Botulinum Toxin a Valuable Prophylactic Agent for Migraines and a Possible Future Option for the Prevention of Hormonal Variations-Triggered Migraines
Abstract
1. Introduction
2. Material and Methods
3. Mechanism of Migraine Development
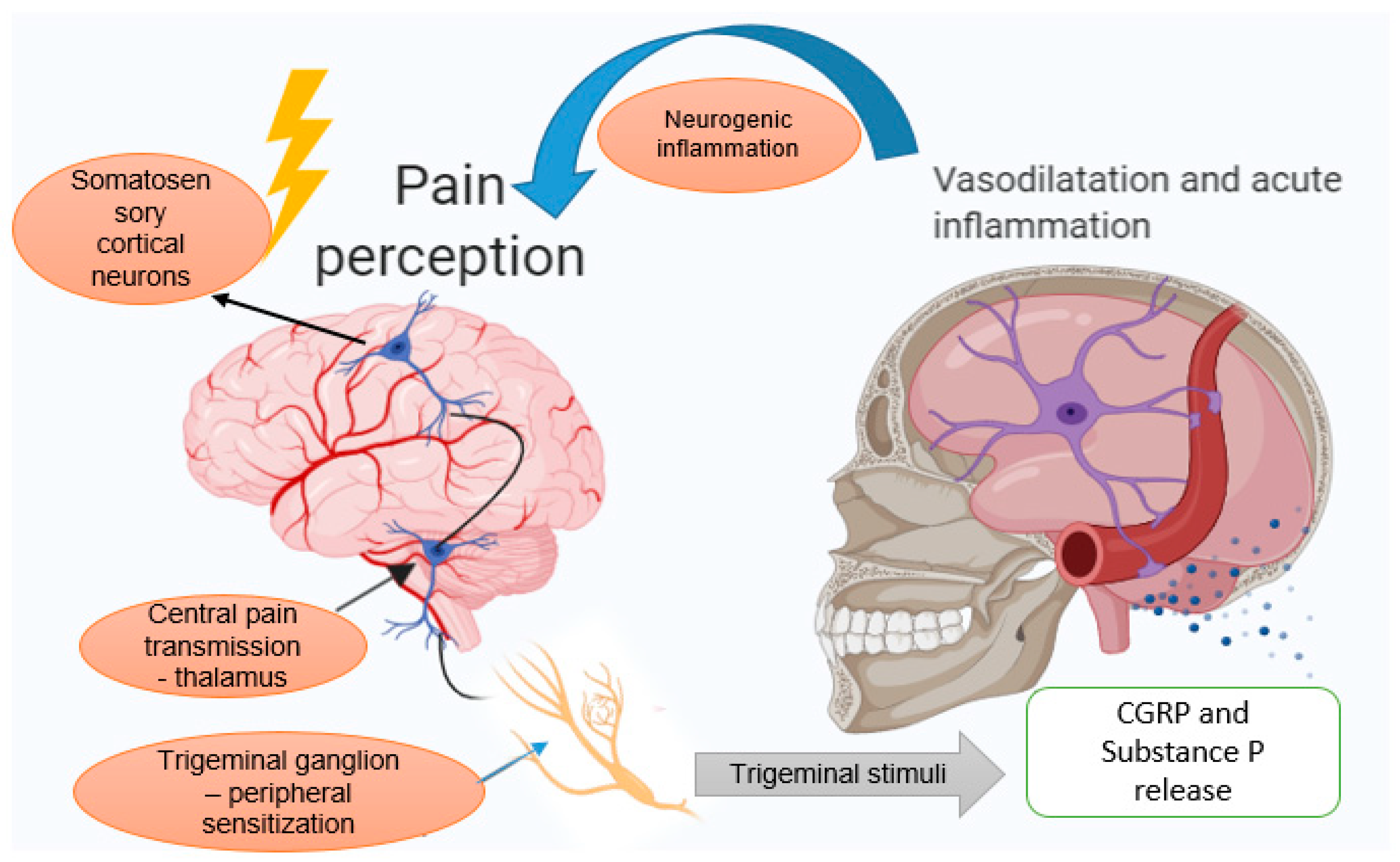
3.1. The Trigeminovascular System
3.2. Neurogenic Inflammation Theory
4. Migraine Induced by Hormonal Variations
5. Botulinum Toxin
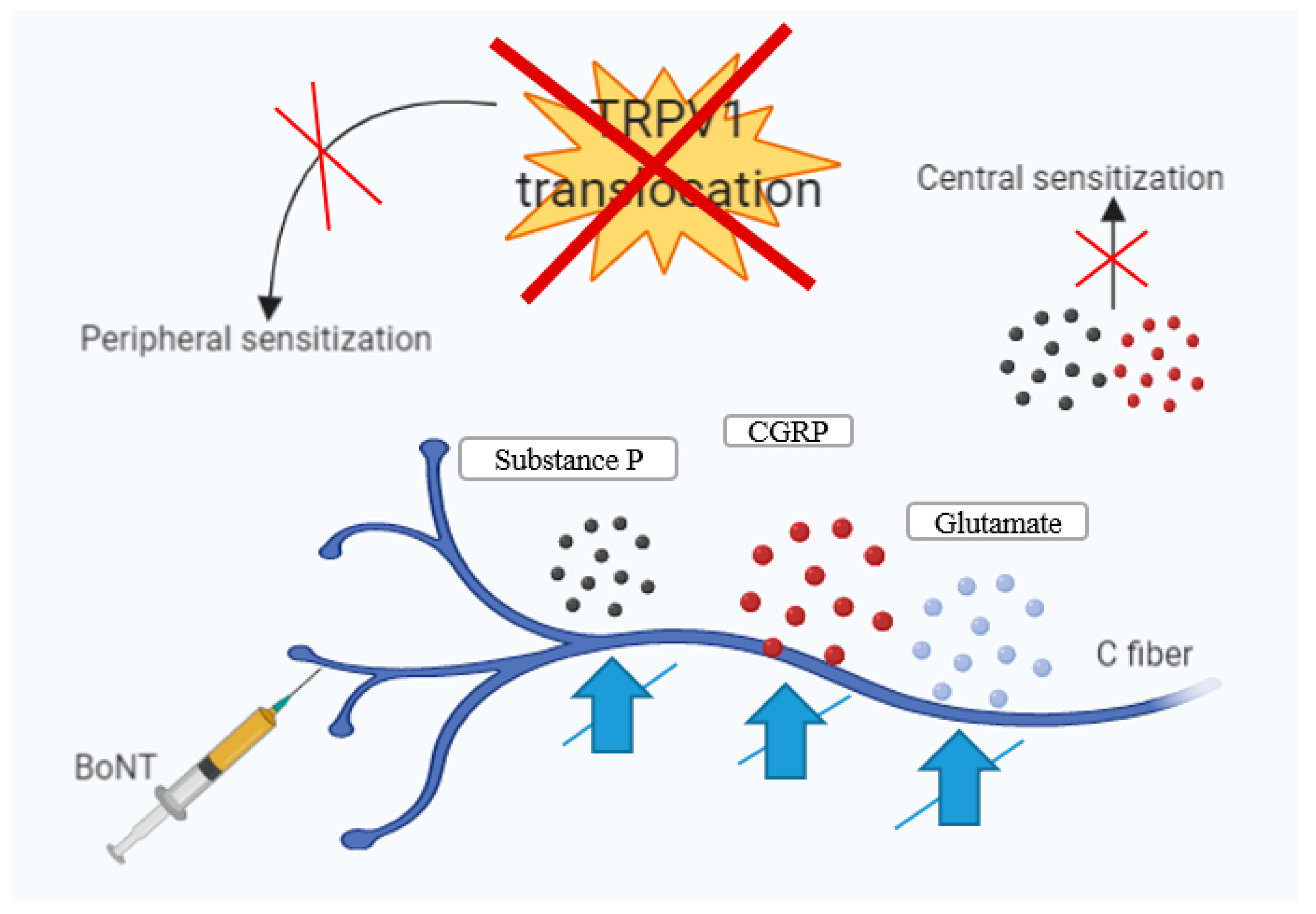
6. BoNT-A—Mechanisms of Action in Chronic Migraine
7. Results and Discussion—Clinical Studies of BoNT-A on Migraine
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACH | Acetylcholine |
| BONT | Botulinum toxin |
| BONT-A | Botulinum toxin serotype A |
| BONT-B | Botulinum toxin serotype B |
| CGRP | Calcitonin gene-related peptide |
| CM | Chronic migraine |
| CNS | Central nervous system |
| DA | Dalton |
| E2 | Estradiol |
| EM | Episodic migraine |
| FDA | Food and Drug Administration |
| FHM | Familial hemiplegic migraine |
| FSH | Follicular-stimulant hormone |
| HC | Heavy chain |
| IL | Interleukin |
| LC | Light chain |
| MA | Migraine with aura |
| MIDAS | Migraine Disability Assessment |
| MO | Migraine without aura |
| MU | Mouse Units |
| NO | Nitric Oxide |
| NOS | Nitric oxide synthase |
| NSAID | Non-steroidal anti-inflammatory drug |
| PREEMPT | Phase III Research Evaluating Migraine Prophylaxis Therapy |
| QOL | Quality of Life |
| SNAP-25 | Synaptosomal-associated protein-25 kDa |
| SNARE | Soluble NSF attachment protein receptor |
| SP | Substance P |
| TNF-A | Tumor necrosis factor alpha |
| TRP | Transient receptor potential channel |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TRVP1 | Transient receptor potential cation channel subfamily V member 1 |
| VAMP | Vesicle-associated membrane protein |
| WHO | World Health Organization |
References
- Ferrari, M.D.; Klever, R.R.; Terwindt, G.M.; Ayata, C.; van der Maagdenberg, A.M. Migraine pathophysiology: Lessons from mouse models and human genetics. Neurology 2015, 14, 65–80. [Google Scholar] [CrossRef]
- Lipton, R.B.; Steward, W.F.; Diamond, S.; Diamond, M.L.; Reed, M. Prevalence and Burden of Migraine in the United States: Data From the American Migraine Study II. Headache 2001, 41, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Puri, V.; Puri, S. Serotonin and CGRP in Migraine. Ann. Neurosci. 2012, 19, 88–94. [Google Scholar] [CrossRef]
- Barbanti, P.; Ferroni, P. Onabotulinum toxin A in the treatment of chronic migraine: Patient selection and special considerations. J. Pain Res. 2017, 10, 2319. [Google Scholar] [CrossRef] [PubMed]
- Burch, R. Migraine and Tension-Type Headache: Diagnosis and Treatment. Med. Clin. N. Am. 2019, 103, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Kors, E.; Haan, J.; Ferrari, M. Migraine genetics. Curr. Pain Headache Rep. 2003, 7, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.M. Brain hyperexcitability: The basis for antiepileptic drugs in migraine prevention. Headache 2005, 45, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Fuh, J.L.; Lu, S.R.; Juang, K.D.; Wang, P.H. Migraine Prevalence During Menopausal Transition. Headache 2003, 43, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Merriam, G.R. Estrogens, progestins, and headache. Neurology 1991, 41, 775–793. [Google Scholar] [CrossRef] [PubMed]
- Somerville, B.W. Estrogen withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology 1975, 25, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Neri, I.; Granella, F.; Nappi, R.; Manzoni, G.C.; Facchinetti, F.; Genazzani, A.R. Characteristics of headache at menopause: A clinico-epidemiologic study. Maturitas 1993, 17, 31–37. [Google Scholar] [CrossRef]
- Whitty, C.W.; Hockaday, J.M. Migraine: A follow-up study of 92 patients. BMJ 1968, 1, 735–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stewart, W.F.; Lipton, R.B.; Celentano, D.D.; Reed, M.L. Prevalence of migraine headache in the United States: Relation to age, income, race and other sociodemographic factors. JAMA 1992, 267, 64–69. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A. Migraine and the menopause. Br. Menopause Soc. J. 2006, 12, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, K.; Couturier, E.G.M.; MaassenVanDenBrink, A. Migraine and perimenopause. Maturitas 2014, 78, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Merriam, G. Sex hormones and headache 1999 (menstrual migraine). Neurology 1999, 53, S3–S13. [Google Scholar] [CrossRef]
- Calhoun, A.; Sutapa, F. Elimination of Menstrual-Related Migraine Beneficially Impacts Chronification and Medication Overuse. Headache 2008, 48, 1186–1193. [Google Scholar] [CrossRef]
