Paralytic Shellfish Toxins and Ocean Warming: Bioaccumulation and Ecotoxicological Responses in Juvenile Gilthead Seabream (Sparus aurata)
Abstract
1. Introduction
2. Results
2.1. Influence of Warming on PST Accumulation and Depuration
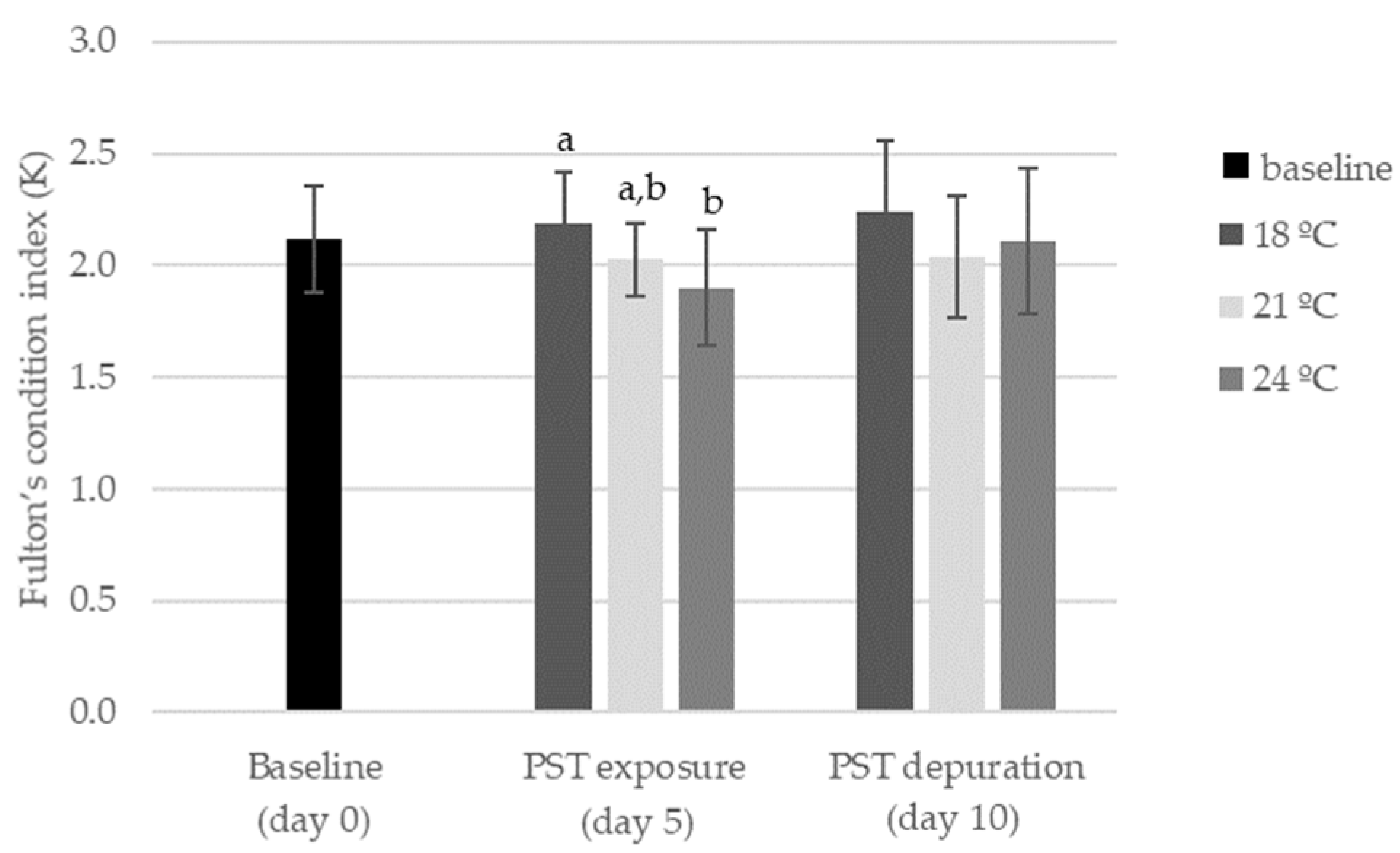
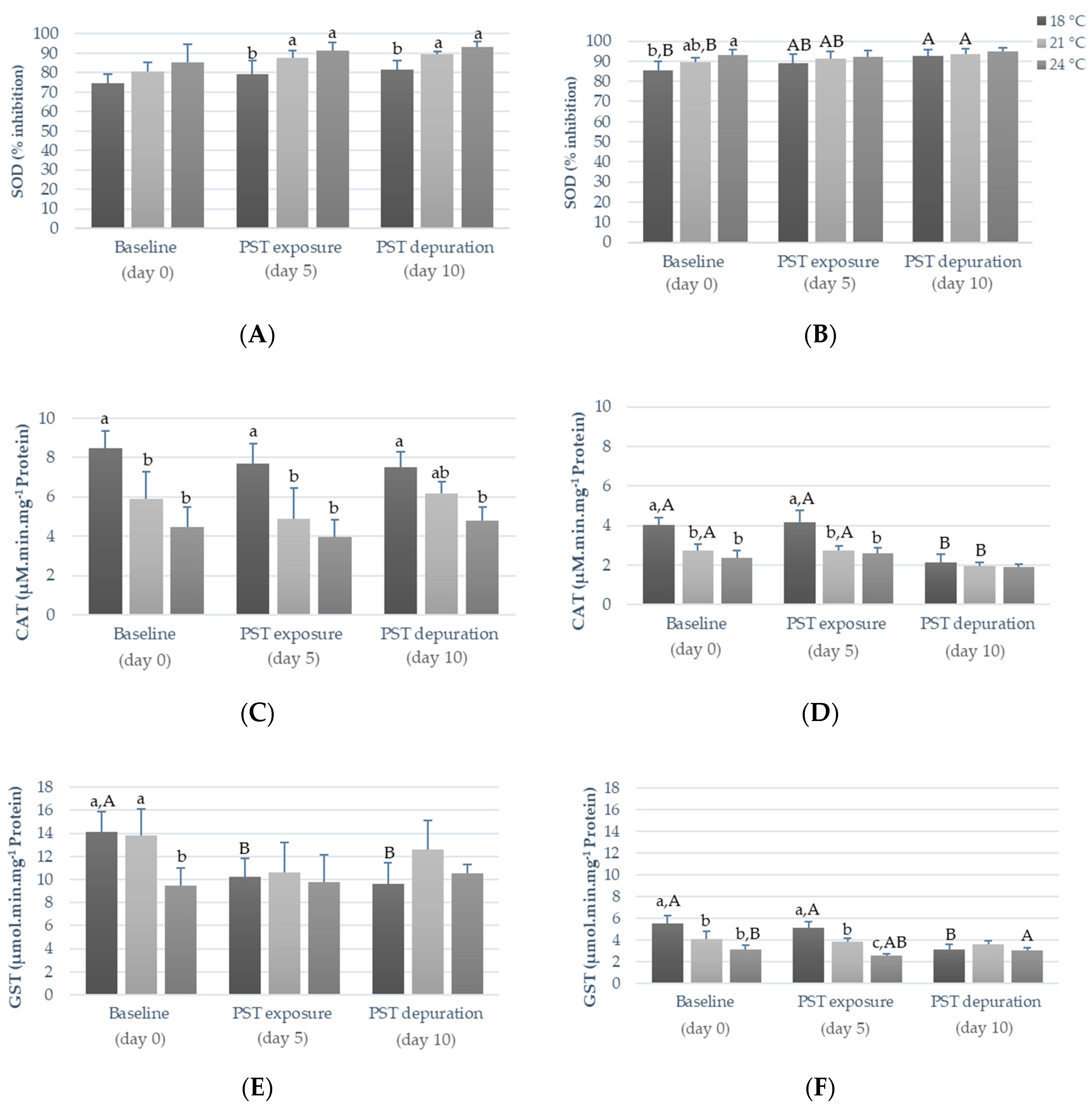
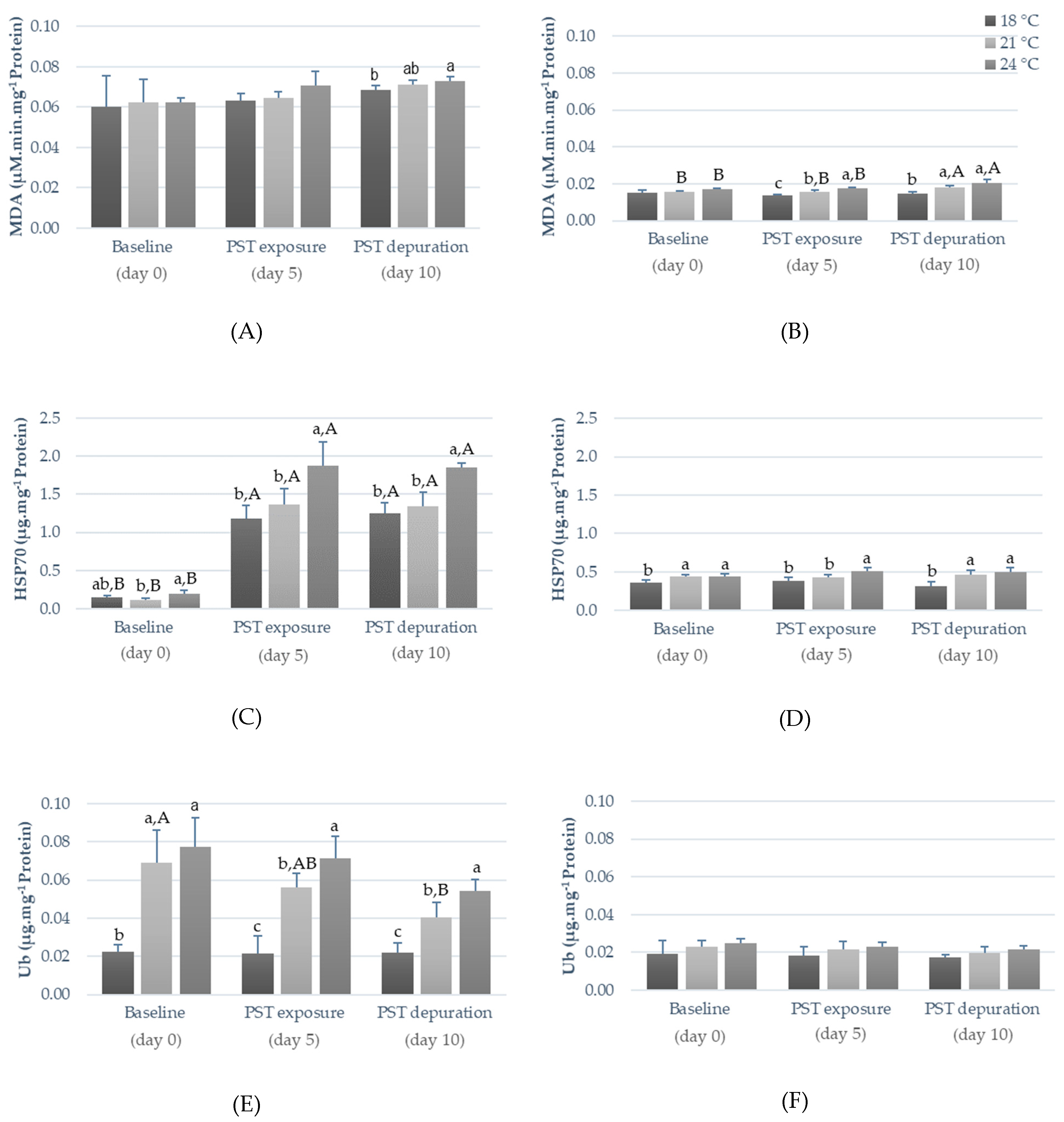
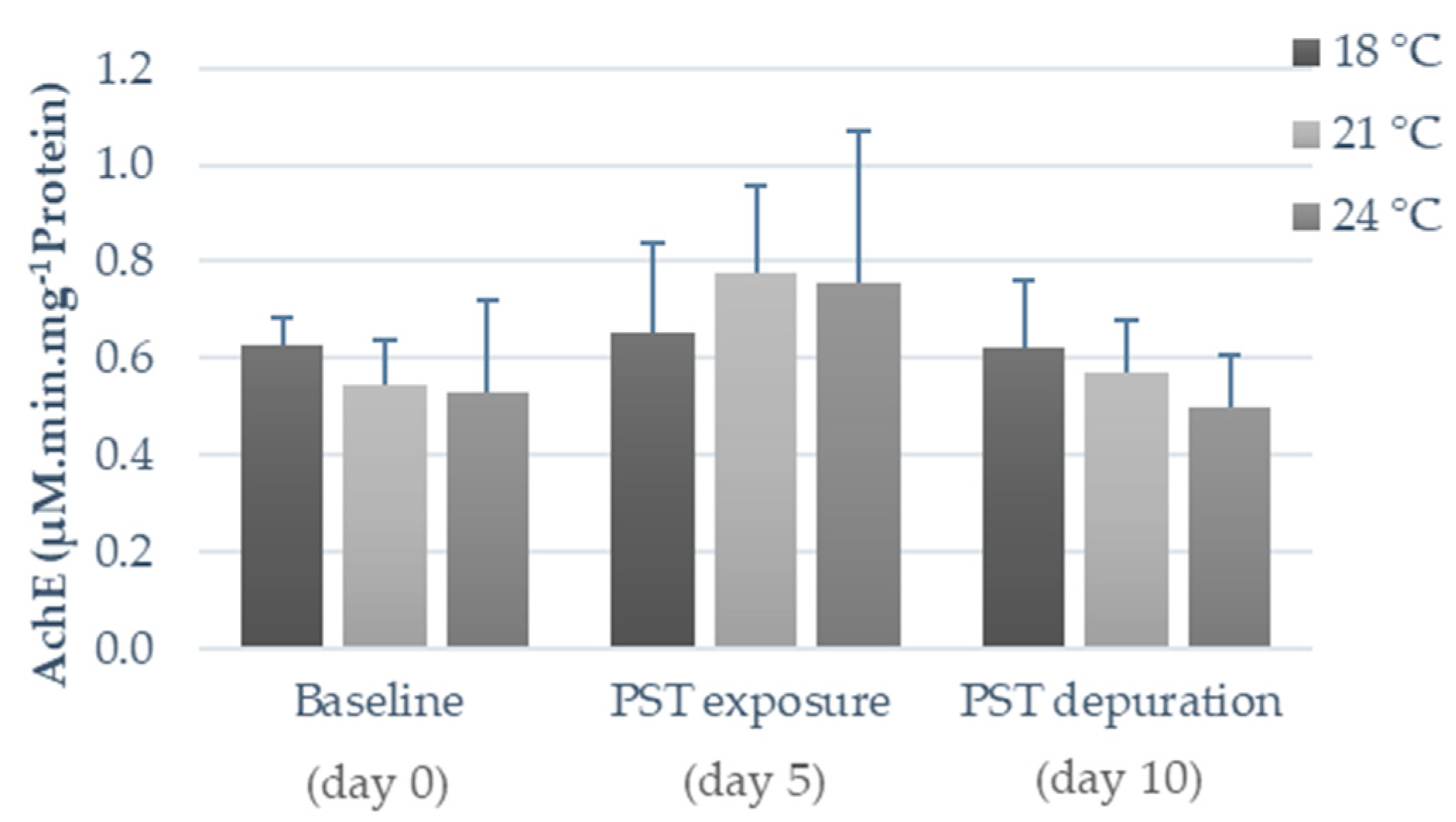
2.2. Influence of Warming and PST Exposure on Fish Ecotoxicological Responses
3. Discussion
3.1. Influence of Warming on PST Accumulation and Depuration
3.2. Influence of Warming and PST Exposure on Fish Biochemical Responses
4. Conclusions
5. Materials and Methods
5.1. Preparation of PST Contaminated Diet
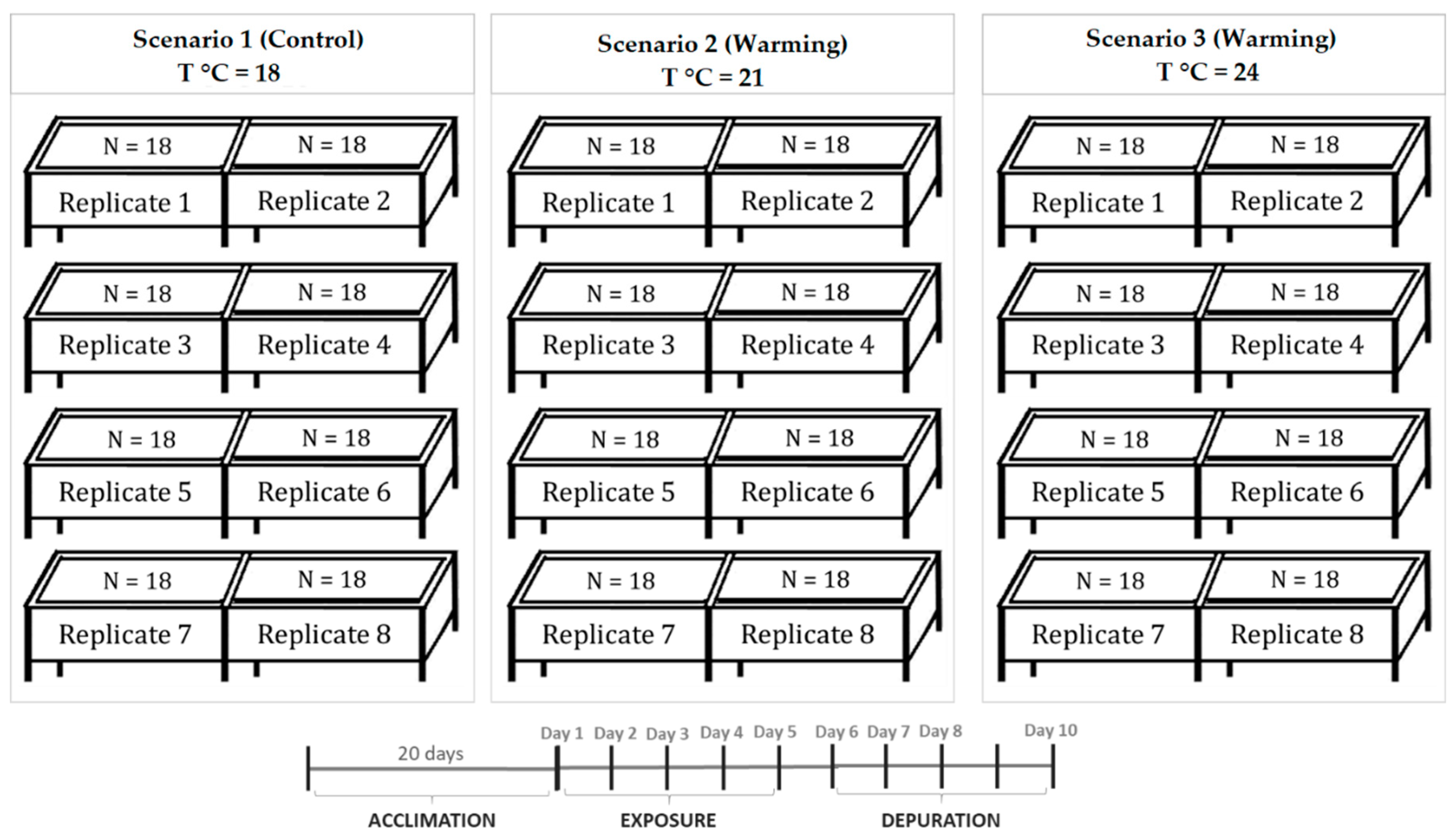
5.2. Experimental Design and Biological Sampling
5.3. Toxins Extraction and Quantification
5.4. Biochemical Assays
5.5. Animal Fitness Index (Fulton’s K index)
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Dolah, F.M. Marine Algal Toxins: Origins, Health Effects, and Their Increased Occurrence. Environ. Health Perspect. 2000, 108, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Nunes, M.L.; Moore, S.K.; Strom, M.S. Climate change and seafood safety: Human health implications. Food Res. Int. 2010, 43, 1766–1779. [Google Scholar] [CrossRef]
