévalo, F.; Salgado, C.; Moroño, A. Depuration of mussels (Mytilus galloprovincialis) contaminated with domoic acid. Aquat. Living Resour. 2002, 15, 53–60. [Google Scholar] [CrossRef]
- Mafra, L.L.; Bricelj, V.M.; Fennel, K. Domoic acid uptake and elimination kinetics in oysters and mussels in relation to body size and anatomical distribution of toxin. Aquat. Toxicol. 2010, 100, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Mafra, L.L.; Bricelj, V.M.; Ouellette, C.; Bates, S.S. Feeding mechanics as the basis for differential uptake of the neurotoxin domoic acid by oysters, Crassostrea virginica, and mussels. Mytilus edulis. Aquat. Toxicol. 2010, 97, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Wohlgeschaffen, G.D.; Mann, K.H.; Subba Rao, D.V.; Pocklington, R. Dynamics of the phycotoxin domoic acid: Accumulation and excretion in two commercially important bivalves. J. Appl. Phycol. 1992, 4, 297–310. [Google Scholar] [CrossRef]
- Shumway, S.E.; Cembella, A.D. The impact of toxic algae on scallop culture and fisheries. Rev. Fish. Sci. 1993, 1, 121–150. [Google Scholar] [CrossRef]
- Álvarez, G.; Uribe, E.; Regueiro, J.; Martin, H.; Gajardo, T.; Jara, L.; Blanco, J. Depuration and anatomical distribution of domoic acid in the surf clam Mesodesma donacium. Toxicon 2015, 102, 1–7. [Google Scholar] [CrossRef]
- Dusek Jennings, E.; Parker, M.S.; Simenstad, C.A. Domoic acid depuration by intertidal bivalves fed on toxin-producing Pseudo-nitzschia multiseries. Toxicon X 2020, 6, 100027. [Google Scholar] [CrossRef]
- James, K.J.; Gillman, M.; Amandi, M.F.; Lopez-Rivera, A.; Puente, P.F.; Lehane, M.; Mitrovic, S.; Furey, A. Amnesic Shellfish Poisoning Toxins in Bivalve Molluscs in Ireland. Toxicon 2005, 46, 852–858. [Google Scholar] [CrossRef]
- Douglas, D.J.; Kenchington, E.R.; Bird, C.J.; Pocklington, R.; Bradford, B.; Silvert, W. Accumulation of domoic acid by the sea scallop (Placopecten magellanicus) fed cultured cells of toxic Pseudo-nitzschia multiseries. Can. J. Fish. Aquat. Sci. 1997, 54, 907–913. [Google Scholar] [CrossRef]
- Blanco, J. Modelling as a mitigation strategy for harmful algal blooms. In Shellfish Safety and Quality; Shumway, S.E., Rodrick, G.E., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 200–227. [Google Scholar]
- Novaczek, I.; Madhyastha, M.S.; Ablett, R.F.; Donald, A.; Johnson, G.; Nijjar, M.S.; Sims, D.E. Depuration of domoic acid from live blue mussels (Mytilus edulis). Can. J. Fish. Aquat. Sci. 1992, 49, 312–318. [Google Scholar] [CrossRef]
- Blanco, J.; Acosta, C.P.; Bermúdez de la Puente, M.; Salgado, C. Depuration and anatomical distribution of the amnesic shellfish poisoning (ASP) toxin domoic acid in the king scallop Pecten maximus. Aquat. Toxicol. 2002, 60, 111–121. [Google Scholar] [CrossRef]
- Gilgan, M.W.; Burns, B.G.; Landry, G.J. Distribution and Magnitude of Domoic Acid Contamination of Shellfish in Atlantic Canada during 1988; Elsevier Science Publishing, Co.: Amsterdam, The Netherlands, 1990; pp. 469–474. [Google Scholar]
- Drum, A.S.; Siebens, T.L.; Crecelius, E.A.; Elston, R.A. Domoic acid in the Pacific razor clam Siliqua patula (Dixon, 1789). J. Shellfish Res. 1993, 12, 443–450. [Google Scholar]