- Martin, V.; Wernke, S.; Mandell, K.; Ramadan, N.; Kao, L.; Bean, J.; Liu, J.; Zoma, W.; Rebar, R. Defining the relationship between ovarian hormones and migraine headache. Headache 2005, 45, 1190–1201. [Google Scholar] [CrossRef]
- Lipton, R.B.; Bigal, M.E.; Diamond, M.; Freitag, F.; Reed, M.L.; Stewart, W.F. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007, 68, 343–349. [Google Scholar] [CrossRef]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Parikh, S.K.; Young, W.B. Migraine: Stigma in Society. Curr. Pain Headache Rep. 2019, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Afra, J.; Frese, A.; Goadsby, P.J.; Linde, M.; May, A.; Sandor, P.S. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force. Eur. J. Neurol. 2009, 16, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Linde, M.; Mulleners, W.M.; Chronicle, E.P.; McCrory, D.C. Gabapentin or pregabalin for the prophylaxis of episodic migraine in adults. Cochrane Database Syst. Rev. 2013, 6, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.; Keam, S.J. Escitalopram. Drugs 2005, 65, 2379. [Google Scholar] [CrossRef] [PubMed]
- Tarlaci, S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin. Neuropharmacol. 2009, 32, 254–2588. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.E.; Olson, R.D.; Cusack, B.J. Randomized, controlled trial of phytoestrogen in the prophylactic treatment of menstrual migraine. Biomed. Pharmacother. 2002, 56, 283–288. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Balan, A.; Scarneciu, I.; Barabas, B.; Ples, L. Therapeutic Approaches of Botulinum Toxin in Gynecology. Toxins 2018, 10, 169. [Google Scholar] [CrossRef]
- Sarchielli, P.; Mancini, M.L.; Calabresi, P. Practical Considerations for the Treatment of Elderly Patients with Migraine. Drugs Aging. 2006, 23, 461–489. [Google Scholar] [CrossRef]
- Ramachandran, R.; Yaksh, T.L. Therapeutic use of botulinum toxin in migraine: Mechanisms of action. Br. J. Pharmacol. 2014, 171, 4177–4192. [Google Scholar] [CrossRef]
- Wheeler, A.H. Botulinum Toxin A, Adjunctive Therapy for Refractory Headaches Associated with Pericranial Muscle Tension. Headache 2003, 38, 468–471. [Google Scholar] [CrossRef]
- Brin, M.F. Botulinum toxin: Chemistry, pharmacology, toxicity, and immunology. Muscle Nerve 1997, 20, 146–168. [Google Scholar] [CrossRef]
- Nigam, P.K.; Anjana, N. Botulinum toxin. Indian J. Dermatol. 2010, 55, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon 2018, 147, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Eleopra, R. Clinical use of non-a botulinum toxins: Botulinum toxin type B. Neurotox. Res. 2006, 9, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Peroutka, S.J. Neurogenic inflammation and migraine: Implications for the therapeutics. Mol. Interv. 2005, 5, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Ashina, M. Emerging migraine treatments and drug targets. Trends Pharmacol. Sci. 2011, 32, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kainz, V.; Zhao, J.; Strassman, A.M.; Levy, D. Vascular ERK mediates migraine-related sensitization of meningeal nociceptors. Ann. Neurol. 2013, 73, 741–750. [Google Scholar] [CrossRef]
- May, A.; Goadsb, P.J. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J. Cereb. Blood Flow Metab. 1999, 19, 115–127. [Google Scholar] [CrossRef]
- McNaughton, F.L.; Feindel, W.H. Innervation of Intracranial Structures: A Reappraisal. In Physiological Aspects of Clinical Neurology; Rose, F.C., Ed.; Blackwell Science: Hoboken, NJ, USA, 1977; pp. 279–293. [Google Scholar]
- Ray, B.S.; Wolff, H.G. Experimental studies on headache; pain-sensitive structures of the head and their significance in headache. Arch. Surg. 1940, 41, 813–856. [Google Scholar] [CrossRef]
- Pietrobon, D.; Moskowitz, M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013, 75, 365–391. [Google Scholar] [CrossRef]
- Tajti, J.; Vecsei, L. The mechanism of peripheral and central sensitization in migraine. A literature review. Neuropsychopharmacol. Hung. 2009, 11, 15–21. [Google Scholar] [PubMed]
- Burstein, R.; Zhang, X.; Levy, D.; Aoki, K.R.; Brin, M.F. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia 2014, 34, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Strassman, A.M.; Raymond, S.A.; Burstein, R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996, 384, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Mathew, N.T. Pathophysiology of chronic migraine and mode of action of preventive medications. Headache 2011, 51, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Strassman, A.M. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J. Neurophysiol. 2002, 88, 3021–3031. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Burstein, R.; Kainz, V.; Jakubowski, M.; Strassman, A.M. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain 2007, 130, 166–176. [Google Scholar] [CrossRef]