- Costa, P.R.; Botelho, M.J.; Lefebvre, K.A. Characterization of paralytic shellfish toxins in seawater and sardines (Sardina pilchardus) during blooms of Gymnodinium catenatum. Hydrobiologia 2010, 655, 89–97. [Google Scholar] [CrossRef]
- Mincarelli, L.F.; Paula, J.R.; Pousão-Ferreira, P.; Rosa, R.; Costa, P.R. Effects of acute waterborne exposure to harmful algal toxin domoic acid on foraging and swimming behaviours of fish early stages. Toxicon 2018, 156, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Costa, S.T.; Braga, A.C.; Rodrigues, S.M.; Vale, P. Relevance and challenges in monitoring marine biotoxins in non-bivalve vectors. Food Control 2017, 76, 24–33. [Google Scholar] [CrossRef]
- Etheridge, S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M.; Berti, M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine Biotoxins: Occurrence, Toxicity, Regulatory Limits and Reference Methods. Front. Microbiol. 2016, 7, 1051. [Google Scholar] [CrossRef] [PubMed]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; 1132p. [Google Scholar]
- Hong, H.Z.; Lam, P.K.; Hsieh, D.P. Interactions of paralytic shellfish toxins with xenobiotic-metabolizing and antioxidant enzymes in rodents. Toxicon 2003, 42, 425–431. [Google Scholar] [CrossRef]
- Madeira, D.; Vinagre, C.; Diniz, M.S. Are fish in hot water? Effects of warming on oxidative stress metabolism in the commercial species Sparus aurata. Ecol. Indic. 2016, 63, 324–331. [Google Scholar] [CrossRef]
- Perreault, F.; Matias, M.S.; Melegari, S.P.; Pinto, C.R.; Creppy, E.E.; Popovic, R.; Matias, W.G. Investigation of animal and algal bioassays for reliable saxitoxin ecotoxicity and cytotoxicity risk evaluation. Ecotoxicol. Environ. Saf. 2011, 74, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.L.; Wunderlin, D.A.; Bistoni, M.A. Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan. Ecotoxicol. Environ. Saf. 2009, 72, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. A 2013, 166, 237–243. [Google Scholar] [CrossRef] [PubMed]
- FAO. Cultured Aquatic Species Information Programme: Sparus aurata. In Cultured Aquatic Species Information Programme; Colloca, F., Cerasi, S., Eds.; FAO Fisheries and Aquaculture Department: Rome, Italy, 2005. [Google Scholar]
