Abstract
A number of pathogenic bacteria utilize toxins to mediate disease in a susceptible host. The foodborne pathogen Salmonella is one of the most important and well-studied bacterial pathogens. Recently, whole genome sequence characterizations revealed the presence of multiple novel ADP-ribosylating toxins encoded by a variety of Salmonella serovars. In this review, we discuss both the classical (SpvB) and novel (typhoid toxin, ArtAB, and SboC/SeoC) ADP-ribosylating toxins of Salmonella, including the structure and function of these toxins and our current understanding of their contributions to virulence.
Key Contribution:
Here we summarize both the classical and novel ADP ribosylating toxins of Salmonella and what is known about their contributions to virulence.
1. Introduction
Salmonella is one of the most diverse and successful pathogens. Although the genus Salmonella includes just two species, enterica and bongori, there are 2,659 different serovars [1]. A total of 6 subspecies are included within species enterica: (I) enterica, (II) salamae, (IIIa) arizonae, (IIIb) diarizonae, (IV) houtenae, and (VI) indica [1,2]. As the majority of animal and human clinical cases result from infection with S. enterica—although infections with S. bongori are occasionally reported [3]—research efforts have primarily focused on the characterization of S. enterica, and more specifically on S. enterica subsp. enterica (abbreviated here as “S.” followed by the serovar). Salmonellae are further classified based on the disease that they cause. While the majority of serovars cause a mild gastroenteritis (generally caused by nontyphoidal Salmonella [NTS] serovars), a select few (Typhi, Sendai, and Paratyphi A, B, or C) cause an invasive, severe infection known as enteric fever [4]. Exceptions to this generalization exist, as some NTS serovars, such as Dublin and Choleraesuis, are frequently associated with invasive disease [5].
Each year, Salmonella causes an estimated 88 million combined illnesses (including NTS, Paratyphi, and Typhi) worldwide [6]. The burden of salmonellosis varies by geographical region [7], as do the specific serovars that are associated with human clinical disease in those regions [8,9]. Salmonella is also an important cause of animal disease, as infection with some serovars is associated with severe clinical disease in select animals, such as S. Gallinarum in chickens [10] and S. Dublin in cows [11]. Although animals can be colonized by Salmonella, and direct contact with animals is a risk factor for salmonellosis [12], the vast majority of infections are foodborne [13] and are often the result of sporadic infection (i.e., not direct contact with animals) [14].
S. Typhimurium, although somewhat serendipitously identified based on its ability to cause mouse typhoid, has become the model for studying NTS serovars [15]. S. Typhi has also been extensively characterized, due to the severity of disease that it causes [16]. As such, conclusions about nontyphoidal and typhoid Salmonella are generally extrapolated from studies with S. Typhimurium and S. Typhi. In contrast to other pathogens that cause a toxin-mediated disease, nontyphoidal salmonellosis is largely the result of the proinflammatory reactions triggered by interactions with the host’s immune system. Salmonella uses a T3SS encoded within Salmonella pathogenicity island (SPI) 1 to secrete effectors into host cells, resulting in membrane ruffling and subsequent engulfment of Salmonella. Once inside a host cell, the activation of SPI-2 genes allows Salmonella to survive and multiply. This allows NTS serovars to promote an inflammatory response in the host’s intestine, resulting in the generation of alternate terminal electron acceptors, thereby enabling NTS to compete with the resident anaerobic bacteria of the gut microbiota [17]. In contrast, S. Typhi utilizes a more stealth approach by (i) expressing a Vi capsular antigen that is associated with reductions in IL-8 expression of both immune and epithelial cells [18], (ii) down-regulating flagellin, resulting in reduced pyroptosis [19], a pro-inflammatory form of cell death, and (iii) producing a novel toxin (typhoid toxin), which has been shown to reduce the levels of circulating immune cells in the blood [20]. Our current understanding about the dichotomous pathogenesis of typhoid and nontyphoidal serovars illustrates the importance of the immune response in creating an inflammatory environment in which nontyphoidal serovars can excel and an environment in which Typhi and other invasive serovars can hide.
ADP-ribosylating toxins play an important role in pathogenesis for a number of bacterial pathogens, including Bordetella pertussis [21], Clostridium botulinum [22], Corynebacterium diphtheriae [23], Vibrio cholerae [24], and others [25,26]. Despite having a common activity (ADP-ribosylation of target proteins), bacterial ADP-ribosylating toxins (bARTTs [25]) target a wide range of host proteins, therefore resulting in a variety of cytotoxic effects, ranging from modulation of the cytoskeleton to promoting host cell death. These toxins constitute two general classes: (i) the diphtheria toxin-like (DT-like) group composed of an active (A) and binding (B) domain present in a single chain (AB), and (ii) the cholera toxin-like (CT-like) group, composed of a single A domain that is non-covalently bound to a pentameric subunit of 5 B domains (AB5) [25]. CT-like toxins can further be categorized into C-2-like binary toxins, in which the A-domain and B-domain subunits are expressed and synthesized separately [25], and C3-like exoenzymes consisting of a single A-domain subunit [25]. Recently, a number of novel bARTTs were discovered and characterized [20,27,28], expanding the diversity of this toxin family [25].
While salmonellosis is not traditionally considered a toxin-mediated disease, certain strains of Salmonella utilize bARTTs (Table 1) to alter host responses in order to promote pathogenesis in their respective hosts. SpvB, perhaps the most well-known of these toxins encoded by Salmonella, is a C2-like bARTT that ADP-ribosylates actin monomers, preventing their polymerization [29]. Three novel bARTT’s—the typhoid toxin [30], ArtAB [31], and SboC/SeoC [32]—were only recently discovered, and will be discussed in further detail in this review. Interestingly, the typhoid toxin produced by S. Typhi was reported to recapitulate many of the clinical signs of typhoid fever in a mouse model [20]. However, recent genomic characterizations of a wide range of nontyphoidal and paratyphoidal serovars revealed the presence of this toxin in many different serovars, most of which do not cause typhoid-like disease in humans, therefore suggesting that the typhoid toxin likely contributes to virulence but is not the sole driving force of typhoid fever.

Table 1.
Current understanding of bacterial ADP-ribosylating toxins (bARTTs) in Salmonella spp.
In this review, we discuss the current knowledge regarding the bARTT’s produced by Salmonella serovars, and our current understanding of how these toxins contribute to Salmonella’s success as a pathogen.
2. SpvB Toxin
The presence of a high molecular weight plasmid encoded by S. Typhimurium isolates was initially reported in the 1970s, although the plasmid was initially characterized as ‘cryptic’ and was solely associated with its observed incapability with the F plasmid [38]. It was later discovered that this ‘cryptic’ plasmid was associated with enhanced adhesion and invasion of HeLa cells, and pathogenesis in mice [39]. Cloning of random fragments from endonuclease digests identified a 7.8 kb segment essential for virulence in plasmid-cured strains of S. Dublin and S. Typhimurium [40], which was later identified and named spvRABCD for Salmonella plasmid virulence (Figure 1) [38]. Further characterization demonstrated that SpvB functions as an ADP-ribosyltransferase that catalyzes ADP-ribosylation of host actin monomers, leading to their depolymerization [41]. spv genes have been identified in some S. enterica subsp. arizonae strains as well, but these genes are chromosomally-encoded and lack spvD [42].