- Eftekhari, S.; Warfvinge, K.; Blixt, F.W.; Edvinsson, L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J. Pain 2013, 14, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Strassman, A.M.; Burstein, R.; Levy, D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J. Pharmacol. Exp. Ther. 2007, 322, 806–812. [Google Scholar] [CrossRef]
- Roch, M.; Messlinger, K.; Kulchitsky, V.; Tichonovich, O.; Azev, O.; Koulchitsky, S. Ongoing activity in trigeminal wide-dynamic range neurons is driven from the periphery. Neuroscience 2007, 150, 681–691. [Google Scholar] [CrossRef]
- Burstein, R.; Yamamura, H.; Malick, A.; Strassman, A.M. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol. 1998, 79, 964–982. [Google Scholar] [CrossRef]
- Schueler, M.; Messlinger, K.; Dux, M.; Neuhuber, W.L.; De, C.R. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain 2013, 154, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Blumberg, P.M.; Szallasi, A. Endovanilloid signaling in pain. Curr. Opin. Neurobiol. 2002, 12, 372–379. [Google Scholar] [CrossRef]
- Uddman, R.; Tajti, J.; Hou, M.; Sundler, F.; Edvinsson, L. Neuropeptide expression in the human trigeminal nucleus caudalis and in the cervical spinal cord C1 and C2. Cephalalgia 2002, 22, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.; Uddman, R.; Ekman, R.; Olesen, J.; Ottosson, A.; Edvinsson, L. Distribution and effects of neuropeptide Y, vasoactive intestinal peptide, substance P, and calcitonin gene-related peptide in human middle meningeal arteries: Comparison with cerebral and temporal arteries. Peptides 1992, 13, 527–536. [Google Scholar] [CrossRef]
- Arulmani, U.; MassenVanDenBrink, A.; Villalon, C.M.; Saxena, P.R. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur. J. Pharmacol. 2004, 500, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Han, D. Association of serum levels of calcitonin gene-related peptide and cytokines during migraine attacks. Ann. Indian Acad. Neurol. 2019, 22, 277–281. [Google Scholar] [CrossRef]
- Mayberg, M.R.; Zervas, N.T.; Moskowitz, M.A. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J. Comp. Neurol. 1984, 223, 46–56. [Google Scholar] [CrossRef]
- Edwards, R.M.; Stack, E.J.; Trizna, W. Calcitonin gene-related peptide stimulates adenylate cyclase and relaxes intracerebral arterioles. J. Pharmacol. Exp. Ther. 1991, 257, 1020–1024. [Google Scholar]
- Goadsby, P.J.; Edvinsson, L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993, 33, 48–56. [Google Scholar] [CrossRef]
- Ashina, M.; Bendtsen, L.; Jensen, R.; Schifter, S.; Olesen, J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain 2000, 86, 133–138. [Google Scholar] [CrossRef]
- Buchanan, J.E.; Phillis, J.W. The role of nitric oxide in the regulation of cerebral blood flow. Brain Res. 1993, 610, 248–255. [Google Scholar] [CrossRef]
- Strecker, T.; Dux, M.; Messlinger, K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephali promoting increases in meningeal blood flow. J. Vasc. Res. 2002, 39, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Buture, A.; Gooriah, R.; Nimeri, R.; Ahmed, F. Current Understanding on Pain Mechanism in Migraine and Cluster Headache. Anesth. Pain Med. 2016, 6, e35190. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, M. On the possible relation of spreading cortical depression to classical migraine. Cephalalgia 1985, 5, 47–51. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, A.F.M.; Granziera, C.; Tuch, D.S.; Snyder, J.; Vincent, M.; Hadjikhani, N. Interictal alterations of the trigeminal somatosensory pathway and PAG in migraine. Neuroreport 2007, 18, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.; Silberstein, S. Central sensitization theory of migraine: Clinical implications. Headache 2006, 46, S182–S191. [Google Scholar] [CrossRef] [PubMed]
- Buzzi, M.G.; Moskowitz, M.A. The trigeminovascular system and migraine. Pathol. Biol. 1992, 40, 313–317. [Google Scholar] [PubMed]
- Gao, Y.J.; Ji, R.R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef]
- Stewart, W.F.; Wood, C.; Reed, M.L.; Roy, J.; Lipton, R.B. Cumulative lifetime migraine incidence in women and men. Cephalalgia 2008, 28, 1170–1178. [Google Scholar] [CrossRef]
