Mycotoxin Contamination of Edible Non-Timber Forest Products in Cameroon
Abstract
1. Introduction
2. Results
2.1. Moisture Content
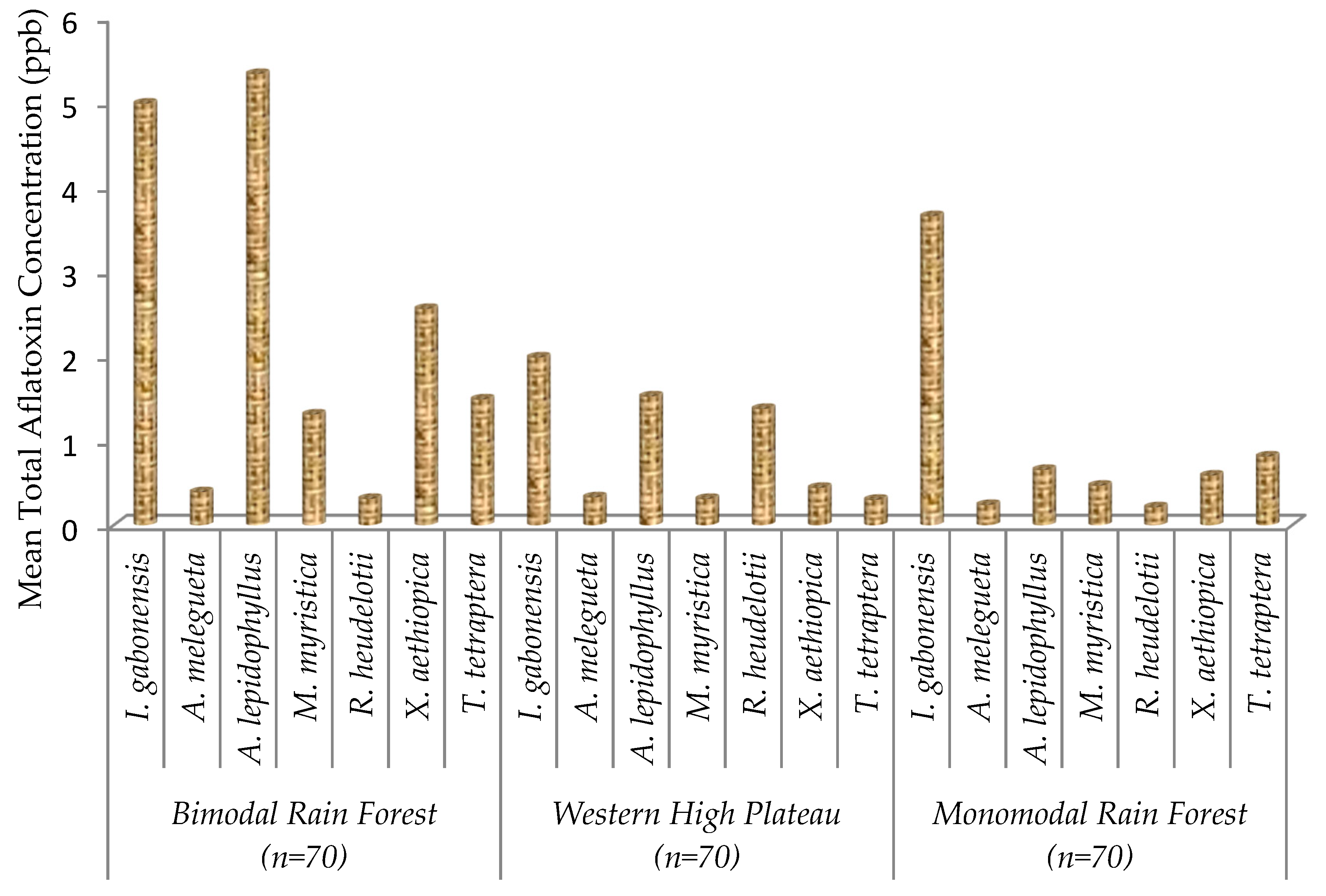
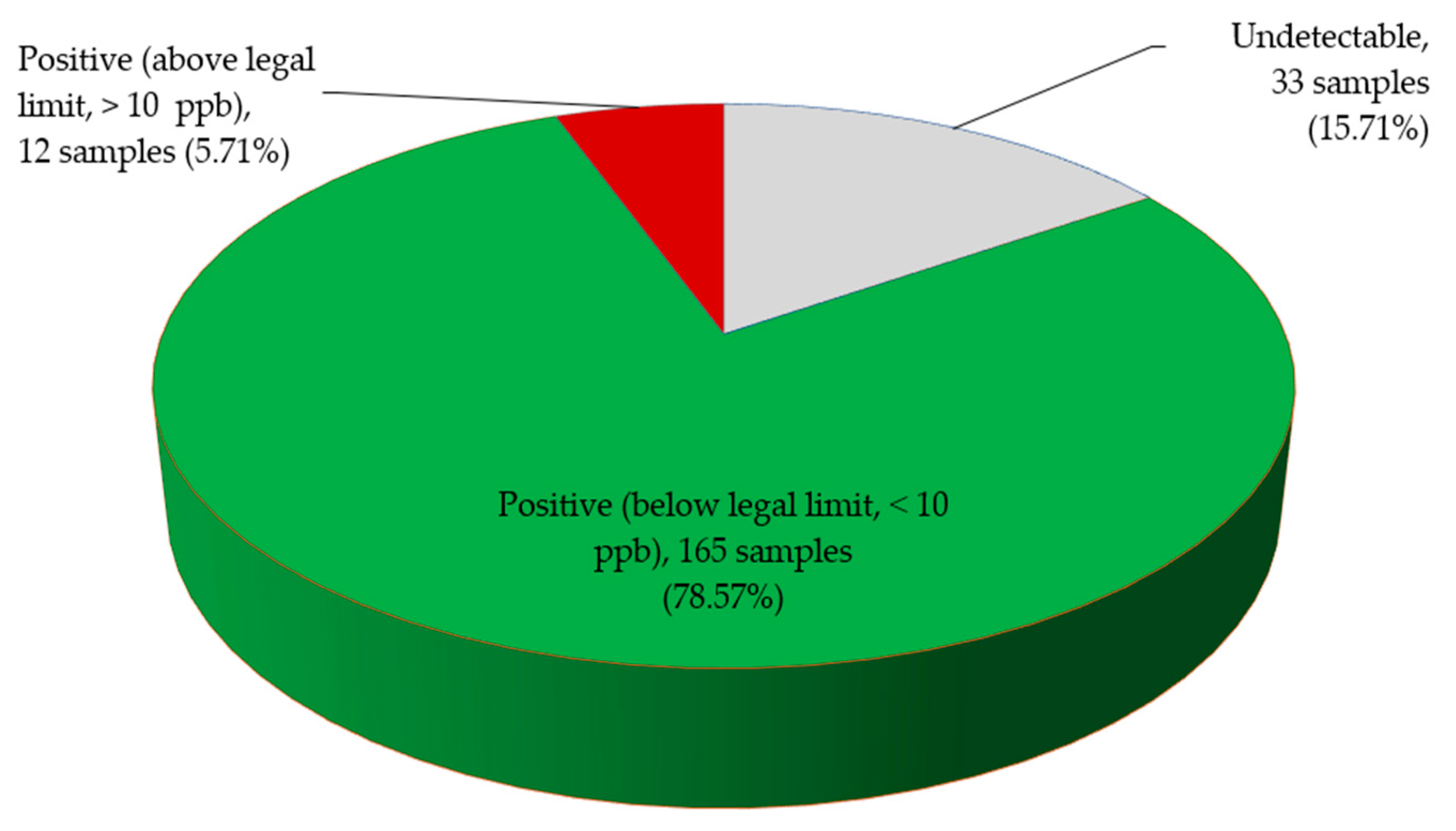
2.2. Total Aflatoxin Contamination of Edible Non-Timber Forest Products
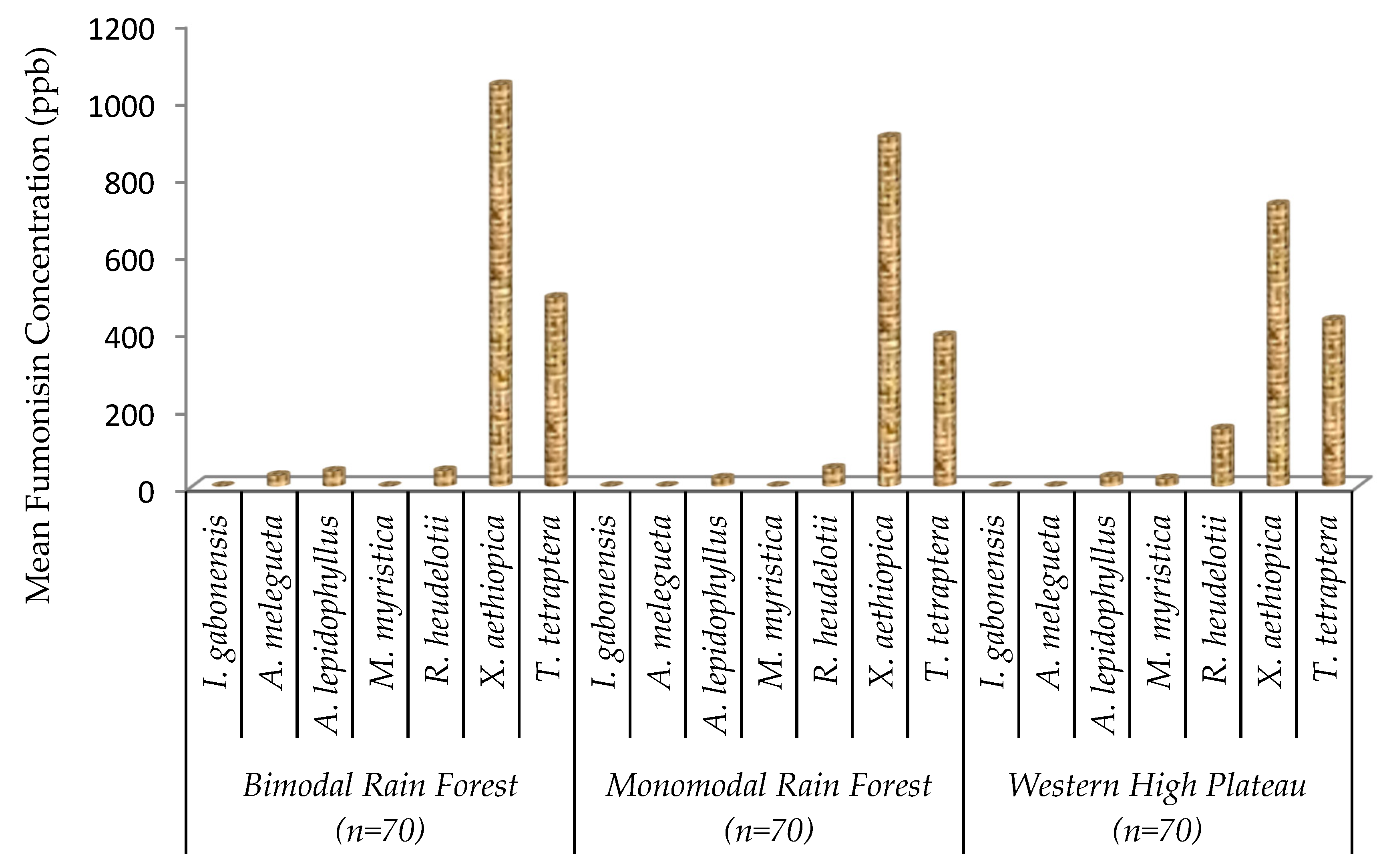
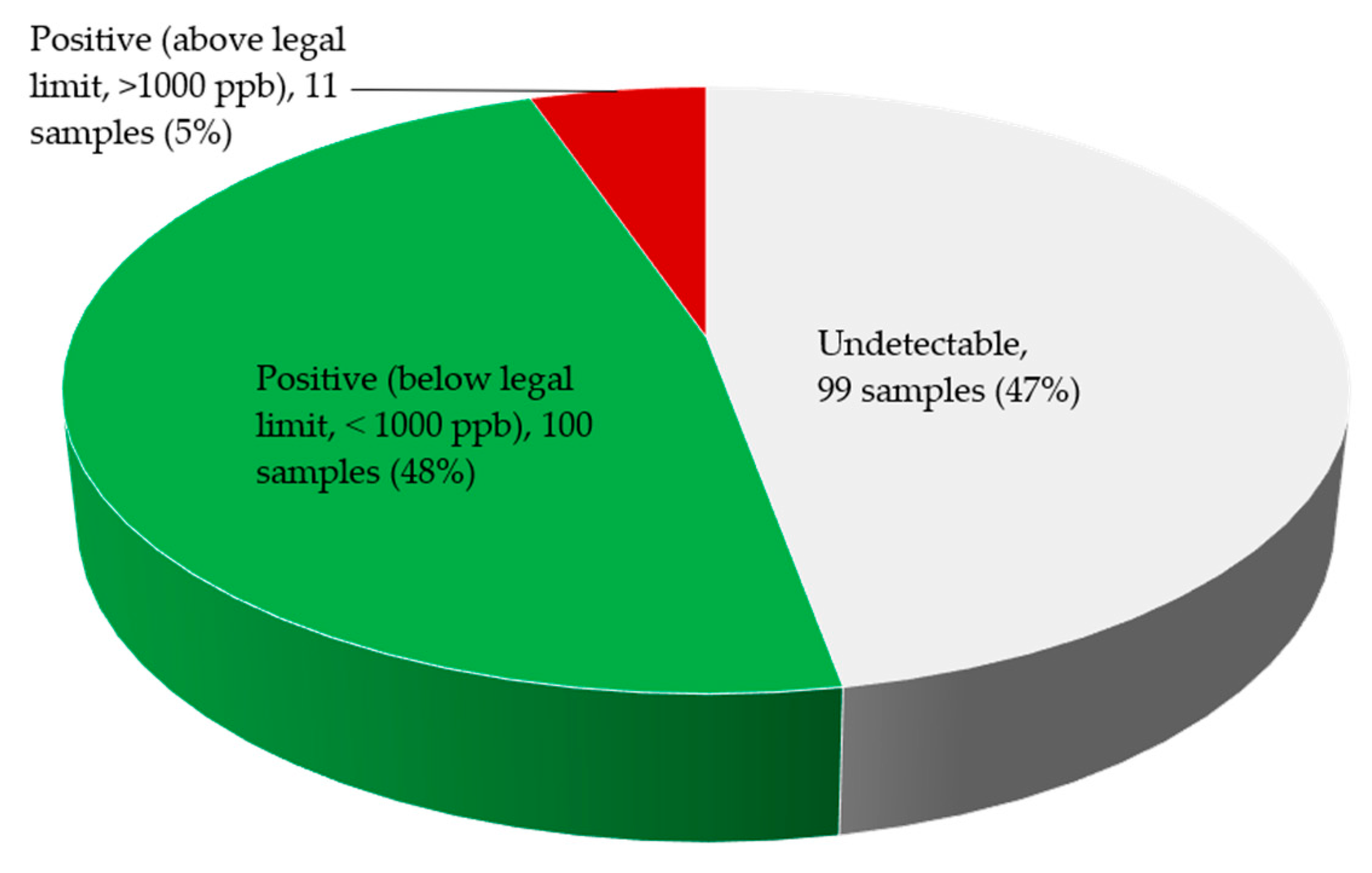
2.3. Fumonisin Contamination of Edible Non-Timber Forest Products
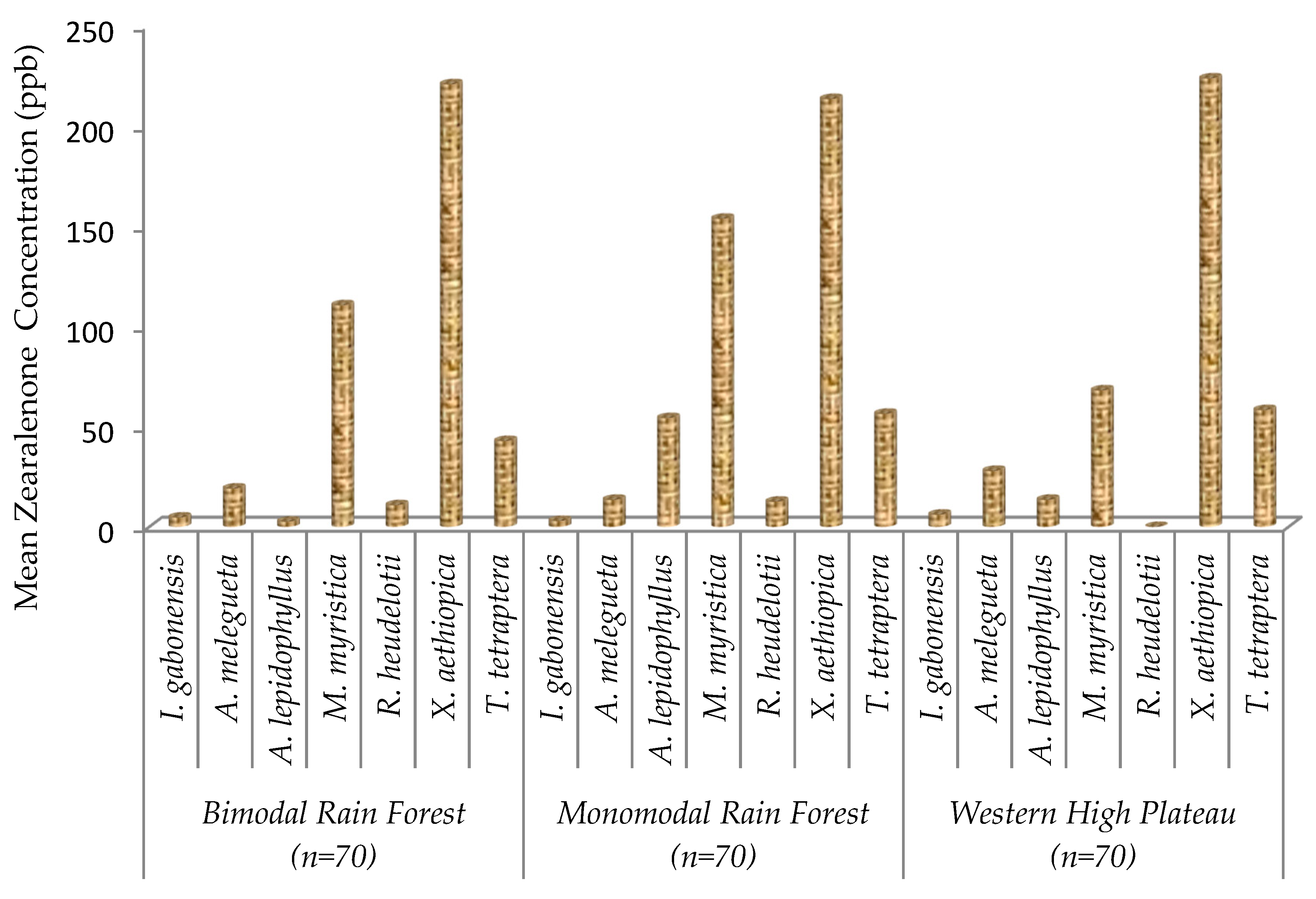
2.4. Zearalenone Contamination on Edible Non-Timber Forest Products
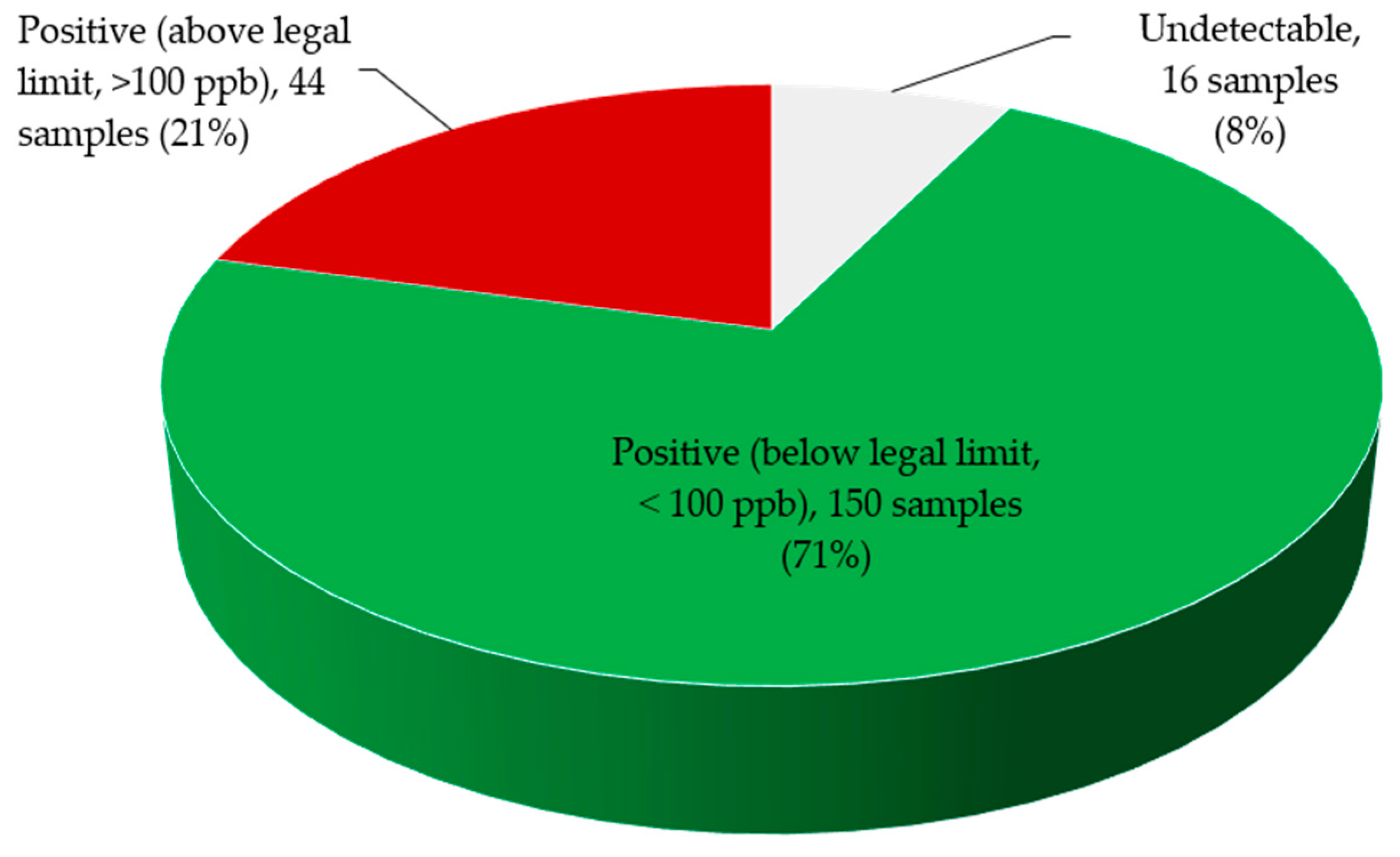
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Collection Areas
5.2. Sampling
5.3. Determination of Moisture Content
5.4. Mycotoxin Extraction Procedures
5.4.1. Total Aflatoxin Extraction
5.4.2. Fumonisin Extraction