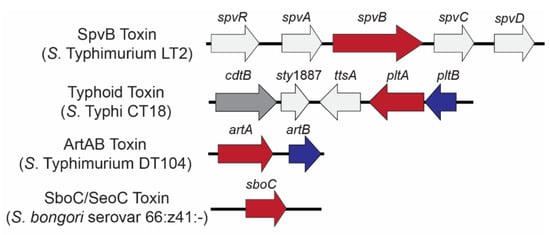
Figure 1.
Genetic loci encoding Salmonella ADP-ribosylating toxins. Genes are color coded to represent toxin components: A domain-encoding (ADP-ribosyltransferase) genes (red) and B (binding) domain-encoding genes (blue); the genes colored light gray are co-located with bARTT genes but are not part of the final holotoxin. Typhoid toxin has an additional A subunit (CdtB; dark gray) which acts as a nuclease.
Virulence plasmids from at least 10 different serovars have been identified as encoding spv genes (Table 2). It is important to note that these virulence plasmids also carry other virulence factors, including plasmid-encoded fimbriae (pef) and rcK (resistance to complement killing), as well as antimicrobial resistance genes [43]. Among the spv genes, spvC encodes a phosphothreonine lyase that inhibits host MAP kinases, [44] and spvD is associated with the suppression of proinflammatory responses [45].

Table 2.
Distribution of ArtAB, typhoid toxin, SboC/SeoC, and SpvB toxin genes.
2.1. SpvB Toxin Structure and Activity
In contrast to other known actin-ADP-ribosylating toxins, SpvB is a single-chain A-domain toxin (i.e., it lacks a binding subunit, as seen in Figure 2) [25]. SpvB is secreted by intracellular Salmonella directly into the cytoplasm of host cells via the SPI-2 T3SS [56], where it catalyzes the mono-ADP-ribosylation of the Arg177 residue of actin monomeric subunits, thereby inhibiting actin’s ability to hydrolyze ATP, which prevents actin polymerization and ultimately results in the depolymerization of actin [36]. This activity opposes the role of several SPI-1- and SPI-2-encoded virulence factors, whose roles are to encourage the polymerization of actin for successful invasion in non-phagocytic epithelial cells, and to maintain the integrity of the SCV membrane, respectively [57,58].
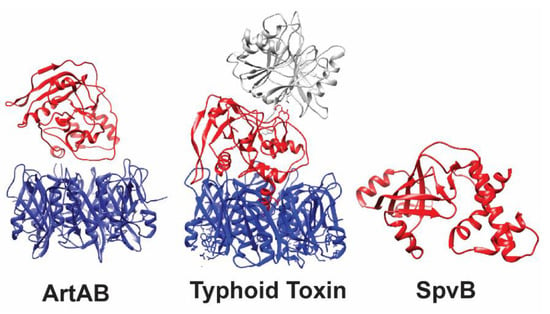
Figure 2.
3-D Structures of ADP-ribosylating toxins of Salmonella. ArtAB and typhoid toxin are secreted exotoxins, while SpvB is translocated directly into the cytoplasm of host cells, and therefore lacks a binding domain. Active domains are shown in red, binding domains in blue, and other toxin components are shown in gray (typhoid toxin CdtB). The typhoid toxin (PDB accession: 4K6L) and SpvB (PDB accession: 2GWL) crystal structures were solved; the crystal structure of ArtA (PDB accession: 4Z9C; used as a template for modeling DT-104 ArtA) and the ArtB pentamer (PDB accession: 5WHV) were resolved as independent subunits. Therefore, the A and B domains of ArtAB are shown as two separate subunits and not as the final conformation of the toxin.
2.2. Implications of SpvB-Mediated Salmonella Virulence
SpvB-mediated actin depolymerization is associated with an accumulation of cells in the G2/M phase, eventually resulting in apoptotic cell death [59]. As inhibition of actin polymerization may also cause arrest in the G1 phase [60], it is likely that SpvB’s activity also prolongs entry into, if not prevents entry into the G2 phase, although previous studies have not conclusively shown this. SpvB also inhibits vacuole-associated actin polymerization (VAP) [61], which plays an important role in maintenance of the SCV membrane [62]. This suggests that SpvB-mediated inhibition of actin depolymerization is associated with a breakdown of the SCV membrane, followed by escape of Salmonella into the host cytosol [61]. This is in contrast to the current understanding that Salmonella multiply and reside within the protective environment of the SCV [58], and instead suggests that SpvB mediates an intra-cytosolic Salmonella lifestyle more akin to that of other pathogens such as Listeria monocytogenes [63], Shigella spp. [64], and Rickettsia spp. [65]. Characterizations of the intracellular growth of SpvB-producing Salmonella will be important for understanding the implications of cytosolic Salmonella. Assessment of the proportion of SpvB-producing Salmonella cells that escape the SCV would provide important insight into the role of SpvB in modulating Salmonella’s intracellular lifestyle.
The primary observation that the spv locus plays an important role in the severity of disease stems from the fact that a number of spv-encoding serovars cause systemic disease in their respective hosts. With the exception of serovars Bovismorbificans, Enteritidis, and Typhimurium, most of the serovars that encode spv genes are host adapted (i.e., Choleraesuis to pigs [66], Dublin to cows [11], Abortusequi to horses and donkeys [67], and Abortusovis to sheep and goats [68]), or host restricted (i.e., Gallinarum to chickens [10] and Paratyphi C and Sendai to humans [53]). In general, infections with these serovars either in the hosts which they have adapted to, or in humans, often results in a more severe infection characterized by invasive disease [5]. An important exception to this is S. Gallinarum, which is restricted to chickens [10]. As a number of studies characterized the role of the virulence plasmid in pathogenesis, and not the role of spvB, conclusions drawn about SpvB’s specific role in pathogenesis should be considered in the context of the other plasmid-encoded virulence factors, especially considering that previous studies have shown that spvBC, but not spvB alone, is sufficient for replacing the entire virulence plasmid during infection of BALB/c mice, suggesting that both SpvB and SpvC are essential for mediating the toxin-associated phenotype in vivo [69]. One study used a mouse model to show that SpvB was required for efficient colonization of the intestinal lamina propia, and although SpvB and SpvC were not essential, they were associated with increased gut inflammation [37]. Another study demonstrated that deletion of spvB in S. Typhimurium resulted in a decrease in bacterial levels in the spleens of infected mice, but only at 14 days post-infection [70], suggesting that spvB may play a role in the systemic spread, or in persistence, but it is unlikely to affect the development of acute salmonellosis. In general, our understanding of SpvB’s role in virulence (based primarily on tissue culture models) has not been translated into obvious roles in pathogenesis, aside from a select few studies that demonstrated a potential role for SpvB in colonization and persistence in vivo, phenotypes that could be associated with other virulence factors that are encoded on the same virulence plasmid as the spv genes (i.e., the pef operon).
3. The Typhoid Toxin
The typhoid toxin, so named because it was originally identified in S. Typhi [30,71], is a novel A2B5 toxin that incorporates the nuclease activity of CdtB from the cytolethal distending toxin [72] with the ADP-ribosyltransferase activity from the pertussis toxin’s active subunit, PtxA (also called S1; Figure 2). The typhoid toxin is chromosomally encoded on a putative mobile element within SPI-11 [30,47] and includes the accessory genes ttsA and STY1887 (Figure 1). While ttsA is predicted to play a role in typhoid toxin secretion, a role for STY1887 has yet to be defined [73].
