Serum P-Cresyl Sulfate Is a Predictor of Central Arterial Stiffness in Patients on Maintenance Hemodialysis
Abstract
1. Introduction
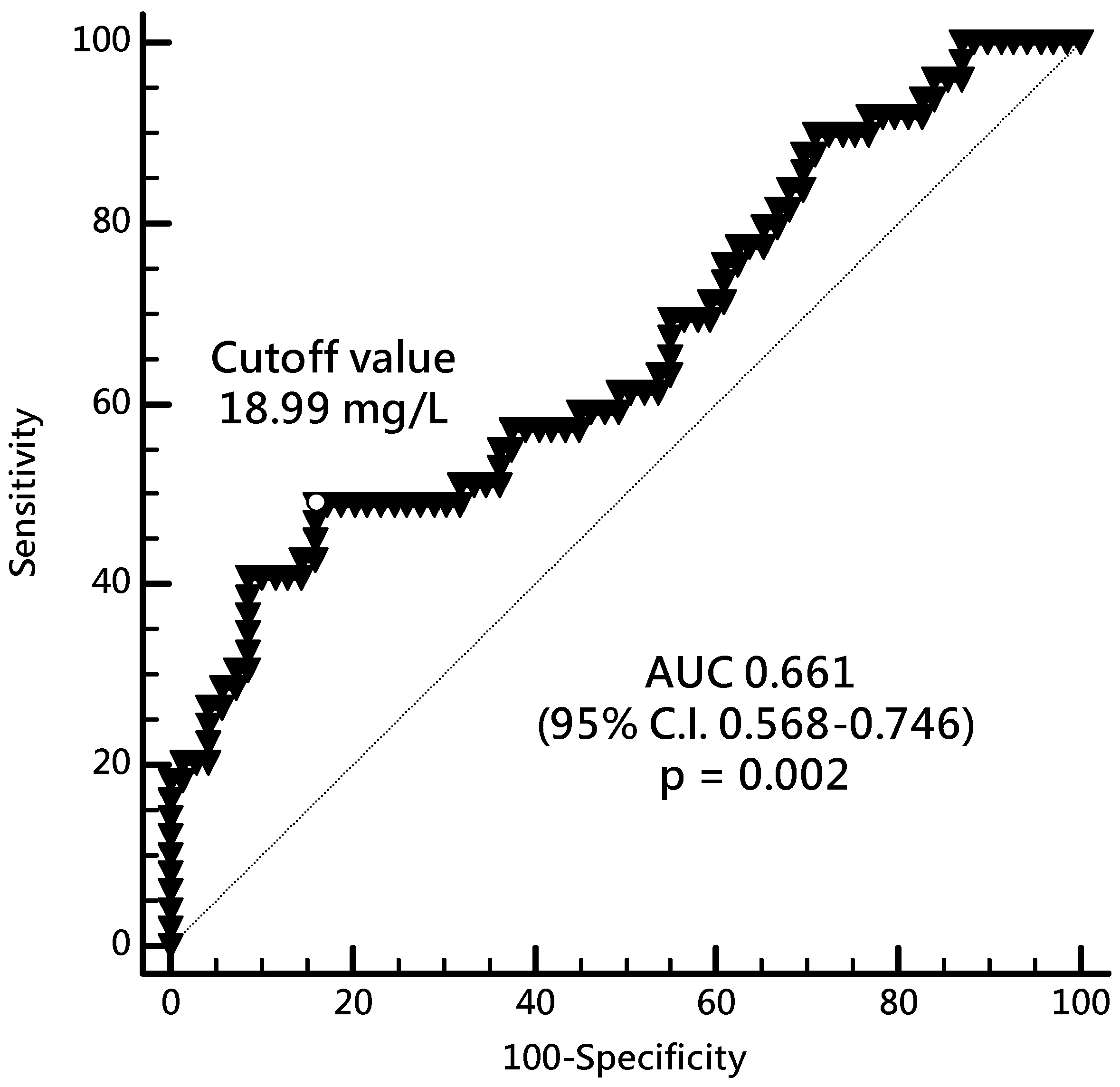
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Participants
5.2. Biochemical and Anthropometic Analyses
5.3. Determination of Serum P-Cresyl Sulfate Levels
5.4. Carotid–Femoral PWV Measurements
5.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Wen, C.P.; Cheng, T.Y.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; Safar, M.E.; London, G.M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999, 99, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef]
- Ossareh, S. Vascular calcification in chronic kidney disease: Mechanisms and clinical implications. Iran. J. Kidney Dis. 2011, 5, 285–299. [Google Scholar]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Townsend, R.R.; Anderson, A.H.; Chirinos, J.A.; Feldman, H.I.; Grunwald, J.E.; Nessel, L.; Roy, J.; Weir, M.R.; Wright, J.T., Jr.; Bansal, N.; et al. Association of pulse wave velocity with chronic kidney disease progression and mortality: Findings from the cric study (chronic renal insufficiency cohort). Hypertension 2018, 71, 1101–1107. [Google Scholar] [CrossRef]
- Wu, I.W.; Hsu, K.H.; Lee, C.C.; Sun, C.Y.; Hsu, H.J.; Tsai, C.J.; Tzen, C.Y.; Wang, Y.C.; Lin, C.Y.; Wu, M.S. P-cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 938–947. [Google Scholar] [CrossRef]
- Sun, C.Y.; Chang, S.C.; Wu, M.S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. P-cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of nadph oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef]
- Cerini, C.; Dou, L.; Anfosso, F.; Sabatier, F.; Moal, V.; Glorieux, G.; De Smet, R.; Vanholder, R.; Dignat-George, F.; Sampol, J.; et al. P-cresol, a uremic retention solute, alters the endothelial barrier function in vitro. Thromb. Haemost. 2004, 92, 140–150. [Google Scholar] [PubMed]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.; Claes, K.; Bammens, B.; de Loor, H.; Viaene, L.; Verbeke, K.; Kuypers, D.; Vanrenterghem, Y.; Evenepoel, P. P-cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Karras, A.; Haymann, J.P.; Bozec, E.; Metzger, M.; Jacquot, C.; Maruani, G.; Houillier, P.; Froissart, M.; Stengel, B.; Guardiola, P.; et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension 2012, 60, 1451–1457. [Google Scholar] [CrossRef]
- Moe, S.M.; Chen, N.X. Mechanisms of vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2008, 19, 213–216. [Google Scholar] [CrossRef]