5.4.3. Zearalenone Extraction
5.5. Mycotoxin Quantification
5.6. Validation of ELISA Data
5.7. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barany, M.; Hammett, A.L.; Sene, A.; Amichev, B. Non-timber forest benefits and HIV/AIDS in Sub-Saharan Africa. J. For. 2001, 12, 36–41. [Google Scholar]
- Marshall, E.; Newton, A.; Schreckenberg, K. Commercialization of non-timber forest products: First steps in analysing the factors influencing success. Int. For. Rev. 2003, 5, 128–137. [Google Scholar]
- Anshu, S.; Prodyut, B.; Pradeep, V.; Sarvashish, R. Contribution of NTFPs in the livelihood of mangrove forest dwellers of Sundarban. J. Hum. Ecol. 2010, 29, 191–200. [Google Scholar] [CrossRef]
- Ahenkan, A.; Boon, E. Improving nutrition and health through non-timber forest products in Ghana. J. Health Popul. Nutr. 2011, 29, 141. [Google Scholar] [CrossRef]
- Ingram, V.; Schure, J. Review of Non-Timber Forest Products (NTFPs) in Central Africa: Cameroon; Center for International Forestry Research Technical Report; Center for International Forestry Research: Bogor, Indonesia, 2010; p. 177. [Google Scholar]
- FAO. Beyond Wood Improving Policies to Promote Sustainable use of Non-Wood Forest Products in Europe; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; 12p. [Google Scholar]
- Djeugap, F.J.; Akoula, N.C.; Kyalo, M.; Njukeng, A.P.; Galani Yamdeu, J.H.; Kuiate, J.R.; Ghimire, S. Morphological and molecular identification of pathogenic fungi of Calabash nutmeg (Monodora myristica Dunal) kernels and their response to different phytoextracts. Int. J. Adv. Agric. Res. 2017, 5, 66–75. [Google Scholar]