- MacGregor, E.A. Menstrual migraine: A clinical review. J. Fam. Plann. Reprod. Health Care 2007, 33, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Couturier, E.G.; Bomhof, M.A.; Neven, A.K.; Van Duijn, N.P. Menstrual migraine in a representative Dutch population sample: Prevalence, disability and treatment. Cephalalgia 2003, 23, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Ricci, S.; Degan, D.; Carolei, A. Migraine in women: The role of hormones and their impact on vascular diseases. J. Headache Pain 2012, 13, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Wöber, C.; Brannath, W.; Schmidt, K.; Kapitan, M.; Rudel, E.; Wessely, P.; Wöber-Bingöl, C. PAMINA Study Group. Cephalalgia 2007, 27, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Sulak, P.J. The perimenopause: A critical time in a woman’s life. Int. J. Fertil Menopausal Stud. 1996, 41, 85–89. [Google Scholar] [PubMed]
- Finocchi, C.; Ferrari, M. Female reproductive steroids and neuronal excitability. Neurol. Sci. 2011, 32, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A. Migraine headache in perimenopausal and menopausal women. Curr. Pain Headache Rep. 2009, 13, 399–403. [Google Scholar] [CrossRef]
- Ebner, S.; Dunbar, M.; McKinnon, R.D. Distinct Roles for PI3K in proliferation and survival of oligodedrocyte progenitor cells. J. Neurosci. Res. 2000, 62, 336–345. [Google Scholar] [CrossRef]
- Couse, J.F.; Lindzey, J.; Grandien, K.; Gustafsson, J.A.; Korach, K.S. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ) messenger ribonucleic acid in the wild-type and ERα–knockout mouse. Endocrinology 1997, 138, 4613–4621. [Google Scholar] [CrossRef]
- Bjornstrom, L.; Sjoberg, M. Estrogen receptor-dependent activation of AP-1 via non-genomic signaling. Nucl. Recept. 2004, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Simpson, D.; Hallett, M.; Ashman, E.; Comella, C.; Green, M.; Gronseth, G. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Nappi, R.E.; Berga, S.L. Migraine and reproductive life. Handbook Clin. Neurol. 2010, 97, 303–322. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.K.; Smith, T.J. Genetic diversity within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. Curr. Top. Microbiol. Immunol. 2013, 364, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.S. Notes on Bacillus botulinus. J. Bacteriol. 1919, 4, 555–570. [Google Scholar] [PubMed]
- Montal, M. Botulinum neurotoxin: A marvel of protein design. Annu. Rev. Biochem. 2010, 79, 591–617. [Google Scholar] [CrossRef] [PubMed]
- Turton, K.; Chaddock, J.A.; Acharya, K.R. Botulinum and tetanus neurotoxins: Structure, function and therapeutic utility. Trends Biochem. Sci. 2002, 27, 552–558. [Google Scholar] [CrossRef]
- Fischer, A.; Montal, M. Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc. Natl. Acad. Sci. USA 2007, 104, 10447–10452. [Google Scholar] [CrossRef]
- Jahn, R.; Scheller, R.H. SNAREs-engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef]
- Sudhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef]
- Dolly, J.O.; Lawrence, G.W.; Meng, J.; Wang, J.; Ovsepian, S.V. Neuro-exocytosis: Botulinum toxins as inhibitory probes and versatile therapeutics. Curr. Opin. Pharmacol. 2009, 9, 326–335. [Google Scholar] [CrossRef]
- Johnson, E.A.; Montecucco, C. Botulism. In Handbook of Clinical Neurology; Andrew, G.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 333–368. [Google Scholar]
- Montecucco, C.; Schiavo, G. Mechanism of action of tetanus and botulinum neurotoxins. Mol. Microbiol. 1994, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Argoff, C.E. A Focused Review on the Use of Botulinum Toxins for Neuropathic Pain. Clin. J. Pain. 2002, 18, S177–S181. [Google Scholar] [CrossRef] [PubMed]
- Sloop, R.R.; Bradley, A.C.; Escutin, R.O. Human response to botulinum toxin injection: Type B compared with type A. Neurology 1997, 49, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Menstrual Migraine. New York Headache Center. Available online: https://nyheadache.com/educational-materials/menstrual-migraine/ (accessed on 19 July 2019).
- Gupta, S.; Amrutkar, D.V.; Mataji, A.; Salmasi, H.; Hay-Schmidt, A.; Sheykhzade, M.; Messlinger, K.; Olesen, J.; Jansen-Olesen, I. Evidence for CGRP re-uptake in rat dura mater encephali. Br. J. Pharmacol. 2010, 161, 1885–1898. [Google Scholar] [CrossRef][Green Version]
- Park, J.H.; Park, H.J. Botulinum toxin for the treatment of neuropathic pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef]
- Takasusuki, T.; Yaksh, T.L. Regulation of spinal substance p release by intrathecal calcium channel blockade. Anesthesiology 2011, 115, 153–164. [Google Scholar] [CrossRef]
- Baulmann, J.; Spitznagel, H.; Herdegen, T.; Unger, T. and Culman, J. Tachykinin receptor inhibition and c-Fos expression in the rat brain following formalin-induced pain. Neuroscience 2000, 95, 813–820. [Google Scholar] [CrossRef]