- Cecelja, M.; Chowienczyk, P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension 2009, 54, 1328–1336. [Google Scholar] [CrossRef]
- Schram, M.T.; Henry, R.M.; van Dijk, R.A.; Kostense, P.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Westerhof, N.; Stehouwer, C.D. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: The hoorn study. Hypertension 2004, 43, 176–181. [Google Scholar] [CrossRef]
- Agnoletti, D.; Mansour, A.S.; Zhang, Y.; Protogerou, A.D.; Ouerdane, S.; Blacher, J.; Safar, M.E. Clinical interaction between diabetes duration and aortic stiffness in type 2 diabetes mellitus. J. Hum. Hypertens. 2017, 31, 189–194. [Google Scholar] [CrossRef]
- McEniery, C.M.; McDonnell, B.J.; So, A.; Aitken, S.; Bolton, C.E.; Munnery, M.; Hickson, S.S.; Yasmin; Maki-Petaja, K.M.; Cockcroft, J.R.; et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension 2009, 53, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Peyster, E.; Chen, J.; Feldman, H.I.; Go, A.S.; Gupta, J.; Mitra, N.; Pan, Q.; Porter, A.; Rahman, M.; Raj, D.; et al. Inflammation and arterial stiffness in chronic kidney disease: Findings from the cric study. Am. J. Hypertens. 2017, 30, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.; Van Kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am. J. Kidney Dis. 2009, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Mare, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Vesey, D.A.; Coombes, J.S.; Weston, K.S.; Hawley, C.M.; McWhinney, B.C.; Ungerer, J.P.; et al. Protein-bound uremic toxins, inflammation and oxidative stress: A cross-sectional study in stage 3–4 chronic kidney disease. Arch. Med. Res. 2014, 45, 309–317. [Google Scholar] [CrossRef]
- Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Lin, Y.L.; Tsai, J.P.; Hsu, B.G. Association between serum indoxyl sulfate levels and endothelial function in non-dialysis chronic kidney disease. Toxins 2019, 11, 589. [Google Scholar] [CrossRef]
- Wang, J.H.; Lee, C.J.; Chen, M.L.; Yang, C.F.; Chen, Y.C.; Hsu, B.G. Association of serum osteoprotegerin levels with carotid-femoral pulse wave velocity in hypertensive patients. J. Clin. Hypertens. 2014, 16, 301–308. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 esc/esh guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 118) | Control Group (n = 69) | High Arterial Stiffness Group (n = 49) | p |
|---|---|---|---|---|
| Carotid–femoral PWV (m/s) | 9.63 ± 2.55 | 7.83 ± 1.30 | 12.16 ± 1.48 | <0.001 * |
| Age (years) | 63.05 ± 13.28 | 61.32 ± 13.74 | 65.49 ± 12.34 | 0.093 |