- Dongmo, G.Z.; Djeugap, J.F.; Fenohi, N.; Kenfack, N.D.; Takuete, R.; Teguefouet, P. Contribution à l’identification des champignons de post-récolte associés aux amandes de Ricinodendron heudelotii et Garcinia kola collectées dans les Hauts Plateaux de l’Ouest Cameroun. Int. J. Boil. Chem. Sci. 2017, 11, 1840–1850. [Google Scholar] [CrossRef][Green Version]
- Jiofack, T.; Fokunang, C.; Guedje, N.M.; Kemeuze, V.; Fongnzossie, E.; Nkongmeneck, B.A.; Mapongmetsem, P.M.; Tsabang, N. Ethnobotanical uses of medicinal plants of two ethnoecological regions of Cameroon. Int. J. Med. Med Sci. 2010, 2, 60–79. [Google Scholar]
- Mpondo, M.E.; Dibong, D.S.; Priso, R.J.; Ngoye, A.; Yemeda, F.L. Etat actuel de la médécine traditionnelle dans le système de santé des populations rurales et urbaines de Douala (Cameroun). J. Appl. Biosci. 2012, 55, 4036–4045. [Google Scholar]
- Shiembo, P.; Newton, A.; Leakey, R. Vegetative propagation of Irvingia gabonensis, a West African fruit tree. For. Ecol. Manag. 1996, 87, 185–192. [Google Scholar] [CrossRef]
- Omokolo, N.D. Preliminary results on the in-vitro regeneration of Ricinodendron Heudelotii (Baill). In Proceedings of the First PROTA International Workshop, Nairobi, Kenya, 3–25 September 2002; 326p. [Google Scholar]
- FAO/COMIFAC. Projet Renforcement de la Sécurité Alimentaire en Afrique Centrale à Travers la Gestion et L’utilisation Durable des Produits Forestiers non Ligneux; Note d’Information No1. 2006. Available online: http://www.fao.org/3/al032f/al032f00.pdf (accessed on 14 July 2019).