- Aoki, K.R.; Francis, J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism Relat. Disord. 2011, 17, S28–S33. [Google Scholar] [CrossRef]
- Durham, P.L.; Cady, R.; Cady, R. Regulation of Calcitonin Gene-Related Peptide Secretion From Trigeminal Nerve Cells by Botulinum Toxin Type A: Implications for Migraine Therapy. Headache 2004, 44, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K. Therapeutic use of botulinum toxin in pain treatment. Neur. Signal. 2018, 2, NS20180058. [Google Scholar] [CrossRef]
- Sulak, M.A.; Ghosh, M.; Sinharoy, P.; Andrei, S.R. Modulation of TRPA1 channel activity by Cdk5 in sensory neurons. Channels 2017, 12, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Cheng, J.; Zhuang, Y.; Qu, W.; Muir, J.; Liang, H.; Zhang, D. Botulinum toxin type A reduces hyperalgesia and TRPV1 expression in rats with neuropathic pain. Pain Med. 2013, 14, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Dhaliwal, H.P.; Kukreja, R.V.; Singh, B.R. The Botulinum Toxin as a Therapeutic Agent: Molecular Structure and Mechanism of Action in Motor and Sensory Systems. Semin. Neurol. 2016, 36, 10–19. [Google Scholar] [CrossRef]
- Lawrence, G.W.; Ovsepian, S.V.; Wang, J.; Aoki, K.R.; Dolly, J.O. Extravesicular intraneuronal migration of internalized botulinum neurotoxins without detectable inhibition of distal neurotransmission. Biochem. J. 2012, 441, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Restani, L.; Giribaldi, F.; Manich, M.; Bercsenyi, K.; Menendez, G.; Rossetto, O.; Caleo, M.; Schiavo, G. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 2012, 8, e1003087. [Google Scholar] [CrossRef]
- Restani, L.; Antonucci, F.; Gianfranceschi, L.; Rossi, C.; Rossetto, O.; Caleo, M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). J. Neurosci. 2011, 31, 15650–15659. [Google Scholar] [CrossRef]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-distance retrograde effects of botulinum neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef]
- Favre-Guilmard, C.; Auguet, M.; Chabrier, P.E. Different antinociceptive effects of botulinum toxin type A in inflammatory and peripheral polyneuropathic rat models. Eur. J. Pharmacol. 2009, 617, 48–53. [Google Scholar] [CrossRef]
- Costa, C.; Tozzi, A.; Rainero, I.; Cupini, L.M.; Calabresi, P.; Sarchielli, P. Cortical spreading depression as a target for anti-migraine agents. J. Headache Pain 2013, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Schmieg, N.; Menendez, G.; Schiavo, G.; Terenzio, M. Signalling endo-somes in axonal transport: Travel updates on the molecular highway. Semin. Cell Dev. Biol. 2014, 27, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Bach-Rojecky, L.; Salković-Petrisić, M.; Lacković, Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neu-ropathy: Bilateral effect after unilateral injection. Eur. J. Pharmacol. 2010, 633, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Bach-Rojecky, L.; Lacković, Z. Central origin of the antinocicep-tive action of botulinum toxin type A. Pharmacol. Biochem. Behav. 2009, 94, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Khanijou, S.; Rubino, J.; Aoki, K. Subcutaneous administra-tion of botulinum toxin A reduces formalin- induced pain. Pain 2004, 107, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T.; Goadsby, P.J. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain 2002, 125, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Aurora, S.K.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F.; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double- blind, randomized, placebo- controlled phases of the PREEMPT clinical program. Headache 2010, 50, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Do, T.P.; Hvedstrup, J.; Schytz, H.W. Botulinum toxin: A review of the mode of action in migraine. Acta Neur. Scand. 2018, 137, 442–451. [Google Scholar] [CrossRef]
- Aguila, M.E.R.; Rebbeck, T.; Leaver, A.M.; Lagopoulos, J.; Brennan, P.C.; Hübscher, M.; Refshauge, K.M. The association between clinical characteristics of migraine and brain GABA levels: An explor-atory study. J. Pain 2016, 17, 1058–1067. [Google Scholar] [CrossRef]
- Janis, J.E.; Barker, J.C.; Palettas, M. Targeted Peripheral Nerve-directed Onabotulinumtoxin A Injection for Effective Long-term Therapy for Migraine Headache. Plat. Reconstr. Surg. Glob. Open 2017, 5, e1270. [Google Scholar] [CrossRef]
- Wollina, U. Botulinum Toxin Type A: Anabotulinum A, Onabotulinum A, and Incabotulinum A–Differences and Common Sense. Kosmetische Medizin 2015, 36, 112–114. [Google Scholar]