- Šegvić-Bubić, T.; Grubišić, L.; Karaman, N.; Tičina, V.; Jelavić, K.M.; Katavić, I. Damages on mussel farms potentially caused by fish predation—Self-service on the ropes? Aquaculture 2011, 319, 497–504. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Marine Biotoxins in Shellfish—Saxitoxin Group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- Costa, P.R.; Lage, S.; Barata, M.; Pousão-Ferreira, P. Uptake, transformation, and elimination kinetics of paralytic shellfish toxins in white seabream (Diplodus sargus). Mar. Biol. 2011, 158, 2805–2811. [Google Scholar] [CrossRef]
- Kwong, R.W.M.; Wang, W.X.; Lam, P.K.S.; Yu, P.K.N. The uptake, distribution and elimination of paralytic shellfish toxins in mussels and fish exposed to toxic dinoflagellates. Aquat. Toxicol. 2006, 80, 82–91. [Google Scholar] [CrossRef]
- Costa, P.R.; Pereira, P.; Guilherme, S.; Barata, M.; Nicolau, L.; Santos, M.A.; Pacheco, M.; Pousão-Ferreira, P. Biotransformation modulation and genotoxicity in white seabream upon exposure to paralytic shellfish toxins produced by Gymnodinium catenatum. Aquat. Toxicol. 2012, 106–107, 42–47. [Google Scholar] [CrossRef]