The vast majority of characterizations have focused on the DNase activity of the typhoid toxin, primarily because the Dnase activity of CdtB is associated with DNA damage. Furthermore, mutations at the catalytic site of CdtB are sufficient to abolish the cell cycle arrest associated with this toxin [20,30]. Although first characterized in S. Typhi, genes encoding the typhoid toxin have since been identified in at least 48 different serovars [33,47], including both typhoidal and nontyphoidal serovars, as well as in S. bongori (Table 2). A few studies have demonstrated the high conservation of PltA (average of 98–100% amino acid identity) across different serovars [52,74].
3.1. Typhoid Toxin Structure and ADP-Ribosyltransferase Activity
The typhoid toxin was originally characterized as utilizing a homopentameric binding subunit of PltB monomers [20], however new evidence suggests that the typhoid toxin can also form a functional holotoxin with a homopentameric binding subunit of ArtB monomers [75,76]. As ArtB and PltB preferentially bind to different sialoglycans on host cells, the use of multiple binding subunits of this toxin was proposed to enable the typhoid toxin to target different tissue types in humans [77], as well as different hosts [76]. X-ray crystallography of purified typhoid toxin revealed that CdtB and PltA, the two active subunits of the toxin, are tethered together by a disulfide bond between PltA’s Cys214 and CdtB’s Cys269 residues [20]. An α-helix at PltA’s C-terminus mediates the PltA–PltB association and is stabilized by interactions of six PltA amino acid residues with the hydrophobic lumen of the PltB homopentameric binding subunit, forming a stable pyramid-shaped toxin [20]. Interestingly, PltA shares homology with both PtxA from the pertussis toxin [30], and ArtA encoded by a number of different Salmonella serovars [33].
Intracellularly, host cell reductases in the endoplasmic reticulum reduce the CdtB–PltA disulfide bond, releasing CdtB from the rest of the holotoxin [78]; hypothetically, the Cys56-Cys207 bond in PltA [20] is also reduced, thereby enabling PltA to bind NAD+ [20], and subsequently mono-ADP ribosylate target host proteins in a manner similar to that of other bARTTs, including the pertussis toxin [25]. PltA is able to ADP-ribosylate proteins in extracts from cultured epithelial cells [30], although the target of this activity has yet to be identified. Given its structural homology and the conservation of amino acid residues involved in both NAD+ binding and the catalytic activity of PtxA from the pertussis toxin (Figure 3) [20], the target of PltA mono-ADP-ribosylation may be host heterometric G-proteins, although this has yet to be definitively confirmed.
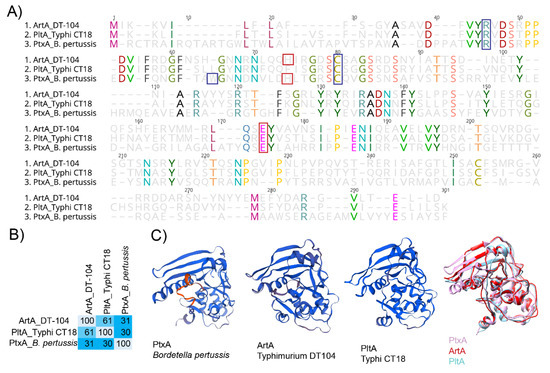
Figure 3.
PltA and ArtA are structurally similar to the active subunit PtxA from the pertussis toxin. (A) Alignment of predicted amino acid sequence of ArtA from S. Typhimurium DT104, PltA from S. Typhi CT18, and PtxA from B. pertussis. Red boxes indicate catalytic residues, and blue boxes represent amino acid residues involved in NAD+ binding. (B) Percent conservation of amino acids in ArtA, PltA, and PtxA; calculations were performed using Geneious software. (C) Structure of ADP-ribosylating subunits for PtxA (PDB accession: 1PRT), ArtA (modeled onto 479C), PltA (4K6L), and all three. Alignment of 3D models was done with Chimera software [79].
3.2. Implications of PltA-Mediated Salmonella Virulence
While the administration of purified typhoid toxin to mice is associated with lethargy, weight loss, neutropenia, and some of the neurologic symptoms associated with typhoid fever, this activity is dependent on CdtB, and not on PltA [20]. One study characterizing the activity of PltA in a tissue culture cell line [30] showed that PltA and PltB are required for activation of the DNA damage response (DDR) and the observed accumulation of cells in the G2/M phase, but PltA need not be catalytically active. This is not surprising given that treatment of cells with purified pertussis toxin does not result in cell death but rather in disruptions in native G-protein signaling via uncoupling of the heterotrimeric G protein complexes [80]. As the pertussis toxin’s systemic effects are what contribute to pertussis disease [21,80], it could be that PltA also contributes to the systemic, or prolonged effects of typhoid fever, but not to the development of acute disease. Therefore, while these studies suggest that the role of PltA in the procurement of the cell cycle arrest phenotype is more or less related to its ability to ensure proper trafficking of CdtB, a role for PltA in pathogenesis cannot be excluded as these studies did not characterize phenotypes that are typically associated with ADP-ribosylation. As typhoid toxin cytotoxicity is dependent on PltA and PltB [30], and studies using a mouse model to assess typhoid toxin activity in vivo have primarily used catalytically-active-PltA [76,77,81,82], it is difficult to assess any potential contributions of PltA-mediated toxicity. Drawing upon what is known about the pertussis toxin’s role in pathogenesis, it would be interesting to determine if PltA is capable of inducing histamine sensitization, lymphocytosis, and insulinemia [83], all of which are established pathologies associated with the pertussis toxin in pertussis disease [21,80].
4. ArtAB Toxin
Saitoh et al. first described genes of a putative bARTT in S. Typhimurium DT104, which they named artAB, for ADP-ribosylating toxin [31]. Today, at least 45 different serovars of Salmonella have been found, using a combination of PCR-based and in silico screening methods to detect artAB, 39 of which (87%) are known to also encode typhoid toxin genes (Table 2). Although the genetic regulatory machinery associated with artAB is not well characterized, multiple studies have found that artAB is encoded within a prophage. When Saitoh et al. treated cultures of artAB-positive S. Typhimurium DT104 with mitomycin C, a DNA-damaging agent that was previously associated with prophage excision [84], they observed a loss of artAB in these isolates [31]. Indeed, artAB was later characterized to be encoded within prophage Gifsy-1 in S. Typhimurium DT-104 isolates [85]. Interestingly, artAB in S. Inverness strain FSL R8-3668 was encoded on the prophage PhInv-1b [86], which was not homologous to Gifsy-1, suggesting that transmission of artAB is likely mediated by multiple prophages. Depending on the serovar, artA and artB are encoded as two discrete genes (Figure 1), or in the case of S. Javiana, artA is a pseudogene that overlaps with artB [81].
Given its structural homology with PtxA from the pertussis toxin (Figure 3) our understanding of ArtAB and its role in virulence has been largely guided by previous work characterizing PtxA.