- Horner, R.A.; Kusske, M.B.; Moynihan, B.P.; Skinner, R.N.; Wekell, J.C. Retention of domoic acid by Pacific razor clams, Siliqua patula (Dixon, 1789): Preliminary study. J. Shellfish Res. 1993, 12, 451–456. [Google Scholar]
- Blanco, J.; Acosta, C.P.; Mariño, C.; Muñíz, S.; Martín, H.; Moroño, A.; Correa, J.; Arévalo, F.; Salgado, C. Depuration of domoic acid from different body compartments of the King Scallop Pecten maximus grown in raft culture and natural bed. Aquat. Living Resour. 2006, 19, 257–265. [Google Scholar] [CrossRef]
- Hess, P.; Brown, N.A.; Bates, L.A.; Turriff, J.J.; Howard, F.G.; Moffat, C.F. A comparison of domoic acid in scallop (Pecten maximus) gonads processed by FRS Ml whith those prepared by a commercial processor. Fish. Res. Serv. 2000, 30, 1–6. [Google Scholar]
- Campbell, D.A.; Kelly, M.S.; Busman, M.; Wiggins, E.; Fernandes, T.F. Impact of preparation method on gonad domoic acid levels in the scallop, Pecten maximus (L.). Harmful Algae 2003, 2, 215–222. [Google Scholar] [CrossRef]
- Campbell, D.A.; Kelly, M.S.; Busman, M.; Bolch, C.J.; Wiggins, E.; Moeller, P.D.R.; Morton, S.L.; Hess, P.; Shumway, S.E. Amnesic shellfish poisoning in the king scallop, Pecten maximus, from the west coast of Scotland. J. Shellfish Res. 2001, 20, 75–84. [Google Scholar]
- Bogan, Y.M.; Bender, K.; Hervas, A.; Kennedy, D.J.; Slater, J.W.; Hess, P. Spatial variability of domoic acid concentration in King Scallops Pecten maximus off the Southeast Coast of Ireland. Harmful Algae 2007, 6, 1–14. [Google Scholar] [CrossRef]
- Bogan, Y.M.; Harkin, A.L.; Gillespie, J.; Kennedy, D.J.; Hess, P.; Slater, J.W. The Influence of Size on Domoic Acid Concentration in King Scallop, Pecten maximus (L.). Harmful Algae 2007, 6, 15–28. [Google Scholar] [CrossRef]
- Bogan, Y.M.; Kennedy, D.J.; Harkin, A.L.; Gillespie, J.; Vause, B.J.; Beukers-Stewart, B.D.; Hess, P.; Slater, J.W. Variation in domoic acid concentration in King Scallop (Pecten maximus) from fishing grounds around the Isle of Man. Harmful Algae 2007, 6, 81–92. [Google Scholar] [CrossRef]
- Blanco, J.; Cano, J.; Marino, M.D.C.; Campos, M.J. Effect of Phytoplankton Containing Paralytic Shellfish and Amnesic Shellfish Toxins on the Culture of the King Scallop Pecten maximus in Málaga (Se Spain). Aquat. Living Resour. 2006, 19, 267–273. [Google Scholar] [CrossRef]
- EU. Commission Decision of 15 March 2002 establishing special health checks for the harvesting and processing of certain bivalve molluscs with a level of amnesic shellfish poison (ASP) exceeding the limit laid down by Council Directive 91/492/EEC. Off. J. Eur. Communities 2002, 75, 65–66. [Google Scholar]
- Paseiro, P. Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) sobre los criterios de seguridad aplicables al contenido de ácido domoico en la vieira (Pecten maximus) para su recolección. Rev. Del Com. Científico 2009, 10, 41–52. [Google Scholar]
- Alamo, V.; Valdivieso, V. Lista sistemática de moluscos marinos del Perú. Bol. Inst. Mar. Perú, Callao. 1987. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1727-99332003000200012 (accessed on 3 November 2020).