- Katsarava, Z.; Buse, D.C.; Manack, A.N.; Lipton, R.B. Defining the Differences Between Episodic Migraine and Chronic Migraine. Curr. Pain Headache Rep. 2012, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Escher, C.M.; Paracka, L.; Dressler, D.; Kollewe, K. Botulinum toxin in the management of chronic migraine: Clinical evidence and experience. Ther. Adv. Neurol. Disord. 2017, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T. Migraine and the menopausal transition. Neurol. Sci. 2014, 35, 65–69. [Google Scholar] [CrossRef]
- Kazerooni, R.; Lim, J.; Blake, A.; Lessig, S. IncobotulinumtoxinA for migraine: A retrospective case series. Clin. Ther. 2015, 37, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P.; Pedersen, N.; Staahl, C.; Drewes, A.; Arendt-Nielsen, L. Subcutaneous Botulinum toxin type A reduces capsaicin- induced trigeminal pain and vasomotor reactions in human skin. Pain 2009, 141, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kollewe, K.; Escher, C.M.; Wulff, D.U.; Fathi, D.; Paracka, L.; Mohammadi, B.; Karst, M.; Dressler, D. Long-term treatment of chronic migraine with OnabotulinumtoxinA: Efficacy, quality of life and tolerability in a real-life setting. J. Neural. Transm. 2016, 123, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F.; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010, 30, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; DeGryse, R.E.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F.; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef]
- Hienn, H.; Gonzalez, A. Migraine Headache Prophylaxis. Am. Fam. Phys. 2019, 99, 17–24. [Google Scholar]
- Cady, R.K.; Schreiber, C.P.; Porter, J.A.; Blumenfeld, A.M.; Farmer, K.U. A multi-center double-blind pilot comparison of onabotulinumtoxinA and topiramate for the prophylactic treatment of chronic migraine. Headache 2011, 51, 21–32. [Google Scholar] [CrossRef]
- Binder, W.J.; Brin, M.F.; Blitzer, A.; SChoenrock, L.D.; Pogoda, J.M. Botulinum toxin type A (BOTOX) for treatment of migraine headaches: An open-label study. Otolaryngol. Head Neck Surg. 2000, 123, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.; Mathew, N.; Saper, J.; Jenkins, S. Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache 2000, 40, 445–450. [Google Scholar] [CrossRef]
- Mathew, N.T.; Frishberg, B.M.; Gawel, M.; Dimitrova, R.; Gibson, J.; Turkel, C.; BOTOX CDH Study Group. Botulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: A randomized, double-blind, placebo-controlled trial. Headache 2005, 45, 293–307. [Google Scholar] [CrossRef]
- Hou, M.; Xie, J.F.; Kong, H.P.; Zhang, Y.; Shao, Y.F.; Wang, C.; Ren, W.T.; Cui, G.F.; Xin, L.; Hou, Y.P. Acupoint Injection of Onabotulinumtoxin A for Migraines. Toxins 2015, 7, 4442–4454. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Varon, S.F.; Grosberg, B.; McAllister, P.J.; Freitag, F.; Aurora, S.K.; Dodick, D.W.; Silberstein, S.D.; Diener, H.C.; DeGryse, R.E.; et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine. Neurology 2011, 77, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, M.; McAllister, P.J.; Bajwa, Z.H.; Ward, T.N.; Smith, P.; Burstein, R. Exploding vs. imploding headache in migraine prophylaxis with botulinum toxin A. Pain 2006, 125, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.L.; Kuriyama, A.; Hayashino, Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: A meta-analysis. JAMA 2012, 307, 1736–1745. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Ramón, C.; Larrosa, D.; Alvarez, R.; Riesco, N.; Pascual, J. Long-term experience with onabotulinumtoxinA in the treatment of chronic migraine: What happens after one year? Cephalalgia 2005, 35, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Saper, J.R.; Mathew, N.T.; Loder, E.W.; DeGryse, R.; VanDenburgh, A.M. A Double-Blind, Randomized, Placebo-Controlled Comparison of Botulinum Toxin Type A Injection Sites and Doses in the Prevention of Episodic Migraine. Pain Med. 2007, 8, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Vollmer-Haase, J.; Schwaag, S.; Rahmann, A.; Husstedt, I.W.; Frese, A. Botulinum toxin A in the prophylactic treatment of migraine—A randomized, double-blind, placebo-controlled study. Cephalalgia 2004, 24, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.H.; Satori, R.; Jabbari, B.; Green, J.; Killgore, W.D.; Labutta, R.; Campbell, W.W. Botulinum toxin type-a in the prevention of migraine: A double-blind controlled trial. Aviat. Space Environ. Med. 2007, 78, 113–118. [Google Scholar]