| Female, n (%) | 59 (50.0) | 36 (52.2) | 23 (46.9) | 0.575 |
| Body mass index (kg/m2) | 24.92 ± 5.13 | 24.92 ± 5.45 | 24.92 ± 4.69 | 0.994 |
| Hemodialysis duration (months) | 56.02 (24.60–111.45) | 80.40 (22.38–133.80) | 45.72 (26.22–74.50) | 0.069 |
| Diabetes mellitus, n (%) | 55 (46.6) | 19 (27.5) | 36 (73.5) | <0.001 * |
| Hypertension, n (%) | 66 (55.9) | 34 (49.3) | 32 (65.3) | 0.084 |
| Systolic blood pressure (mmHg) | 142.13 ± 25.64 | 138.07 ± 26.99 | 147.84 ± 22.38 | 0.021 * |
| Diastolic blood pressure (mmHg) | 76.02 ± 15.61 | 76.17 ± 16.29 | 75.29 ± 15.17 | 0.765 |
| Heart rate (beats per minute) | 75.37 ± 12.79 | 76 ± 13.17 | 74.49 ± 12.32 | 0.629 |
| Blood urea nitrogen (mg/dL) | 60.36 ± 14.75 | 59.77 ± 13.64 | 61.20 ± 16.31 | 0.604 |
| Creatinine (mg/dL) | 9.17 ± 1.99 | 9.47 ± 1.98 | 8.73 ± 1.94 | 0.047 * |
| Urea reduction rate | 0.74 ± 0.04 | 0.74 ± 0.05 | 0.73 ± 0.04 | 0.501 |
| Kt/V (Gotch) | 1.35 ± 0.17 | 1.36 ± 0.19 | 1.33 ± 0.16 | 0.396 |
| Total cholesterol (mg/dL) | 143.19 ± 35.25 | 146.35 ± 38.11 | 138.73 ± 30.60 | 0.244 |
| Triglyceride (mg/dL) | 113.00 (86.75–178.75) | 109.00 (86.50–199.00) | 121.00 (85.00–174.50) | 0.785 |
| Glucose (mg/dL) | 136.50 (113.75–177.00) | 132.00 (110.50–162.00) | 143.00 (119.50–206.00) | 0.042 * |
| Total calcium (mg/dL) | 8.96 ± 0.75 | 8.94 ± 0.76 | 8.99 ± 0.75 | 0.732 |
| Phosphorus (mg/dL) | 4.65 ± 1.32 | 4.69 ± 1.35 | 4.61 ± 1.31 | 0.746 |
| Intact parathyroid hormone (pg/mL) | 186.50 (66.60–353.35) | 211.70 (101.10–413.15) | 136.80 (44.40–281.75) | 0.098 |
| C-reactive protein (mg/dL) | 0.34 (0.09–0.95) | 0.24 (0.08–0.86) | 0.57 (0.13–1.09) | 0.029 * |
| Total p-cresyl sulfate (mg/L) | 16.57 ± 9.07 | 13.95 ± 5.93 | 20.26 ± 11.27 | <0.001 * |
| Angiotensin receptor blocker, n (%) | 35 (29.7) | 19 (27.) | 16 (32.7) | 0.549 |
| β-blocker, n (%) | 39 (33.1) | 22 (31.9) | 17 (34.7) | 0.749 |
| Calcium channel blocker, n (%) | 46 (39.0) | 29 (42.0) | 17 (34.7) | 0.421 |
| Statin, n (%) | 19 (16.1) | 8 (11.6) | 11(22.4) | 0.114 |
| Fibrate, n (%) | 13 (11.0) | 9 (13.0) | 4 (8.2) | 0.404 |
| Variables | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Presence of diabetes mellitus | 4.095 | 1.429–11.739 | 0.009 * |
| Total p-cresyl sulfate, 1 mg/L | 1.072 | 1.002–1.147 | 0.043 * |
| Age, 1 year | 1.026 | 0.987–1.066 | 0.191 |
| Sex (female) | 0.610 | 0.223–1.690 | 0.336 |
| C-reactive protein, 0.1 mg/dL | 1.822 | 0.831–3.997 | 0.134 |
| Glucose, 1 mg/dL | 1.004 | 0.997–1.011 | 0.286 |
| Creatinine, 1 mg/dL | 0.945 | 0.710–1.259 | 0.700 |