- Cosyns, H.; Degrande, A.; De Wulf, R.; Damme, P.V.; Tchoundjeu, Z. Can commercialization of NTFPs alleviate poverty? A case study of Ricinodendron heudelotii (Baill.) Pierre ex Pax. Kernel marketing in Cameroon. J. Agric. Rural Dev. Trop. Subtrop. 2011, 122, 45–56. [Google Scholar]
- Fandohan, P.; Gnonlonfin, B.; Hell, K.; Marasas, W.; Wingfield, M. Natural occurrence of Fusarium and subsequent fumonisin contamination in preharvest and stored maize in Benin, West Africa. Int. J. Food Microbiol. 2005, 99, 173–183. [Google Scholar] [CrossRef]
- Rajarajan, P.; Rajasekaran, K.; Devi, N.A. Isolation and quantification of Aflatoxin from Aspergillus flavus infected stored peanuts. Indian J. Pharm. Boil. Res. 2013, 5, 16–24. [Google Scholar] [CrossRef]
- Kana, J.R.; Gnonlonfin, B.B.J.; Harvey, J.; Wainaina, J.; Wanjuki, I.; Skilton, R.A.; Teguia, A. Assessment of aflatoxin contamination of maize, peanut meal and poultry feed mixtures from different agroecological zones in Cameroon. Toxins 2013, 5, 884–894. [Google Scholar] [CrossRef]
- Lanyasunya, T.P.; Wamae, L.W.; Musa, H.H.; Olowofeso, O.; Lokwaleput, I.K. The risk of mycotoxins contamination of dairy feed and milk on smallholder dairy farms in Kenya. Pak. J. Nutr. 2005, 4, 162–169. [Google Scholar]
- Singh, P.K. Assessment of mycotoxins in edible tree borne oil seeds (TBOS). J. Food Res. 2012, 1, 92–101. [Google Scholar] [CrossRef][Green Version]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Finnegan, D. Mycotoxins in Cereals: Sources and Risks. European Mycotoxins Awareness Network. Available online: http://eman.leatherheadfood.com (accessed on 30 June 2019).
- Ngoko, Z.; Marasas, W.F.O.; Rheeder, J.P.; Shephard, G.S.; Wingfield, M.J.; Cardwell, K.F. Fungal infection and mycotoxin contamination of maize in the Humid forest and the western highlands of Cameroon. Phytoparasites 2001, 29, 352–360. [Google Scholar] [CrossRef]
- Milani, J.M. Ecologicial conditions affecting mycotoxins production in cereals: A review. Vet. Med. 2013, 58, 405–411. [Google Scholar] [CrossRef]
- Bankole, S.; Adebanjo, A. Mycotoxins in food in West Africa: Current situation and possibilities of controlling it. Afr. J. Biotechnol. 2003, 2, 254–263. [Google Scholar]
- Cotty, P.J.; Mellon, J.E. Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination. Mycotoxin Res. 2006, 22, 110–117. [Google Scholar] [CrossRef]
- Bankole, S.A. Changes in moisture content, fungal infection and kernel germinability of maize in storage. International J. Trop. Plant Dis. 1994, 12, 213–218. [Google Scholar]
- Adebayo-Tayo, B.C.; Onilude, A.A.; Ogunjobi, A.A.; Gbolagade, J.S.; Oladapo, M.O. Detection of fungi and aflatoxin in shelved bush mango seeds (Irvingia spp.) stored for sale in Uyo, Nigeria. Afr. J. Biotechnol. 2006, 5, 1729–1732. [Google Scholar]
- Seetha, A.; Munthali, W.; Msere, H.W.; Swai, E.; Muzanila, Y.; Sichone, E.; Tsusaka, T.W.; Rathore, A.; Okori, P. Occurrence of aflatoxins and its management in diverse cropping systems of central Tanzania. Mycotoxin Res. 2017, 33, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Singh, P.K. Mycotoxin producing potential of seed myco-flora of some forest trees. Indian For. 2000, 126, 1231–1233. [Google Scholar]
- Singh, P.K.; Khan, S.; Harsh, N.; Pandey, R. Incidence of myco-flora and mycotoxins in some edible fruits and seeds of forest origin. Mycotoxin Res. 2001, 17, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Sage, L.; Krivobok, S.; Delbos, É.; Seigle-Murandi, F.; Creppy, E.E. Fungal flora and Ochratoxin A production in grapes and musts from France. J. Agric. Food Chem. 2002, 50, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Taveira, J.A.; Midio, A.F. Incidence of aflatoxin M1 in milk marketed in Sao Paulo, Brazil. Ital. J. Food Sci. 2001, 13, 443–447. [Google Scholar]