- Petri, S.; Tolle, T.; Straube, A.; Pfaffenrath, V.; Stefenelli, U.; Ceballos-Baumann, A. Botulinum Toxin as Preventive Treatment for Migraine: A Randomized Double-Blind Study. Eur. Neurol. 2009, 62, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Chankrachang, S.; Arayawichanont, A.; Poungvarin, N.; Nidhinandana, S.; Boonkongchuen, P.; Towanabut, S.; Sithinamsuwan, P.; Kongsaengdao, S. Prophylactic Botulinum Type A Toxin Complex (Dysport®) for Migraine Without Aura. Headache 2011, 51, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.S.; Prasad, A.; Singh, M.M.; Sharma, S.; Bala, K. Botulinum toxin type A in prophylactic treatment of migraine. Am. J. Ther. 2006, 13, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Relja, M.; Poole, A.C.; Schoenen, J.; Pascual, J.; Lei, X.; Thompson, C. A multicentre, double-blind, randomized, placebo-controlled, parallel group study of multiple treatments of botulinum toxin type A (BoNTA) for the prophylaxis of episodic migraine headaches. Cephalalgia 2007, 27, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Elkind, A.H.; O’Carroll, P.; Blumenfeld, A.; DeGryse, R.; Dimitrova, R. A series of three sequential, randomized, controlled studies of repeated treatments with botulinum toxin type A for migraine prophylaxis. J. Pain 2006, 7, 688–696. [Google Scholar] [CrossRef]
- Barrientos, N.; Chana, P. Botulinum toxin type A in prophylactic treatment of migraine headaches: A preliminary study. J. Headache Pain 2003, 4, 146–151. [Google Scholar] [CrossRef]
- Freitag, F.G.; Diamond, S.; Diamond, M.; Urban, G. Botulinum Toxin Type A in the treatment of chronic migraine without medication overuse. Headache 2008, 48, 201–209. [Google Scholar] [CrossRef]
- Magalhaes, E.; Menezes, C.; Cardeal, M.; Melo, A. Botulinum toxin type A versus amitriptyline for the treatment of chronic daily migraine. Clin. Neurol. Neurosurg. 2010, 112, 463–466. [Google Scholar] [CrossRef]
- Mathew, N.T.; Jaffri, S.F. A double-blind comparison of onabotulinumtoxina (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: A pilot study. Headache 2009, 49, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Winner, P.; Freeman, M.C.; Spierings, E.L.; Heiring, J.O.; DeGryse, R.E.; Van Denburgh, A.M.; Nolan, M.E.; Turkel, C.C. Onabotulinumtoxin A for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache 2011, 51, 1358–1373. [Google Scholar] [CrossRef] [PubMed]
 |  |  |  |  |  |  |
|---|---|---|---|---|---|---|
| Ophthalmic Disorders | Movement Disorders | Cosmetic Applications | Chronic Pain | Genito-Urinary System Disorders | Gastrointestinal Disorders | Other Conditions |
| Established Indications Of Bont (Approved by FDA) | Tried Applications of Bont | |||||
| Strabismus | Cervical dystonia | Wrinkles | Migraine | Chronic pelvic pain | Achalasia | Cerebral palsy |
| Concomitant misalignment | Oromandibular dystonia | Axillary Hyperhidrosis | Tension type headache | Vulvodynia | Bruxism | Spinal cord injury |
| Restrictive or myogenic strabismus | Torticollis | Lateral canthal lines | Lower back ache | Detrusor-sphincter dyssynergia | Palatal myoclonus | Various brain injuries after trauma |
| Eyelid retraction | Tardive dystonia | Glabellar lines | Myofascial pain | Spasms of perineal musculature | Chronic anal fissures | Upper-limb spasticity |
| Duane’s syndrome | Other focal dystonias | Browlift | Tennis elbow | Dyspareunia | Larynx affections | Hemifacial spasms |
| Non-concomitant Misalignment | Trigeminal neuropathy | Painful bladder syndrome | Temporomandibular joint dysfunction | Stoke-induced spasticity | ||
| Author, Reference | Study Design | No. of Patients | Type of Migraine | Dose of BoNT-A | Results | Follow-Up Period |
|---|---|---|---|---|---|---|
| Saper et al., 2007 [142] | Randomized, Double-Blind, placebo-control study | 232 patients (45 placebo) | EM | 25 U BoNT-A | Both BoNT-A and placebo had similar efficiency, and showed greater reduction of migraine severity | 3 months |
| Evers et al., 2004 [143] | Randomized, Double-Blind, placebo-control study | 60 patients (20 placebo) | EM | 16 U or 100 U BoNT-A | Both BoNT-A and placebo decreased the number of migraine days and the frequency of the attacks | 3 months |
| Vo et al., 2007 [144] | Randomized, Double-Blind, placebo-control study | 32 patients (17 placebo) | EM | 205 U BoNT-A | No significant reduction of migraine frequency and severity was registered The headache pattern index indicated a protective effect for BoNT-A against attacks severity | 3 months |
| Petri et al., 2009 [145] | Randomized, Double-Blind, placebo-control study | 127 patients (63 placebo) | EM | 80–120 U BoNT-A into cervical and pericranial muscles | BoNT-A was not useful as a prophylactic treatment; the reduction of headache did not reach statistically significance | 3 months |
| Chankrachang et al., 2011 [146] | Randomized, Double-Blind, placebo-control study | 128 patients (37 placebo) | EM | 120–240 U BoNT-A | BoNT-A was significantly useful over placebo | 8–12 weeks |