- Costa, P.R. Impact and effects of paralytic shellfish poisoning toxins derived from harmful algal blooms to marine fish. Fish 2016, 17, 226–248. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Shumway, S.E. Paralytic shellfish toxins in bivalve mollusks: Occurrence, transfer kinetics and biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Camacho, C.; Barbosa, V.; Alves, R.; Anacleto, P.; Cunha, S.C.; Fernandes, J.O.; Pousão-Ferreira, P.; Paula, J.R.; Rosa, R.; et al. Bioaccumulation and ecotoxicological responses of juvenile white seabream (Diplodus sargus) exposed to triclosan, warming and acidification. Environ. Pollut. 2019, 245, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.; Seebacher, F.; O’Connor, W.; Zammit, A.; Harwood, D.T.; Murray, S. Warm temperature acclimation impacts metabolism of paralytic shellfish toxins from Alexandrium minutum in commercial oysters. Glob. Chang. Biol. 2015, 21, 3402–3413. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M. The Effect of Temperature on Paralytic Shellfish Toxin Uptake by Blue Mussels (Mytilus Edulis) and Sea Scallops (Placopecten magellanicus). Honors College Paper 216. 2015. Available online: http://digitalcommons.library.umaine.edu/honors/216 (accessed on 23 May 2019).