4.1. ArtAB Toxin’s ADP-Ribosyltransferase Activity and Structure
ArtAB is composed of one subunit of ArtA that uses a pentamer of ArtB subunits for the binding domain (Figure 2) [33]. Although the crystal structure of the holotoxin has not been solved, the crystal structure of individual subunits (both known and modelled) shows an overall conserved structure of ArtA with PltA and PtxA (Figure 3). Alignment of ArtA with PtxA shows the conservation of key amino acid residues that are critical for both NAD+ binding and the catalytic activity of PtxA. Namely, residues Arg9 and Cys41 involved in NAD+ binding, and catalytic residues His35 and Glu129, involved in the mature ArtA polypeptide (i.e., following hypothetical cleavage of the signal peptide [21]) are conserved in ArtA (Figure 3).
Given ArtA’s conserved catalytic and structural amino acid residues, Uchida et al. predicted that the target of ArtA’s ADP-ribosyltransferase activity, like PtxA of the pertussis toxin, would be host G-proteins. Similar to PtxA, reduction of the disulfide bonds is necessary for ArtB activity [34]. Co-incubation of both culture supernatants of S. Typhimurium DT104 and ArtA expressed in E. coli, with CHO cell post-nuclear supernatants, identified at least one 41 kDa protein that was ADP-ribosylated [34]. Using pertussis toxin as a control, which is known to ADP-ribosylate cysteine residues at the carboxy-terminus of host Gαi2 and Gαi3 proteins [87], ArtA-treated lysates prevented pertussis toxin-mediated ADP-ribosylation of host proteins [34], suggesting that ArtA and PtxA ADP-ribosylate the same residues in G proteins. In vitro ADP-ribosylation of purified Gαi2, and to a lesser extent Gαi3, proteins, confirmed that ArtA acts in a similar manner to PtxA from the pertussis toxin [88]. Taken together, the pertussis toxin’s well-established role in virulence [80,83,89] and the parallels between ArtA and PtxA’s structure and conservation of key amino acid residues, this novel bARTT may play an important role in Salmonella virulence.
4.2. Implications of ArtAB-Mediated Salmonella Virulence
Treatment of various cell lines with purified ArtAB from S. Typhimurium DT104 recapitulates some of the phenotypes established for pertussis toxin cytotoxicity [80,83,90], including a cell clustering phenotype in CHO-K1 cells [34], increased levels of intracellular cAMP in isoproterenol-induced RAW 264.7 macrophage-like cells [33], increased serum insulin levels (e.g., insulinemia), and intraperitoneal injection of neonatal mice (a model system commonly used for pertussis toxin), resulted in premature death [33]. Interestingly, ArtAB from S. bongori showed reduced cytotoxicity in both cellular and mouse models, likely due to differences in ArtB binding affinity to host cells [33]. It is important to note that ArtAB did not increase the levels of circulating white blood cells (WBCs; leukocytosis), which is a key role of the pertussis toxin in promoting pertussis disease [21]. Future studies characterizing ArtAB’s potential role in virulence, including additional analyses, such as histamine sensitization, inflammatory histopathology, cytokine expression, and differences in CD4+ and CD8+ T cell populations, all of which have been demonstrated in mouse models of pertussis [91], are needed to better understand ArtAB’s role in virulence. As NTS serovars promote an inflammatory response in the host in order to take advantage of the production of alternate terminal electron acceptors [17], it is interesting that the pertussis toxin is associated with reductions in recruited neutrophils and decreases in pro-inflammatory cytokine production at the onset of disease, which is likely reflective of the inactivation of G-protein-coupled chemokine receptors due to ADP-ribosylation [89]. Therefore, ArtAB’s role in pathogenesis is likely dependent on Salmonella’s ability to both balance pro-inflammatory responses and delay Salmonella clearance by immune cells. Studies examining ArtAB in the context of Salmonella infection will be important for understanding what roles this toxin might play.
5. SboC/SeoC Toxin
Whole genome sequence characterization of S. bongori identified several novel SPI-1-encoded genes not found in S. enterica subsp. enterica strains [46]. One such gene, sboC, shared 57% sequence homology with EspJ from enteropathogenic E. coli (EPEC) and the rodent pathogen Citrobacter rodentium [32]. EspJ is a type III secretion effector of EPEC strains and is encoded on the cryptic prophage CP-933U [92]; EspJ simultaneously ADP-ribosylates and amidates the conserved kinase-domain residue Glu310 of Src kinase [35]. While homologues of SboC have not been identified in S. enterica subsp. enterica, screening of other S. enterica subspecies identified an additional homolog, called SeoC, in S. enterica subsp. arizonae isolates (8/9 isolates screened) and S. enterica subsp. salamae (4/7 isolates screened) [32,46]. Alignment of the translated amino acid sequences of SeoC from both subsp. arizonae (83% identity) and subsp. salamae (77–78% identity) strains demonstrates a high level of homology with SboC [32]. Alignment with both E. coli and C. rodentium EspJ showed 56–57% amino acid identity among SeoC/SboC from Salmonella [32]. Although infections with S. bongori and S. enterica subsp. arizonae and salamae are relatively rare, the discovery of SboC/SeoC is important, as it demonstrates the utility of whole genome sequencing analysis in the identification of novel virulence factors in lesser studied salmonellae.
5.1. SboC/SeoC Toxin’s ADP-Ribosyltransferase Activity and Structure
Given the recent discovery of these toxins, formal crystallization studies have not been performed for SboC or SeoC or its homolog EspJ, although alignment of amino acid sequences shows conservation of Arg79 and Asp187 residues involved in the catalytic function [32]. Translocation of SboC and SeoC was mediated by the T3SS encoded within Salmonella pathogenicity island 1, as ΔinvA mutants failed to translocate SboC and SeoC into HeLa cells [32,46]. Interestingly, inactivation of either the SPI-2 or the locus of enterocyte effacement (LEE)-encoded T3SSs also marginally decreased translocation of SeoC, suggesting that multiple T3SSs may be involved in its translocation [32]. Upon entering the cell, SboC/SeoC ADP-ribosylates residue Glu310 of Src kinase [32] and the Glu236 residue of Csk [93], and potentially other nonreceptor tyrosine kinases.
5.2. Implications of SboC/SeoC-Mediated Salmonella Virulence
Although infections with S. bongori and S. enterica subspecies arizonae and salamae occur in humans [94], these salmonellae are more frequently associated with the resident microbiota of reptiles [95]. Furthermore, while S. bongori and S. enterica subspecies arizonae and salamae encode SPI-1 genes, which are essential for invasion of host cells [96], S. bongori and subsp. arizonae lack SPI-2 genes that would be necessary for intracellular survival [46], therefore decreasing the likelihood of their causing salmonellosis in healthy animals/humans. Infection of J774.A1 macrophage-like cells with sboC/seoC-encoding Salmonella reduced phagocytosis of IgG opsonized beads, suggesting that similar to EspJ, ADP-ribosylation of nonreceptor tyrosine kinases is inhibitory to opsonophagocytosis [32], which could reduce the number of bacteria phagocytized by immune cells. One study found that intestinal epithelial cells collected from C57BL/6 mice infected with wild-type C. rodentium had an altered proteome, having significantly lower levels of proteins involved in cytokine responses, immune cell migration and adhesion, and phagocytosis, compared to the levels of these proteins among intestinal epithelial cells of mice infected with the ΔespJ mutant [93]. In the context of S. bongori and S. enterica subsp. arizonae and salamae, SboC/SeoC may reduce phagocytic killing, allowing the bacteria to persist in a susceptible host, although experimental confirmation will be necessary to assert a role for SboC/SeoC.