| Systolic blood pressure, 1 mmHg | 1.002 | 0.983–1.022 | 0.838 |
| Heart rate, 1 beat per minute | 0.991 | 0.954–1.030 | 0.657 |
| Hemodialysis duration, 1 month | 0.997 | 0.988–1.005 | 0.433 |
| Variables | Central PWV (m/s) | ||||
|---|---|---|---|---|---|
| Simple Regression | Multivariate Regression | ||||
| r | p | Beta | Adjusted R2 Change | p | |
| Age (years) | 0.078 | 0.402 | - | - | - |
| Female sex | −0.085 | 0.357 | - | ||
| Body mass index (kg/m2) | 0.056 | 0.545 | - | - | - |
| Log-HD duration (months) | −0.255 | 0.005 * | - | - | - |
| Diabetes mellitus | 0.538 | <0.001 * | 0.446 | 0.283 | <0.001 * |
| Hypertension | 0.081 | 0.381 | - | - | - |
| Systolic blood pressure (mmHg) | 0.263 | 0.004 * | - | - | - |
| Diastolic blood pressure (mmHg) | 0.055 | 0.551 | - | - | - |
| Heart rate (beats per minute) | −0.112 | 0.228 | |||
| Blood urea nitrogen (mg/dL) | 0.016 | 0.867 | - | - | - |
| Creatinine (mg/dL) | −0.094 | 0.313 | - | - | - |
| Urea reduction rate | −0.099 | 0.285 | - | - | - |
| Kt/V (Gotch) | −0.104 | 0.264 | - | - | - |
| Total cholesterol (mg/dL) | −0.068 | 0.462 | - | - | - |
| Log-triglyceride (mg/dL) | −0.015 | 0.872 | - | - | - |
| Log-glucose (mg/dL) | 0.198 | 0.031 * | - | - | - |
| Total calcium (mg/dL) | 0.028 | 0.763 | - | - | - |
| Phosphorus (mg/dL) | 0.065 | 0.487 | - | - | - |
| Log-iPTH (pg/mL) | −0.088 | 0.341 | - | - | - |
| Log-CRP (mg/dL) | 0.135 | 0.144 | - | - | - |
| Total p-cresyl sulfate (mg/L) | 0.382 | <0.001 * | 0.174 | 0.028 | 0.018 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-H.; Wang, C.-H.; Kuo, C.-H.; Lin, Y.-L.; Tsai, J.-P.; Hsu, B.-G. Serum P-Cresyl Sulfate Is a Predictor of Central Arterial Stiffness in Patients on Maintenance Hemodialysis. Toxins 2020, 12, 10. https://doi.org/10.3390/toxins12010010
Lai Y-H, Wang C-H, Kuo C-H, Lin Y-L, Tsai J-P, Hsu B-G. Serum P-Cresyl Sulfate Is a Predictor of Central Arterial Stiffness in Patients on Maintenance Hemodialysis. Toxins. 2020; 12(1):10. https://doi.org/10.3390/toxins12010010
Chicago/Turabian StyleLai, Yu-Hsien, Chih-Hsien Wang, Chiu-Huang Kuo, Yu-Li Lin, Jen-Pi Tsai, and Bang-Gee Hsu. 2020. "Serum P-Cresyl Sulfate Is a Predictor of Central Arterial Stiffness in Patients on Maintenance Hemodialysis" Toxins 12, no. 1: 10. https://doi.org/10.3390/toxins12010010
APA StyleLai, Y.-H., Wang, C.-H., Kuo, C.-H., Lin, Y.-L., Tsai, J.-P., & Hsu, B.-G. (2020). Serum P-Cresyl Sulfate Is a Predictor of Central Arterial Stiffness in Patients on Maintenance Hemodialysis. Toxins, 12(1), 10. https://doi.org/10.3390/toxins12010010