- Sanzana, J. Estudios Preliminares del Ostión (Chlamys Purpurata, Lamarck, 1819) de la zona de Valparaíso; Universidad Católica de Valparaíso: Valparaíso, Chile, 1978. [Google Scholar]
- Wolff, M.; Taylor, M.H.; Mendo, J.; Yamashiro, B. A catch forecast model for the Peruvian scallop (Argopecten purpuratus) based on estimators of spawning stock and settlement rate. Ecol. Model. 2007, 209, 333–341. [Google Scholar] [CrossRef][Green Version]
- Valenzuela, A.; Yáñez, C.G.; Golusda, C. The scallop from the chilean north (Argopecten purpuratus), A food with a high nutritional value. Rev. Chil. Nutr. 2011, 38, 148–155. [Google Scholar]
- Mendo, J.; Wolff, M.; Mendo, T.; Ysla, L. Scallop Fishery and Culture in Peru. In Scallops: Biology, Ecology, Aquaculture, and Fisheries; Shumway, S.E., Parson, G.J., Eds.; Elsevier: Amsterdam, The Netehrlands, 2016; Volume 40, pp. 1089–1109. [Google Scholar]
- Wolff, M. Population dynamics of the Peruvian scallop Argopecten purpuratus during the El Niño phenomenon of 1983. Can. J. Fish. Aquat. Sci. 1987, 44, 1684–1691. [Google Scholar] [CrossRef]
- Kluger, L.C.; Taylor, M.H.; Wolff, M.; Stotz, W.; Mendo, J. From an open-access fishery to a regulated aquaculture business: The case of the most important Latin American bay scallop (Argopecten purpuratus). Rev. Aquac. 2019, 11, 187–203. [Google Scholar] [CrossRef]
- von Brand, E.; Abarca, A.; Merino, G.; Stotz, W. Scallop Fishery and Aquaculture in Chile: A History of Developments and Declines. In Scallops: Biology, Ecology, Aquaculture and Fisheries; Shumway, S.E., Parson, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 40, pp. 1047–1071. [Google Scholar]
- Barber, B.; Blake, N. Reproductive Physiology. In Scallops: Biology, Ecology, Aquaculture and Fisheries; Shumway, S.E., Parson, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 253–299. [Google Scholar]
- Bricelj, V.M.; Cembella, A.D.; Laby, D. Temperature effects on kinetics of paralytic shellfish toxin elimination in Atlantic surfclams. Spisula solidissima. Deep-Sea Res. Part Ii: Top. Stud. Oceanogr. 2014, 103, 308–317. [Google Scholar] [CrossRef]
- Peck, L.S. Temperature and basal metabolism in two Antarctic marine herbivores. J. Exp. Mar. Biol. Ecol. 1989, 127, 1–12. [Google Scholar] [CrossRef]
- Grizzle, R.E.; Bricelj, V.M.; Shumway, S.E. Physiological ecology of Mercenaria mercenaria. In Biology of the Hard Clam; Kraeuter, J.N., Castagna, M., Eds.; Elsevier: New York, NY, USA, 2001; pp. 305–382. [Google Scholar]
- Mafra, L., Jr.; Bricelj, V.; Ouellette, C.; Léger, C.; Bates, S. Mechanisms contributing to low domoic acid uptake by oysters feeding on Pseudo-nitzschia cells. I. Filtration and pseudofeces production. Aquat. Biol. 2009, 6, 201–212. [Google Scholar] [CrossRef]
- Beninger, P.; Le Pennec, M. Scallop structure and function. In Scallops: Biology, Ecology, Aquaculture and Fisheries; Shumway, S.E., Parson, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 85–158. [Google Scholar]
- Eggermont, M.; Cornillie, P.; Dierick, M.; Adriaens, D.; Nevejan, N.; Bossier, P.; Van den Broeck, W.; Sorgeloos, P.; Defoirdt, T.; Declercq, A.M. The blue mussel inside: 3D visualization and description of the vascular-related anatomy of Mytilus edulis to unravel hemolymph extraction. Sci. Rep. 2020, 10, 6773. [Google Scholar] [CrossRef]