6. Taking What We Know About Salmonella bARTTs and Moving the Field Forward
Our understanding of the potential role that toxins play in salmonellosis, including the potential for host adaptation/specificity, represents an important and exciting area of research. As novel [30,32,46] and well-characterized [51] bARTTs have been identified from genomic characterizations of more diverse Salmonella strains, the true diversity of Salmonella bARTTs is likely underestimated. One key hurdle in documenting this diversity is related to the lack of publicly available whole genome sequence data for less common Salmonella serovars; two recent studies used whole genome sequence data to analyze 246 [97] and 266 [98] different serovars, representing just ~15–17% of S. enterica subsp. enterica serovars [2]. With the transition from traditional typing schemes (e.g., serology, MLST, PFGE, phage typing) to whole genome sequencing [99,100], bioinformatic screens of previously un-sequenced serovars will likely reveal new serovars encoding known bARTTs as well as novel bARTTs.
Although SpvB was the first Salmonella bARTT to be discovered, our understanding of the mechanistic role that SpvB plays during an infection is surprisingly less studied compared to the novel bARTTs. The application of novel genetic tools and cell and animal models to revisit SpvB’s role in pathogenesis may enhance our understanding of why spvB-encoding serovars are typically more virulent than their spvB-null relatives, especially considering that a number of studies compared the phenotypes of strains before and after plasmid curing, essentially testing the cumulative effects of the entire Spv operon. Given SpvB’s limited distribution among Salmonella serovars and its association with serovars being more likely to cause invasive disease, an investigation into the outcomes of exposure to this toxin, both at the cellular and host levels would be beneficial in furthering our understanding of the biological significance of SpvB and other actin-targeting bARTTs during an infection. Furthermore, characterizations aimed at determining within-serovar conservation of these bARTTs would be beneficial in informing our understanding of why some serovars, and even some strains within a serovar, are more likely to infect, and/or cause disease in a given host. Last, the majority of characterizations of these toxins focused on their role in acute salmonellosis. As these bARTTs may have immunomodulatory effects (e.g., neutropenia associated with the typhoid toxin), an examination of the role(s) that bARTTs play in terms of prolonging Salmonella clearance from the host (i.e., prolonged shedding), and their potential role in recurrent infection as a result of dampening the adaptive immune response, presents an intriguing, and presently, unanswered question.
Overall, extensive characterizations of Salmonella bARTTs have either not been done, or characterizations are reflective of just a handful of serovars or strains. Of particular interest is the potential role that these bARTTs play in regard to (i) enhancing virulence of different serovars, (ii) adaptation to, or restriction to a particular host(s), and (iii) how these bARTTs could be used as potential targets of novel treatment or preventive strategies, such as has been done with pertussis toxin in the dTaP vaccine [101]. Investigation of the roles that Salmonella bARTTs play could also serve to clarify our understanding of the potential roles of homologous bARTTs found in other species.
7. Conclusions
Salmonella remains one of the most important bacterial pathogens worldwide. The discovery of novel bARTTs encoded by a diverse number of Salmonella serovars represents an exciting new avenue with the potential for uncovering both unresolved questions about differences in disease severity, and alternative strategies for reducing the morbidity and mortality associated with salmonellosis. The examination of these, and homologous bARTTs found in other bacterial pathogens, demonstrate the wide array of mechanisms that bacteria have evolved to alter host responses in order to promote their survival and transmission.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brenner, F.; Villar, R.; Angulo, F.; Tauxe, R.; Swaminathan, B. Salmonella nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [PubMed]
- Issenhuth-Jeanjean, S.; Roggentin, P.; Mikoleit, M.; Guibourdenche, M.; de Pinna, E.; Nair, S.; Fields, P.I.; Weill, F.-X. Supplement 2008–2010 (no. 48) to the White–Kauffmann–Le Minor scheme. Res. Microbiol. 2014, 165, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, G.M.; Pignato, S.; Mammina, C.; Grimont, F.; Grimont, P.A.; Nastasi, A.; Giammanco, G. Persistent endemicity of Salmonella bongori 48: z35:-in southern Italy: Molecular characterization of human, animal, and environmental isolates. J. Clin. Microbiol. 2002, 40, 3502–3505. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O. Persistent infection and long-term carriage of typhoidal and nontyphoidal salmonellae. Clin. Microbiol. Rev. 2018, 32, e00088-18. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.F.; Ingram, L.A.; Cieslak, P.R.; Vugia, D.J.; Tobin-D’Angelo, M.; Hurd, S.; Medus, C.; Cronquist, A.; Angulo, F.J. Salmonellosis outcomes differ substantially by serotype. J. Infect. Dis. 2008, 198, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Boore, A.L.; Hoekstra, R.M.; Iwamoto, M.; Fields, P.I.; Bishop, R.D.; Swerdlow, D.L. Salmonella enterica infections in the United States and assessment of coefficients of variation: A novel approach to identify epidemiologic characteristics of individual serotypes, 1996–2011. PLoS ONE 2015, 10, e0145416. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Lo Fo Wong, D.M.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Path. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef]
- Pecoraro, H.L.; Thompson, B.; Duhamel, G.E. Histopathology case definition of naturally acquired Salmonella enterica serovar Dublin infection in young Holstein cattle in the northeastern United States. J. Vet. Diagn. Invest. 2017, 29, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Moreno Switt, A.I.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 32. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Ebel, E.D.; Williams, M.S.; Cole, D.; Travis, C.C.; Klontz, K.C.; Golden, N.J.; Hoekstra, R.M. Comparing characteristics of sporadic and outbreak-associated foodborne illnesses, United States, 2004–2011. Emerg. Infect. Dis. 2016, 22, 1193. [Google Scholar] [CrossRef] [PubMed]
- Tsolis, R.M.; Xavier, M.N.; Santos, R.L.; Baumler, A.J. How to become a top model: Impact of animal experimentation on human Salmonella disease research. Infect. Immun. 2011, 79, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, S.C.; Forest, C.G.; Lepage, C.; Leclerc, J.-M.; Daigle, F. So similar, yet so different: Uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol. Lett. 2010, 305, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chavez, F.; Baumler, A.J. The pyromaniac inside you: Salmonella metabolism in the host gut. Annu. Rev. Microbiol. 2015, 69, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Raffatellu, M.; Chessa, D.; Wilson, R.P.; Dusold, R.; Rubino, S.; Bäumler, A.J. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 2005, 73, 3367–3374. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Atluri, V.; Poon, V.; Romão, E.L.; Tsolis, R.M.; Bäumler, A.J. The flagellar regulator TviA reduces pyroptosis by Salmonella enterica serovar Typhi. Infect. Immun. 2015, 83, 1546–1555. [Google Scholar] [CrossRef]
- Song, J.; Gao, X.; Galán, J.E. Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature 2013, 499, 350–354. [Google Scholar] [CrossRef]