- Braga, A.C.; Camacho, C.; Marques, A.; Gago-Martínez, A.; Pacheco, M.; Costa, P.R. Combined effects of warming and acidification on accumulation and elimination dynamics of paralytic shellfish toxins in mussels Mytilus galloprovincialis. Environ. Res. 2018, 164, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Busato, R.H.; Ribeiro, C.A.O.; Cestaric, M.M.; Ramsdorf, W.A.; Magalhães, V.F.; Wosiack, A.C.; Silva de Assis, H.C. Analyses of paralytic shellfish toxins and biomarkers in a southern Brazilian reservoir. Toxicon 2010, 55, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Maulvault, A.L.; Custodio, C.; Anacleto, P.; Repolho, T.; Pousão, P.; Nunes, M.L.; Diniz, M.; Rosa, R.; Marques, M. Bioaccumulation and elimination of mercury in juvenile seabass (Dicentrarchus labrax) in a warmer environment. Environ. Res. 2016, 149, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.J.; Horsberg, T.E. Kinetic properties of saxitoxin in Atlantic salmon (Salmo salar) and Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 152, 444–450. [Google Scholar] [CrossRef]
- Silva de Assis, H.C.; da Silva, C.A.; Oba, E.T.; Pamplona, J.H.; Mela, M.; Doria, H.B.; Guiloski, I.C.; Ramsdorf, W.; Cestari, M.M. Hematologic and hepatic responses of the freshwater fish Hoplias malabaricus after saxitoxin exposure. Toxicon 2013, 66, 25–30. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Barbosa, V.; Alves, R.; Custódio, A.; Anacleto, P.; Repolho, T.; Pousão-Ferreira, P.; Rosa, R.; Marques, A.; Diniz, M. Ecophysiological responses of juvenile seabass (Dicentrarchus labrax) exposed to increased temperature and dietary methylmercury. Sci. Total Environ. 2017, 586, 551–558. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Barbosa, V.; Alves, R.; Anacleto, P.; Camacho, C.; Cunha, S.C.; Fernades, J.O.; Pousão-Ferreira, P.; Rosa, R.; Marques, A.; et al. Integrated multi-biomarker responses of juvenile seabass to diclofenac, warming and acidification co-exposure. Aquat. Toxicol. 2017, 202, 65–79. [Google Scholar] [CrossRef]