- Jones, T.O.; Whyte, J.N.C.; Townsend, L.D.; Ginter, N.G.; Iwama, G.K. Effects of domoic acid on haemolimph pH, pCO2, and pO2 in the Pacific oyster, Crassostrea gigas and the California mussel. Mytilus californianus. Aquat. Toxicol. 1995, 31, 43–55. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. SIL Commun. 1953–1996 2017, 9, 1–38. [Google Scholar] [CrossRef]
- Navarro, J.M.; Gonzalez, C.M. Physiological responses of the Chilean scallop Argopecten purpuratus to decreasing salinities. Aquaculture 1998, 167, 315–327. [Google Scholar] [CrossRef]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and Size-Classes of Phytoplankton in the Baltic Sea; Helsinki Commission Baltic Marine Environment Protection Commission: Helsinki, Finland, 2006; 144p. [Google Scholar]
- Quilliam, M.A.; Xie, M.; Hardstaff, W.R. Rapid Extraction and Cleanup for Liquid Chromatographic Determination of Domoic Acid in Unsalted Seafood. J. AOAC Int. 1995, 78, 543–554. [Google Scholar] [CrossRef]
- Regueiro, J.; Martin-Morales, E.; Álvarez, G.; Blanco, J. Sensitive determination of domoic acid in shellfish by on-line coupling of weak anion exchange solid-phase extraction and liquid chromatography-diode array detection-tandem mass spectrometry. Food Chem. 2011, 129, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, K.; Petzoldt, T.; Setzer, R.W. Solving Differential Equations in R: Package deSolve. J. Stat. Softw. 2010, 33. [Google Scholar] [CrossRef]
- Soetaert, K.; Petzoldt, T. Inverse Modelling, Sensitivity and Monte Carlo Analysis in R Using Package FME. J. Stat. Softw. 2010, 33. [Google Scholar] [CrossRef]



| Organ | Natural Conditions | Laboratory Conditions | ||
|---|---|---|---|---|
| Depuration Rate | Transfer Rate | Depuration Rate | Transfer Rate | |
| DG | 0.03806 | 0.095 | ||
| Gonad | 0.229144 | 0.06657 | 3.272 | 0.336 |
| Mantle | 0.524622 | 0.10365 | 0.504 | 0.005 |
| Gill | 1.424803 | 0.24322 | 4.202 | 0.048 |
| Muscle | 0.33993 | 0.1412 | 4.201 | 0.286 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, G.; Rengel, J.; Araya, M.; Álvarez, F.; Pino, R.; Uribe, E.; Díaz, P.A.; Rossignoli, A.E.; López-Rivera, A.; Blanco, J. Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs. Toxins 2020, 12, 698. https://doi.org/10.3390/toxins12110698
Álvarez G, Rengel J, Araya M, Álvarez F, Pino R, Uribe E, Díaz PA, Rossignoli AE, López-Rivera A, Blanco J. Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs. Toxins. 2020; 12(11):698. https://doi.org/10.3390/toxins12110698
Chicago/Turabian StyleÁlvarez, Gonzalo, José Rengel, Michael Araya, Francisco Álvarez, Roberto Pino, Eduardo Uribe, Patricio A. Díaz, Araceli E. Rossignoli, Américo López-Rivera, and Juan Blanco. 2020. "Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs" Toxins 12, no. 11: 698. https://doi.org/10.3390/toxins12110698
APA StyleÁlvarez, G., Rengel, J., Araya, M., Álvarez, F., Pino, R., Uribe, E., Díaz, P. A., Rossignoli, A. E., López-Rivera, A., & Blanco, J. (2020). Rapid Domoic Acid Depuration in the Scallop Argopecten purpuratus and Its Transfer from the Digestive Gland to Other Organs. Toxins, 12(11), 698. https://doi.org/10.3390/toxins12110698