- Locht, C.; Coutte, L.; Mielcarek, N. The ins and outs of pertussis toxin. FEBS J. 2011, 278, 4668–4682. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Bärmann, M.; Ohishi, I.; Tsuyama, S.; Jakobs, K.; Habermann, E. Botulinum C2 toxin ADP-ribosylates actin. Nature 1986, 322, 390. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J. Diphtheria toxin: Mode of action and structure. Bacteriol. Rev. 1975, 39, 54–85. [Google Scholar] [PubMed]
- Gill, D.M.; Meren, R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: Basis of the activation of adenylate cyclase. Proc. Natl. Acad. Sci. USA 1978, 75, 3050–3054. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.C.; Aktories, K.; Barbieri, J.T. Novel bacterial ADP-ribosylating toxins: Structure and function. Nat. Rev. Microbiol. 2014, 12, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Barbieri, J.T. Molecular mechanisms of the cytotoxicity of ADP-ribosylating toxins. Annu. Rev. Microbiol. 2008, 62, 271–288. [Google Scholar] [CrossRef]
- Lang, A.E.; Kuhn, S.; Mannherz, H.G. Photorhabdus luminescens toxins TccC3 and TccC5 affect the interaction of actin with actin-binding proteins essential for treadmilling. Curr. Top. Microbiol. Immunol. 2017, 399, 53–67. [Google Scholar]
- Lang, A.E.; Schmidt, G.; Schlosser, A.; Hey, T.D.; Larrinua, I.M.; Sheets, J.J.; Mannherz, H.G.; Aktories, K. Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science 2010, 327, 1139–1142. [Google Scholar] [CrossRef]
- Guiney, D.G.; Fierer, J. The role of the spv genes in Salmonella pathogenesis. Front. Microbiol. 2011, 2, 129–129. [Google Scholar] [CrossRef]
- Spanò, S.; Ugalde, J.E.; Galán, J.E. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell. Host Microbe 2008, 3, 30–38. [Google Scholar] [CrossRef]
- Saitoh, M.; Tanaka, K.; Nishimori, K.; Makino, S.-i.; Kanno, T.; Ishihara, R.; Hatama, S.; Kitano, R.; Kishima, M.; Sameshima, T. The artAB genes encode a putative ADP-ribosyltransferase toxin homologue associated with Salmonella enterica serovar Typhimurium DT104. Microbiology. 2005, 151, 3089–3096. [Google Scholar] [CrossRef]
- Pollard, D.J.; Young, J.C.; Covarelli, V.; Herrera-León, S.; Connor, T.R.; Fookes, M.; Walker, D.; Echeita, A.; Thomson, N.R.; Berger, C.N.; et al. The type III secretion system effector SeoC of Salmonella enterica subsp. salamae and S. enterica subsp. arizonae ADP-ribosylates Src and inhibits opsonophagocytosis. Infect. Immun. 2016, 84, 3618–3628. [Google Scholar]
- Tamamura, Y.; Tanaka, K.; Uchida, I. Characterization of pertussis-like toxin from Salmonella spp. that catalyzes ADP-ribosylation of G proteins. Sci. Rep. 2017, 7, 2653–2653. [Google Scholar] [CrossRef] [PubMed]
- Uchida, I.; Ishihara, R.; Tanaka, K.; Hata, E.; Makino, S.; Kanno, T.; Hatama, S.; Kishima, M.; Akiba, M.; Watanabe, A.; et al. Salmonella enterica serotype Typhimurium DT104 ArtA-dependent modification of pertussis toxin-sensitive G proteins in the presence of [32P]NAD. Microbiology 2009, 155, 3710–3718. [Google Scholar] [CrossRef]
- Young, J.C.; Clements, A.; Lang, A.E.; Garnett, J.A.; Munera, D.; Arbeloa, A.; Pearson, J.; Hartland, E.L.; Matthews, S.J.; Mousnier, A.; et al. The Escherichia coli effector EspJ blocks Src kinase activity via amidation and ADP ribosylation. Nat. Commun. 2014, 5, 5887. [Google Scholar] [CrossRef]
- Hochmann, H.; Pust, S.; von Figura, G.; Aktories, K.; Barth, H. Salmonella enterica SpvB ADP-ribosylates actin at position arginine-177-characterization of the catalytic domain within the SpvB protein and a comparison to binary clostridial actin-ADP-ribosylating toxins. Biochemistry 2006, 45, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Kappeli, R.; Kaiser, P.; Stecher, B.; Hardt, W.D. Roles of spvB and spvC in S. Typhimurium colitis via the alternative pathway. Int. J. Med. Microbiol. 2011, 301, 117–124. [Google Scholar] [CrossRef]
- Gulig, P.A. Virulence plasmids of Salmonella typhimurium and other salmonellae. Microb. Pathog. 1990, 8, 3–11. [Google Scholar] [CrossRef]
- Jones, G.W.; Rabert, D.K.; Svinarich, D.M.; Whitfield, H.J. Association of adhesive, invasive, and virulent phenotypes of Salmonella typhimurium with autonomous 60-megadalton plasmids. Infect. Immun. 1982, 38, 476–486. [Google Scholar] [PubMed]
- Williamson, C.M.; Pullinger, G.D.; Lax, A.J. Identification of an essential virulence region on Salmonella plasmids. Microb. Pathog. 1988, 5, 469–473. [Google Scholar] [CrossRef]
- Lesnick, M.L.; Reiner, N.E.; Fierer, J.; Guiney, D.G. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol. Microbiol. 2001, 39, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Libby, S.J.; Lesnick, M.; Hasegawa, P.; Kurth, M.; Belcher, C.; Fierer, J.; Guiney, D.G. Characterization of the spv locus in Salmonella enterica serovar Arizona. Infect. Immun. 2002, 70, 3290–3294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rotger, R.; Casadesus, J. The virulence plasmids of Salmonella. Int. Microbiol. 1999, 2, 177–184. [Google Scholar]
- Haneda, T.; Ishii, Y.; Shimizu, H.; Ohshima, K.; Iida, N.; Danbara, H.; Okada, N. Salmonella type III effector SpvC, a phosphothreonine lyase, contributes to reduction in inflammatory response during intestinal phase of infection. Cell. Microbiol. 2012, 14, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Rolhion, N.; Furniss, R.C.; Grabe, G.; Ryan, A.; Liu, M.; Matthews, S.A.; Holden, D.W. Inhibition of nuclear transport of NF-kB p65 by the Salmonella type III secretion system effector SpvD. PLoS Pathog. 2016, 12, e1005653. [Google Scholar] [CrossRef] [PubMed]
- Fookes, M.; Schroeder, G.N.; Langridge, G.C.; Blondel, C.J.; Mammina, C.; Connor, T.R.; Seth-Smith, H.; Vernikos, G.S.; Robinson, K.S.; Sanders, M.; et al. Salmonella bongori provides insights into the evolution of the Salmonellae. PLoS Pathog. 2011, 7, e1002191. [Google Scholar] [CrossRef] [PubMed]
- den Bakker, H.C.; Switt, A.I.M.; Govoni, G.; Cummings, C.A.; Ranieri, M.L.; Degoricija, L.; Hoelzer, K.; Rodriguez-Rivera, L.D.; Brown, S.; Bolchacova, E.; et al. Genome sequencing reveals diversification of virulence factor content and possible host adaptation in distinct subpopulations of Salmonella enterica. BMC Genom. 2011, 12, 425. [Google Scholar] [CrossRef]
- Silva, C.; Puente, J.L.; Calva, E. Salmonella virulence plasmid: Pathogenesis and ecology. Pathog. Dis. 2017, 75, ftx070. [Google Scholar] [CrossRef]
- Akiba, M.; Sameshima, T.; Anzai, T.; Wada, R.; Nakazawa, M. Salmonella Abortusequi strains of equine origin harbor a 95kb plasmid responsible for virulence in mice. Vet. Microbiol. 1999, 68, 265–272. [Google Scholar] [CrossRef]
- Uzzau, S.; Gulig, P.A.; Paglietti, B.; Leori, G.; Stocker, B.A.; Rubino, S. Role of the Salmonella abortusovis virulence plasmid in the infection of BALB/c mice. FEMS Microbiol. Lett. 2000, 188, 15–18. [Google Scholar] [CrossRef]
- Bronowski, C.; Fookes, M.C.; Gilderthorp, R.; Ashelford, K.E.; Harris, S.R.; Phiri, A.; Hall, N.; Gordon, M.A.; Wain, J.; Hart, C.A.; et al. Genomic characterisation of invasive non-typhoidal Salmonella enterica subspecies enterica serovar Bovismorbificans isolates from Malawi. PLoS Negl. Trop. Dis. 2013, 7, e2557. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Wiedmann, M. The cytolethal distending toxin produced by nontyphoidal Salmonella serotypes Javiana, Montevideo, Oranienburg, and Mississippi induces DNA damage in a manner similar to that of serotype Typhi. mBio 2016, 7, e02109-16. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Q.; Feng, Y.; Wang, Y.; Zou, Q.H.; Chen, F.; Guo, J.T.; Peng, Y.H.; Jin, Y.; Li, Y.G.; Hu, S.N.; et al. Salmonella paratyphi C: Genetic divergence from Salmonella choleraesuis and pathogenic convergence with Salmonella typhi. PLoS ONE 2009, 4, e4510. [Google Scholar] [CrossRef] [PubMed]
- WHO. Collaborating Centre for Reference and Research on Salmonella. Antigenic Formulae of the Salmonella Serovars; WHO: Geneva, Switzerland, 2007; Available online: https://www.pasteur.fr/sites/default/files/veng_0.pdf (accessed on 17 June 2019).