| Anand et al., 2006 [147] | Randomized, Double-Blind, placebo-control study | 32 patients (16 placebo) | EM | 50 U BoNT-A | 75% of patients reported a complete relief of the symptoms | 3 months |
| Relja et al., 2007 [148] | Randomized, Double-Blind, placebo-control study | 515 patients | EM | 75–225 U BoNT-A | Similar results in both groups | 9 months |
| Silberstein et al., 2000 [135] | Randomized, Double-Blind, placebo-control study | 123 (41 placebo) | EM | 25 U or 75 U BoNT-A | Greater results in both groups | 3 months |
| Elkind et al., 2006 [149] | Randomized, Double-Blind, placebo-control study | 182 patients (100 placebo) | EM | 7.5 U–50 U BoNT-A | No improvements in headache were noted, no differences between BoNT-A and placebo | 120 days |
| Barrientos and Chana, 2003 [150] | Randomized, Double-Blind, placebo-control study | 30 patients (15 placebo) | EM | 50 U BoNT-A | The number of attacks per day and headache frequencies were significantly reduced on day 90 | 3 months |
| Freitag et al., 2008 [151] | Randomized, Double-Blind, placebo-control study | 86 patients | CM | 100 U BoNT-A | BoNT-A was superior to placebo for both endpoints | 4 months |
| Cady et al., 2011 [133] | Randomized, Double-Blind, placebo-control study | 59 patients (30 topiramate) | CM | 300 U BoNT-A | Similar results for both BoNT-A and Topiramate | 26 weeks |
| Diener et al., 2010 [131] | Randomized, Double-Blind, placebo-control study | 679 patients (338 placebo) | CM | 155–195 U BoNT-A | All the secondary endpoints were favoured | 32 weeks |
| Binder et al., 2000 [134] | Non-randomized, open-label | 106 patients | CM | −51% of cases—complete response −38% of cases—partial response −70% of cases—improvements were observed after one hour of injection | 3 months | |
| Magalhaes et al., 2010 [152] | Randomized, Double-Blind, placebo-control study | 72 patients (23 amytriptiline) | CM | 250 U BoNT-A | No difference was observed between BoNT-A and amytriptiline effects | 90 days |
| Mathew et al., 2009 [153] | Randomized, Double-Blind, placebo-control study | 60 patients (29 topiramate) | CM | 200 U BoNT-A | Similar results for both groups. BoNT-A and Topiramate showed similar efficiency | 9 months |
| Aurora et al., 2011 [154] | Randomized, Double-Blind, placebo-control study | 1384 patients (696 placebo) | CM | 155–195 U BoNT-A | BoNT-A was efficient in improvement of the total headache days number | 56 weeks |
| Aurora et al., 2010 [130] | Randomized, Double-Blind, placebo-control study | 679 patients (338 placebo) | CM | 155–195 U BoNT-A | BoNT-A was efficient in improvement of the headache days number but no reduction in the migraine episodes was recorded | 24 weeks |
| Lipton et al., 2011 [138] | Randomized, Double-Blind, placebo-control study | 1384 patients (696 placebo) | CM | 155 U BoNT-A | Significantly reduction in headache compared to placebo | 56 weeks |
| Mathew et al., 2005 [136] | Prospective Study | 571 patients | CM | 105–260 U | 50% or more decrease in the frequency of headache days was registered at 180 days | 11 months |
| Dodick et al., 2009 [119] | Randomized, placebo-control study | 1384 patients | CM | 155–195 U | BoNT-A considerable decreased the number of pain days in comparison to placebo. | 24 weeks |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dima, L.; Bălan, A.; Moga, M.A.; Dinu, C.G.; Dimienescu, O.G.; Varga, I.; Neculau, A.E. Botulinum Toxin a Valuable Prophylactic Agent for Migraines and a Possible Future Option for the Prevention of Hormonal Variations-Triggered Migraines. Toxins 2019, 11, 465. https://doi.org/10.3390/toxins11080465
Dima L, Bălan A, Moga MA, Dinu CG, Dimienescu OG, Varga I, Neculau AE. Botulinum Toxin a Valuable Prophylactic Agent for Migraines and a Possible Future Option for the Prevention of Hormonal Variations-Triggered Migraines. Toxins. 2019; 11(8):465. https://doi.org/10.3390/toxins11080465
Chicago/Turabian StyleDima, Lorena, Andreea Bălan, Marius Alexandru Moga, Cătălina Georgeta Dinu, Oana Gabriela Dimienescu, Ioana Varga, and Andrea Elena Neculau. 2019. "Botulinum Toxin a Valuable Prophylactic Agent for Migraines and a Possible Future Option for the Prevention of Hormonal Variations-Triggered Migraines" Toxins 11, no. 8: 465. https://doi.org/10.3390/toxins11080465
APA StyleDima, L., Bălan, A., Moga, M. A., Dinu, C. G., Dimienescu, O. G., Varga, I., & Neculau, A. E. (2019). Botulinum Toxin a Valuable Prophylactic Agent for Migraines and a Possible Future Option for the Prevention of Hormonal Variations-Triggered Migraines. Toxins, 11(8), 465. https://doi.org/10.3390/toxins11080465






