T cell Receptor Vβ9 in Method for Rapidly Quantifying Active Staphylococcal Enterotoxin Type-A without Live Animals
Abstract
1. Introduction
2. Results
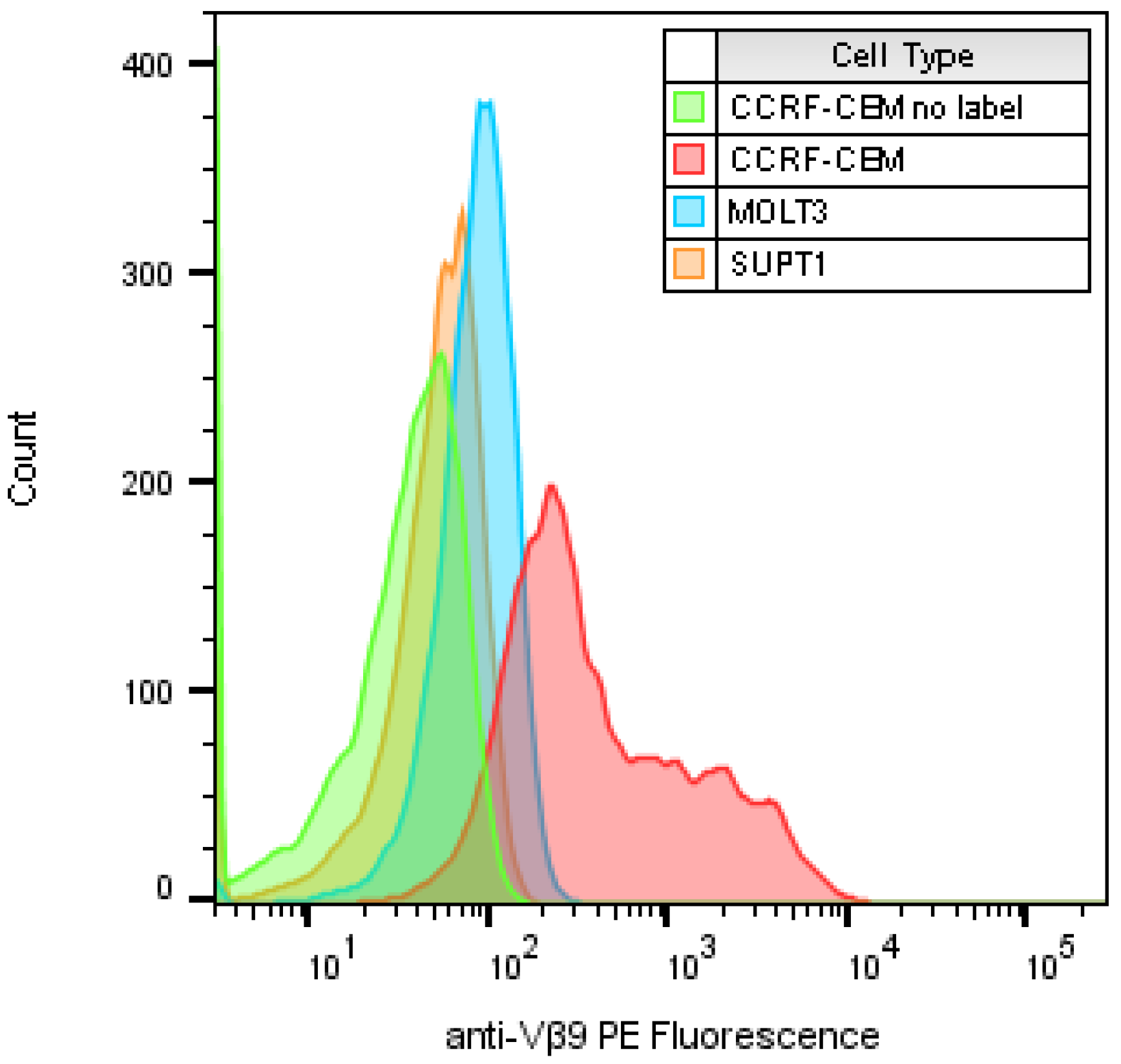