- CDC. National Enteric Disease Surveillance: Salmonella Annual Report, 2016; CDC: Atlanta, GA, USA, 2016. Available online: https://www.cdc.gov/nationalsurveillance/pdfs/2016-Salmonella-report-508.pdf (accessed on 17 June 2019).
- Jennings, E.; Thurston, T.L.M.; Holden, D.W. Salmonella SPI-2 type III secretion system effectors: Molecular mechanisms and physiological consequences. Cell Host Microbe 2017, 22, 217–231. [Google Scholar] [CrossRef] [PubMed]
- LaRock, D.L.; Chaudhary, A.; Miller, S.I. Salmonellae interactions with host processes. Nat. Rev. Microbiol. 2015, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Steele-Mortimer, O. The Salmonella-containing vacuole: Moving with the times. Curr. Opin. Microbiol. 2008, 11, 38–45. [Google Scholar] [CrossRef]
- Mesa-Pereira, B.; Medina, C.; Camacho, E.M.; Flores, A.; Santero, E. Novel tools to analyze the function of Salmonella effectors show that SvpB ectopic expression induces cell cycle arrest in tumor cells. PLoS ONE 2013, 8, e78458. [Google Scholar] [CrossRef] [PubMed]
- Lohez, O.D.; Reynaud, C.; Borel, F.; Andreassen, P.R.; Margolis, R.L. Arrest of mammalian fibroblasts in G1 in response to actin inhibition is dependent on retinoblastoma pocket proteins but not on p53. J. Cell Biol. 2003, 161, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Brittnacher, M.; Haraga, A.; Jeng, R.L.; Welch, M.D.; Miller, S.I. Salmonella effectors translocated across the vacuolar membrane interact with the actin cytoskeleton. Mol. Microbiol. 2003, 48, 401–415. [Google Scholar] [CrossRef]
- Meresse, S.; Unsworth, K.E.; Habermann, A.; Griffiths, G.; Fang, F.; Martinez-Lorenzo, M.J.; Waterman, S.R.; Gorvel, J.P.; Holden, D.W. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell. Microbiol. 2001, 3, 567–577. [Google Scholar] [CrossRef]
- Portnoy, D.A.; Auerbuch, V.; Glomski, I.J. The cell biology of Listeria monocytogenes infection: The intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 2002, 158, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Reeves, A.Z.; Klein, J.A.; Twedt, D.J.; Knodler, L.A.; Lesser, C.F. The type III secretion system apparatus determines the intracellular niche of bacterial pathogens. Proc. Natl. Acad. Sci. USA 2016, 113, 4794–4799. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.; Marteyn, B.; Sansonetti, P.J.; Tang, C.M. Life on the inside: The intracellular lifestyle of cytosolic bacteria. Nat. Rev. Microbiol. 2009, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Sørensen, G.; Löfström, C.; Leekitcharoenphon, P.; Nielsen, B.; Wingstrand, A.; Aarestrup, F.M.; Hendriksen, R.S.; Baggesen, D.L. Reappearance of Salmonella serovar Choleraesuis var. Kunzendorf in Danish pig herds. Vet. Microbiol. 2015, 176, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Grandolfo, E.; Parisi, A.; Ricci, A.; Lorusso, E.; de Siena, R.; Trotta, A.; Buonavoglia, D.; Martella, V.; Corrente, M. High mortality in foals associated with Salmonella enterica subsp. enterica Abortusequi infection in Italy. J. Vet. Diagn. Inv. 2018, 30, 483–485. [Google Scholar]
- Pardon, P.; Sanchis, R.; Marly, J.; Lantier, F.; Pepin, M.; Popoff, M. Ovine salmonellosis caused by Salmonella abortus ovis. Ann. Rech. Vet. 1988, 19, 221–235. [Google Scholar] [PubMed]
- Matsui, H.; Bacot, C.M.; Garlington, W.A.; Doyle, T.J.; Roberts, S.; Gulig, P.A. Virulence plasmid-borne spvB and spvC genes can replace the 90-kilobase plasmid in conferring virulence to Salmonella enterica serovar Typhimurium in subcutaneously inoculated mice. J. Bacteriol. 2001, 183, 4652–4658. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, A.S.; Mushamiri, I.; Niemann, G.S.; Brown, R.N.; Adkins, J.N.; Heffron, F. Diverse secreted effectors are required for Salmonella persistence in a mouse infection model. PLoS ONE 2013, 8, e70753. [Google Scholar] [CrossRef] [PubMed]
- Haghjoo, E.; Galán, J.E. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 4614–4619. [Google Scholar] [CrossRef]
- Jinadasa, R.N.; Bloom, S.E.; Weiss, R.S.; Duhamel, G.E. Cytolethal distending toxin: A conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages. Microbiology 2011, 157, 1851–1875. [Google Scholar] [CrossRef]
- Hodak, H.; Galan, J.E. A Salmonella Typhi homologue of bacteriophage muramidases controls typhoid toxin secretion. EMBO Rep. 2013, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rivera, L.D.; Bowen, B.M.; den Bakker, H.C.; Duhamel, G.E.; Wiedmann, M. Characterization of the cytolethal distending toxin (typhoid toxin) in non-typhoidal Salmonella serovars. Gut Pathoh. 2015, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.; Stack, G.; Jiao, X.; Lara-Tejero, M.; Galán, J.E. Alternate subunit assembly diversifies the function of a bacterial toxin. bioRxiv 2019, 624130. [Google Scholar]
- Gao, X.; Deng, L.; Stack, G.; Yu, H.; Chen, X.; Naito-Matsui, Y.; Varki, A.; Galán, J.E. Evolution of host adaptation in the Salmonella typhoid toxin. Nat. Microbiol. 2017, 2, 1592. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.A.; Lee, S.; Zhao, J.; Thompson, A.J.; McBride, R.; Tsogtbaatar, B.; Paulson, J.C.; Nussinov, R.; Deng, L.; Song, J. In vivo tropism of Salmonella Typhi toxin to cells expressing a multiantennal glycan receptor. Nat. Microbiol. 2018, 3, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Jin, S.C.; Jiao, X.; Galan, J.E. Unique features in the intracellular transport of typhoid toxin revealed by a genome-wide screen. PLoS Pathog. 2019, 15, e1007704. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.A.; Hewlett, E.L. Virulence factors of Bordetella pertussis. Annu. Rev. Microbiol. 1986, 40, 661–686. [Google Scholar] [CrossRef]