- Pandey, S.; Ahmad, I.; Parvez, S.; Bin-Hafeez, B.; Haque, R.; Raisuddin, S. Effect of Endosulfan on Antioxidants of Freshwater Fish Channa punctatus Bloch: 1. Protection Against Lipid Peroxidation in Liver by Copper Preexposure. Arch. Environ. Contam. Toxicol. 2001, 41, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.A.; Oba, E.T.; Ramsdorf, W.A.; Magalhães, V.F.; Cestari, M.M.; Ribeiro, C.A.O.; Silva de Assis, H.C. First report about saxitoxins in freshwater fish Hoplias malabaricus through trophic exposure. Toxicon 2011, 57, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Gubbins, M.J.; Eddy, F.B.; Gallacher, S.; Stagg, R.M. Paralytic shellfish poisoning toxins induce xenobiotic metabolising enzymes in Atlantic salmon (Salmo salar). Mar. Environ. Res. 2000, 50, 479–483. [Google Scholar] [CrossRef]
- Madeira, D.; Vinagre, C.; Costa, P.M.; Diniz, M.S. Histopathological alterations, physiological limits, and molecular changes of juvenile Sparus aurata in response to thermal stress. Mar. Ecol. Prog. Ser. 2014, 505, 253–266. [Google Scholar] [CrossRef]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Chang. 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Niedzwiadek, B. Quantitative determination of paralytic shellfish poisoning toxins in shellfish by using prechromatographic oxidation and liquid chromatography with fluorescence detection. J. AOAC Int. 2001, 84, 1099–1108. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
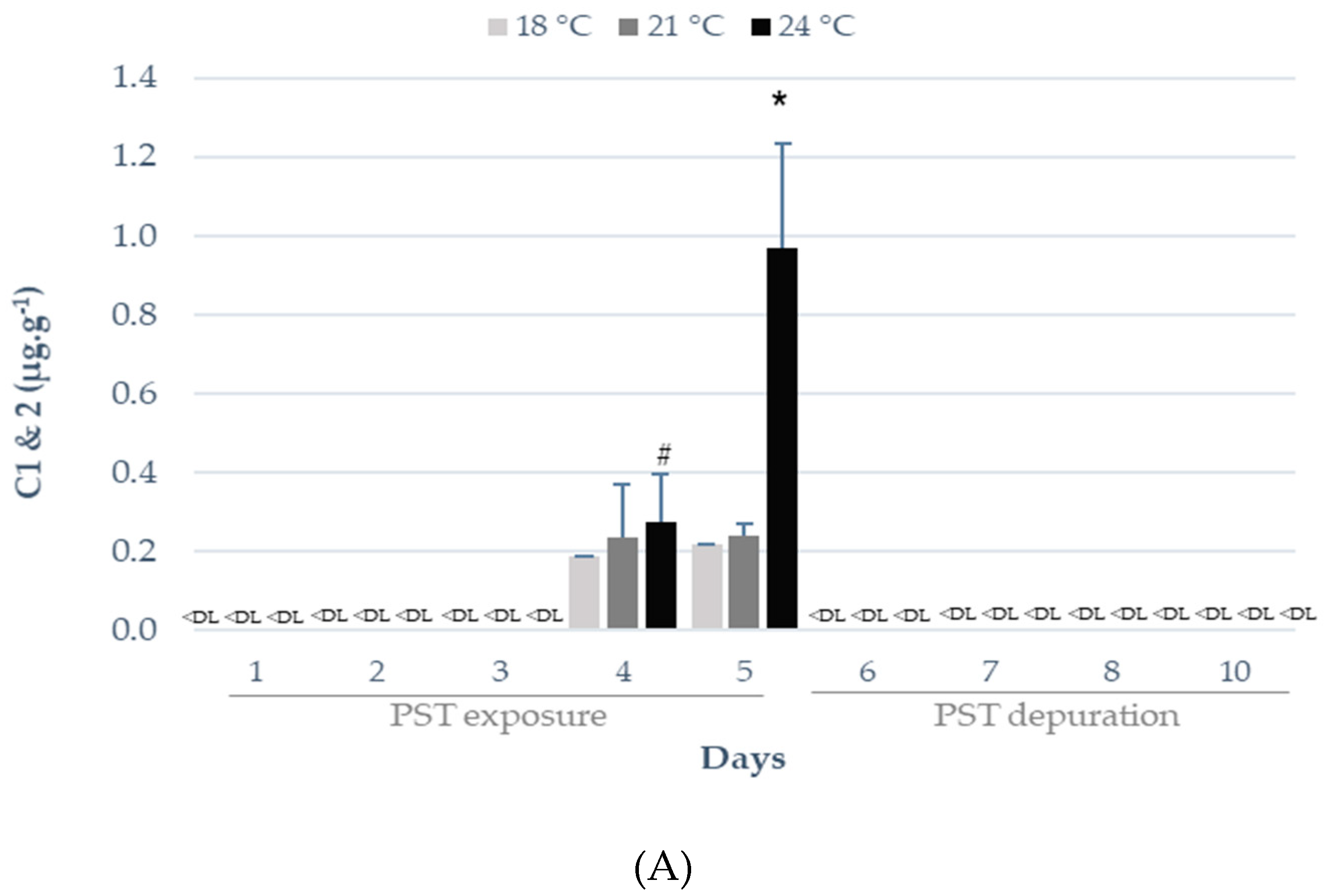
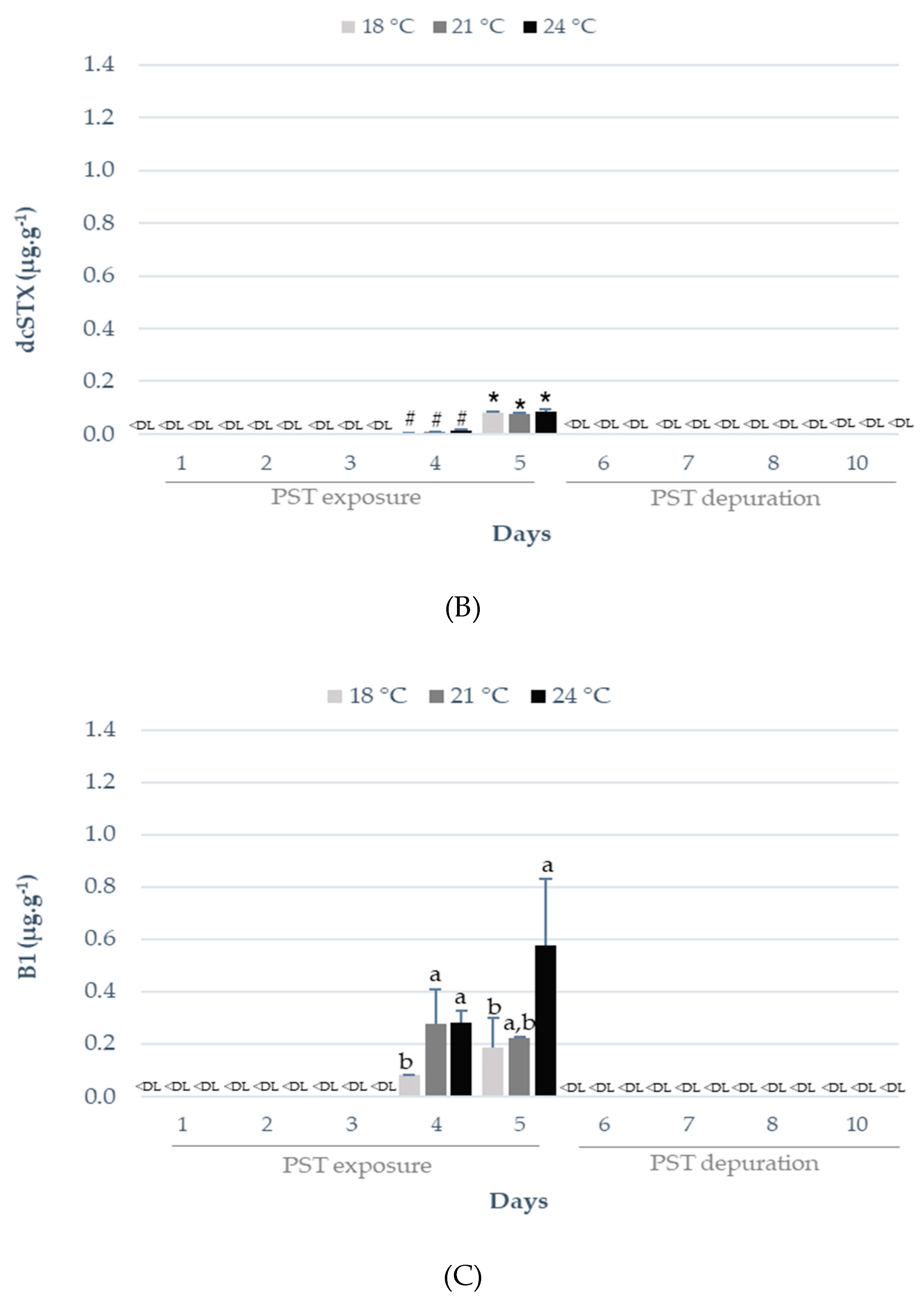
| Sampling Day | Temperature | Toxicity (µg STX eq. kg−1) | EC 853/200411 |
|---|---|---|---|
| Day 4 | 18 °C | 74.7 ± 1.6 | 800 µg STX eq. kg−1 |
| 21 °C | 59.1 ± 8.3 # | ||
| 24 °C | 67.1 ± 6.5 # | ||
| Day 5 | 18 °C | 113.4 ± 17.2 b | |
| 21 °C | 154.1 ± 1.2 b,* | ||
| 24 °C | 275.4 ± 3.0 a,* |
| Toxins Analogues | DW | WW |
|---|---|---|
| dcGTX2 and dcGTX3 | 4.6 | 1.1 |
| C1 and C2 | 248.9 | 60.8 |
| dcSTX | 50.4 | 12.3 |
| GTX2 and GTX3 | 1.5 | 0.4 |
| B1 | 156.1 | 38.1 |
| STX | 0.6 | 0.1 |
| Total Toxicity (mg STX eq. kg−1) | 111.5 | 27.23 |