- Eaton, D.L.; Groopman, J.D. The Toxicology of Aflatoxin; Academic Press: New York, NY, USA, 1994; 426p. [Google Scholar]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Ono, E.Y.; Sugiura, Y.; Homechin, M.; Kamogae, M.; Vizzoni, E.; Ueno, Y.; Hirooka, E.Y. Effect of climatic conditions on natural microflora and fumonisins in freshly harvested corn of the state of Parana, Brazil. Mycopathologia 1999, 147, 139–148. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Sabino, M. Mycotoxin research in Brazil: The last decade in review. Braz. J. Microbiol. 2002, 33, 1–11. [Google Scholar] [CrossRef]
- Chulze, S.N.; Ramirez, M.L.; Farnochi, M.C.; Pascale, M.; Visconti, A.; March, G. Fusarium and fumonisin occurrence in Argentinian corn at different ear maturity stages. J. Agric. Food Chem. 1996, 44, 2797–2801. [Google Scholar] [CrossRef]
- Marasas, W.F.; Kellerman, T.S.; Gelderblom, W.C.; A Coetzer, J.; Thiel, P.G.; Van Der Lugt, J.J. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet. Res. 1988, 55, 197–203. [Google Scholar] [PubMed]
- Turner, P.; Nikiema, P.; Wild, C. Fumonisin contamination of food: Progress in development of biomarkers to better assess human health risks. Mutat. Res. Toxicol. Environ. Mutagen. 1999, 443, 81–93. [Google Scholar] [CrossRef]
- D’Mello, J.; Placinta, C.; Macdonald, A. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed. Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Jinap, S.; Rashid, U. Detection of aflatoxins and zearalenone contamination in wheat derived products. Food Control 2014, 35, 223–226. [Google Scholar] [CrossRef]
- Phuong, N.H.; Thieu, N.Q.; Ogle, B.; Pettersson, H. Aflatoxins, Fumonisins and Zearalenone contamination of maize in the south-eastern and central highlands provinces of Vietnam. Agriculture 2015, 5, 1195–1203. [Google Scholar] [CrossRef]
- El Hoshy, S.M. Occurrence of Zearalenone in milk, meat and their products with emphasis on influence of heat treatments on its level. Archiv für Lebensm. 1999, 50, 140–143. [Google Scholar]
- Nuryono, N.; Noviandi, C.; Böhm, J.; Razzazi-Fazeli, E. A limited survey of zearalenone in Indonesian maize-based food and feed by ELISA and high-performance liquid chromatography. Food Control 2005, 16, 65–71. [Google Scholar] [CrossRef]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health. Organ. 1999, 77, 754–766. [Google Scholar] [PubMed]
- Frazier, W.C.; Westhoff, D.C. Food Microbiology 4th ed; International Edition; McGraw-Hill: Singapore, 1988; 440p.AOAC. In Official Methods of Analysis of AOAC International, 6th ed.; 5th Revision; AOAC International Publishers: Gaithersburg, MA, USA, 1999; Volume 11. [Google Scholar]

| Commodities | Agroecological Zones (AEZs) | Number of Samples | Mean within AEZs (%) | Mean (%) |
|---|---|---|---|---|
| Irvingia gabonensis (n = 30) | Bimodal Rainforest | 10 | 5.94 | 5.83 ± 0.6 c |
| Monomodal Rainforest | 10 | 6.43 | ||
| Western High Plateau | 10 | 5.11 | ||
| Ricinodendron heudelotii (n = 30) | Bimodal Rainforest | 10 | 5.24 | 5.29 ± 0.04 c |
| Monomodal Rainforest | 10 | 5.29 | ||
| Western High Plateau | 10 | 5.33 | ||
| Afrostyrax lepidophyllus (n = 30) | Bimodal Rainforest | 10 | 10.66 | 12.03 ± 1.1 b |
| Monomodal Rainforest | 10 | 12.67 | ||
| Western High Plateau | 10 | 12.77 | ||
| Aframomum melegueta (n = 30) | Bimodal Rainforest | 10 | 13.52 | 12.09 ± 1.6 b |
| Monomodal Rainforest | 10 | 11.71 | ||
| Western High Plateau | 10 | 11.06 | ||
| Monodora myristica (n = 30) | Bimodal Rainforest | 10 | 9.46 | 10.08 ± 1.5 b |
| Monomodal Rainforest | 10 | 11.62 | ||
| Western High Plateau | 10 | 9.15 | ||
| Xylopia aethiopica (n = 30) | Bimodal Rainforest | 10 | 10.48 | 6.99 ± 2.2 c |