- Miller, R.A.; Betteken, M.I.; Guo, X.; Altier, C.; Duhamel, G.E.; Wiedmann, M. The typhoid toxin produced by the nontyphoidal Salmonella enterica serotype Javiana is required for induction of a DNA damage response in vitro andsystemic spread in vivo. mBio 2018, 9, e00467-18. [Google Scholar] [CrossRef]
- Belluz, L.D.B.; Guidi, R.; Pateras, I.S.; Levi, L.; Mihaljevic, B.; Rouf, S.F.; Wrande, M.; Candela, M.; Turroni, S.; Nastasi, C.; et al. The typhoid toxin promotes host Ssurvival and the establishment of a persistent asymptomatiinfection. PLoS Pathog. 2016, 12, e1005528. [Google Scholar]
- Carbonetti, N.H. Pertussis toxin and adenylate cyclase toxin: Key virulence factors of Bordetella pertussis and cell biology tools. Future Microbiol. 2010, 5, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Otsuji, N.; Sekiguchi, M.; Iijima, T.; Takagi, Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature 1959, 184, 1079. [Google Scholar] [CrossRef] [PubMed]
- Hiley, L.; Fang, N.X.; Micalizzi, G.R.; Bates, J. Distribution of Gifsy-3 and of variants of ST64B and Gifsy-1 prophages amongst Salmonella enterica serovar Typhimurium isolates: Evidence that combinations of prophages promote clonality. PLoS ONE 2014, 9, e86203. [Google Scholar] [CrossRef] [PubMed]
- Moreno Switt, A.I.; den Bakker, H.C.; Cummings, C.A.; Rodriguez-Rivera, L.D.; Govoni, G.; Raneiri, M.L.; Degoricija, L.; Brown, S.; Hoelzer, K.; Peters, J.E.; et al. Identification and characterization of novel Salmonella mobile elements involved in the dissemination of genes linked to virulence and transmission. PLoS ONE 2012, 7, e41247. [Google Scholar] [CrossRef] [PubMed]
- Avigan, J.; Murtagh, J.J., Jr.; Stevens, L.A.; Angus, C.W.; Moss, J.; Vaughan, M. Pertussis toxin-catalyzed ADP-ribosylation of G(o) alpha with mutations at the carboxyl terminus. Biochemistry 1992, 31, 7736–7740. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Barbieri, J.T. Pertussis toxin-catalyzed ADP-ribosylation of Gi-2 and Gi-3 in CHO cells is modulated by inhibitors of intracellular trafficking. Infect. Immun. 1996, 64, 593–599. [Google Scholar] [PubMed]
- Melvin, J.A.; Scheller, E.V.; Miller, J.F.; Cotter, P.A. Bordetella pertussis pathogenesis: Current and future challenges. Nat. Rev. Microbiol. 2014, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, E.L.; Sauer, K.T.; Myers, G.A.; Cowell, J.L.; Guerrant, R.L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 1983, 40, 1198–1203. [Google Scholar]
- Scanlon, K.M.; Snyder, Y.G.; Skerry, C.; Carbonetti, N.H. Fatal pertussis in the neonatal mouse model is associated with pertussis toxin-mediated pathology beyond the airways. Infect. Immun. 2017, 85, e00355-17. [Google Scholar] [CrossRef]
- Dahan, S.; Wiles, S.; La Ragione, R.M.; Best, A.; Woodward, M.J.; Stevens, M.P.; Shaw, R.K.; Chong, Y.; Knutton, S.; Phillips, A.; et al. EspJ is a prophage-carried type III effector protein of attaching and effacing pathogens that modulates infection dynamics. Infect. Immun. 2005, 73, 679–686. [Google Scholar] [CrossRef]
- Pollard, D.J.; Berger, C.N.; So, E.C.; Yu, L.; Hadavizadeh, K.; Jennings, P.; Tate, E.W.; Choudhary, J.S.; Frankel, G. Broad-spectrum regulation of nonreceptor tyrosine kinases by the bacterial ADP-ribosyltransferase EspJ. mBio 2018, 9, e00170-18. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.L.; Ni, F.C.Y.; Janda, J.M. Increase in extraintestinal infections caused by Salmonella enterica subspecies II-IV. Emerg. Infect. Dis. 2012, 18, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.; Miranda, J.M.; Regal, P.; Vazquez, B.; Franco, C.M.; Cepeda, A. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiolog. Res. 2018, 206, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Res. 2013, 26, 308–341. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.E.; Kruczkiewicz, P.; Laing, C.R.; Lingohr, E.J.; Gannon, V.P.; Nash, J.H.; Taboada, E.N. The Salmonella in silico typing resource (SISTR): An open web-accessible tool for rapidly typing and subtyping draft Salmonella genome assemblies. PLoS ONE 2016, 11, e0147101. [Google Scholar] [CrossRef]
- Worley, J.; Meng, J.; Allard, M.W.; Brown, E.W.; Timme, R.E. Salmonella enterica phylogeny based on whole-genome sequencing reveals two new clades and novel patterns of horizontally acquired genetic elements. mBio 2018, 9, e02303-18. [Google Scholar] [CrossRef]
- Alikhan, N.-F.; Zhou, Z.; Sergeant, M.J.; Achtman, M. A genomic overview of the population structure of Salmonella. PLOS Genet. 2018, 14, e1007261. [Google Scholar] [CrossRef]
- Yachison, C.A.; Yoshida, C.; Robertson, J.; Nash, J.H.E.; Kruczkiewicz, P.; Taboada, E.N.; Walker, M.; Reimer, A.; Christianson, S.; Nichani, A.; et al. The validation and implications of using whole genome sequencing as a replacement for traditional serotyping for a National Salmonella Reference Laboratory. Front. Microbiol. 2017, 8, 1044. [Google Scholar] [CrossRef]
- Wagner, L.D.; Corvette, L.J.; Ngundi, M.M.; Burns, D.L. Towards replacement of the acellular pertussis vaccine safety test: Comparison of in vitro cytotoxic activity and in vivo activity in mice. Vaccine 2017, 35, 7160–7165. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).