| Molecular Biomarker | Ecotoxicological Response | Methodology Used | References |
|---|---|---|---|
| Superoxide dismutase (SOD) | Oxidative stress | Enzymatic assay | [12,24,32] |
| Catalase (CAT) | Oxidative stress | Enzymatic assay | [12,24,32] |
| Glutathione S-transferase (GST) | Oxidative stress and xenobiotic detoxification phase II | Enzymatic assay | [12,24,32] |
| Heat shock response (HSP70) | Chaperoning, heat shock response | Indirect ELISA | [24,32] |
| Ubiquitin (Ub) | Protein degradation and DNA repair | Direct ELISA | [24,32] |
| Lipid peroxidation (LPO) | Oxidative stress and cellular damage | Thiobarbituric acid reactive substances (TBARS) method | [12,24,32] |
| Acetylcholinesterase (AChE) | Neurotoxicity | Enzymatic assay | [24,32] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, V.; Santos, M.; Anacleto, P.; Maulvault, A.L.; Pousão-Ferreira, P.; Costa, P.R.; Marques, A. Paralytic Shellfish Toxins and Ocean Warming: Bioaccumulation and Ecotoxicological Responses in Juvenile Gilthead Seabream (Sparus aurata). Toxins 2019, 11, 408. https://doi.org/10.3390/toxins11070408
Barbosa V, Santos M, Anacleto P, Maulvault AL, Pousão-Ferreira P, Costa PR, Marques A. Paralytic Shellfish Toxins and Ocean Warming: Bioaccumulation and Ecotoxicological Responses in Juvenile Gilthead Seabream (Sparus aurata). Toxins. 2019; 11(7):408. https://doi.org/10.3390/toxins11070408
Chicago/Turabian StyleBarbosa, Vera, Marta Santos, Patrícia Anacleto, Ana Luísa Maulvault, Pedro Pousão-Ferreira, Pedro Reis Costa, and António Marques. 2019. "Paralytic Shellfish Toxins and Ocean Warming: Bioaccumulation and Ecotoxicological Responses in Juvenile Gilthead Seabream (Sparus aurata)" Toxins 11, no. 7: 408. https://doi.org/10.3390/toxins11070408
APA StyleBarbosa, V., Santos, M., Anacleto, P., Maulvault, A. L., Pousão-Ferreira, P., Costa, P. R., & Marques, A. (2019). Paralytic Shellfish Toxins and Ocean Warming: Bioaccumulation and Ecotoxicological Responses in Juvenile Gilthead Seabream (Sparus aurata). Toxins, 11(7), 408. https://doi.org/10.3390/toxins11070408