| Monomodal Rainforest | 10 | 5.47 | ||
| Western High Plateau | 10 | 5.01 | ||
| Tetrapleura tetraptera (n = 30) | Bimodal Rainforest | 10 | 21.36 | 20.84 ± 1.6 a |
| Monomodal Rainforest | 10 | 22.55 | ||
| Western High Plateau | 10 | 18.62 |
| Commodities | Percentage of Positive Samples | Mean (ppb) | |
|---|---|---|---|
| Irvingia gabonensis | <1 ppb | 11.11% | 3.54 ± 0.9 a |
| 1–10 ppb | 77.77% | ||
| 10–20 ppb | 11.11% | ||
| Aframomum melegueta | <1 ppb | 94.44% | 0.32 ± 0.1 c |
| 1–10 ppb | 5.56% | ||
| 10–20 ppb | 0% | ||
| Afrostyrax lepidophyllus | <1 ppb | 55.55% | 2.5 ± 0.7 a,b |
| 1–10 ppb | 38.9% | ||
| 10–20 ppb | 5.56% | ||
| Monodora myristica | <1 ppb | 94.44% | 0.7 ± 0.2 c,b |
| 1–10 ppb | 5.56% | ||
| 10–20 ppb | 0% | ||
| Ricinodendron heudelotii | <1 ppb | 83.33% | 0.63 ± 0.2 c,b |
| 1–10 ppb | 16.67% | ||
| 10–20 ppb | 0% | ||
| Xylopia aethiopica | <1 ppb | 58.33% | 1.2 ± 0.2 c,b |
| 1–10 ppb | 33.33% | ||
| 10–20 ppb | 8.33% | ||
| Tetrapleura tetraptera | <1 ppb | 72.22% | 0.9 ± 0.4 c,b |
| 1–10 ppb | 27.78% | ||
| 10–20 ppb | 0% | ||
| Commodities | Percentage of Positive Samples | Mean (ppb) | |
|---|---|---|---|
| Irvingia gabonensis | <100 ppb | 0% | 0.00 f |
| 100–1000 ppb | 0% | ||
| 1000–6000 ppb | 0% | ||
| Aframomum melegueta | <100 ppb | 94.44% | 9.30 ± 2.4 e |
| 100–1000 ppb | 5.56% | ||
| 1000–6000 ppb | 0% | ||
| Afrostyrax lepidophyllus | <100 ppb | 94.44% | 28.23 ± 8.7 d |
| 100–1000 ppb | 5.56% | ||
| 1000–6000 ppb | 0% | ||
| Monodora myristica | <100 ppb | 100% | 6.52 ± 2.9 e |
| 100–1000 ppb | 0% | ||
| 1000–6000 ppb | 0% | ||
| Ricinodendron heudelotii | <100 ppb | 94.44% | 78.62 ± 12.3 c |
| 100–1000 ppb | 5.56% | ||
| 1000–6000 ppb | 0% | ||
| Xylopia aethiopica | <100 ppb | 0% | 891.97 ± 84.9 a |
| 100–1000 ppb | 50.0% | ||
| 1000–6000 ppb | 50.0% | ||
| Tetrapleura tetraptera | <100 ppb | 11.11% | 437.08 ± 78.6 b |
| 100–1000 ppb | 83.33% | ||
| 1000–6000 ppb | 5.55% | ||
| Commodities | Percentage of Positive Samples | Mean (ppb) | |
|---|---|---|---|
| Irvingia gabonensis | <15 ppb | 100% | 4.61 ± 1.6 d |
| 15–100 ppb | 0% | ||
| 100–500 ppb | 0% | ||
| Aframomum melegueta | <15 ppb | 44.44% | 20.24 ± 7.7 c,d |
| 15–100 ppb | 55.56% | ||
| 100–500 ppb | 0% | ||
| Afrostyrax lepidophyllus | <15 ppb | 77.78% | 23.51 ± 9.8 c,d |
| 15–100 ppb | 16.67% | ||
| 100–500 ppb | 5.55% | ||
| Monodora myristica | <15 ppb | 0% | 110.89 ± 22.7 b |
| 15–100 ppb | 33.33% | ||
| 100–500 ppb | 66.67% | ||
| Ricinodendron heudelotii | <15 ppb | 77.78% | 7.84 ± 3.5 d |
| 15–100 ppb | 22.22% | ||
| 100–500 ppb | 0% | ||
| Xylopia aethiopica | <15 ppb | 0% | 219.47 ± 35.2 a |
| 15–100 ppb | 0% | ||
| 100–500 ppb | 100% | ||
| Tetrapleura tetraptera | <15 ppb | 0% | 52.56 ± 16.1 c |
| 15–100 ppb | 100% | ||
| 100–500 ppb | 0% | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djeugap, J.F.; Ghimire, S.; Wanjuki, I.; Muiruri, A.; Harvey, J. Mycotoxin Contamination of Edible Non-Timber Forest Products in Cameroon. Toxins 2019, 11, 430. https://doi.org/10.3390/toxins11070430
Djeugap JF, Ghimire S, Wanjuki I, Muiruri A, Harvey J. Mycotoxin Contamination of Edible Non-Timber Forest Products in Cameroon. Toxins. 2019; 11(7):430. https://doi.org/10.3390/toxins11070430
Chicago/Turabian StyleDjeugap, Joseph Fovo, Sita Ghimire, Immaculate Wanjuki, Anne Muiruri, and Jagger Harvey. 2019. "Mycotoxin Contamination of Edible Non-Timber Forest Products in Cameroon" Toxins 11, no. 7: 430. https://doi.org/10.3390/toxins11070430
APA StyleDjeugap, J. F., Ghimire, S., Wanjuki, I., Muiruri, A., & Harvey, J. (2019). Mycotoxin Contamination of Edible Non-Timber Forest Products in Cameroon. Toxins, 11(7), 430. https://doi.org/10.3390/toxins11070430