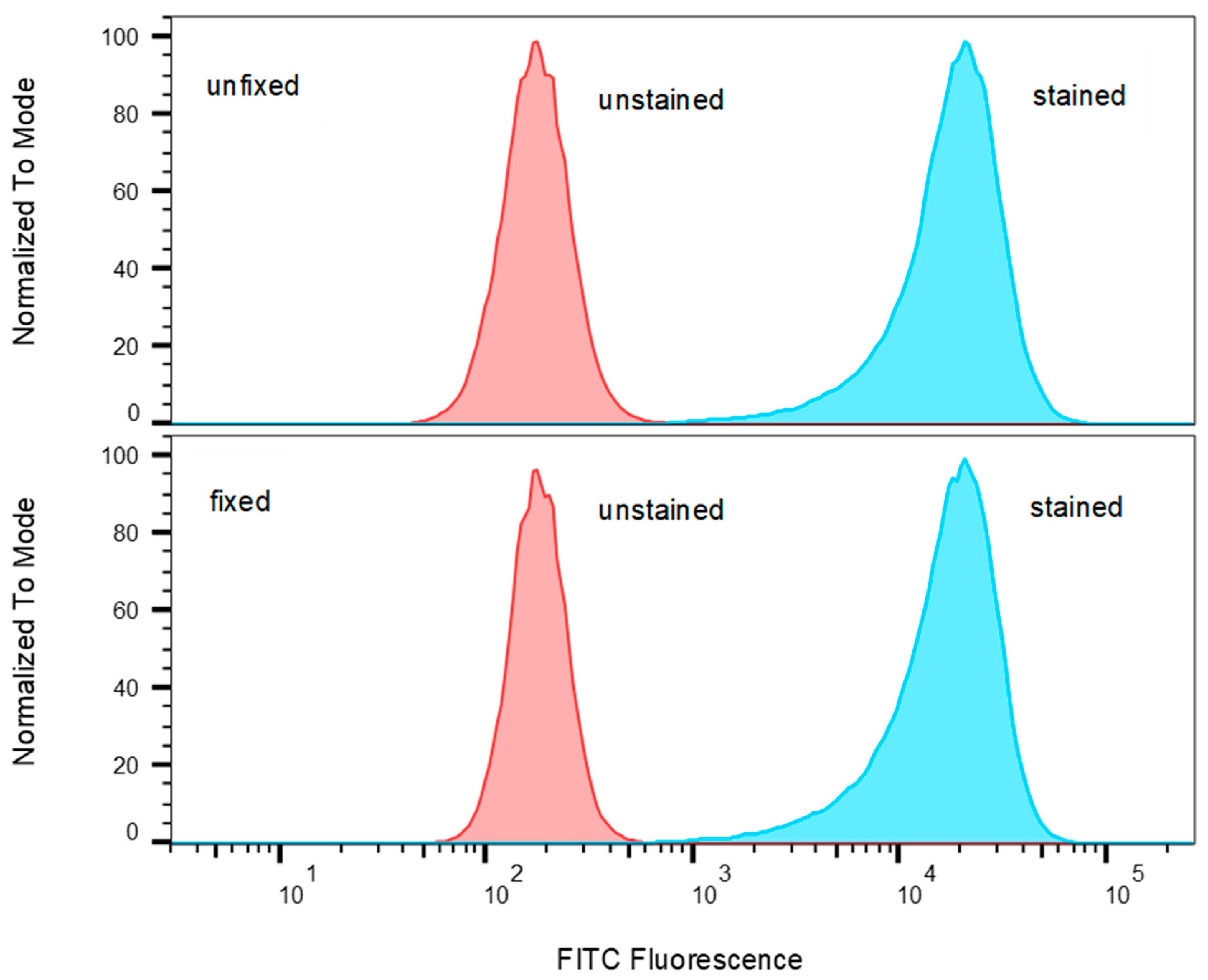
2.1. Identify T-Cell Lines that Highly Express TCR Vβ9 Receptor on Their Surface
2.2. Evaluation of TCR Vβ9 Based Assay for Specific, Rapid Detection of SEA
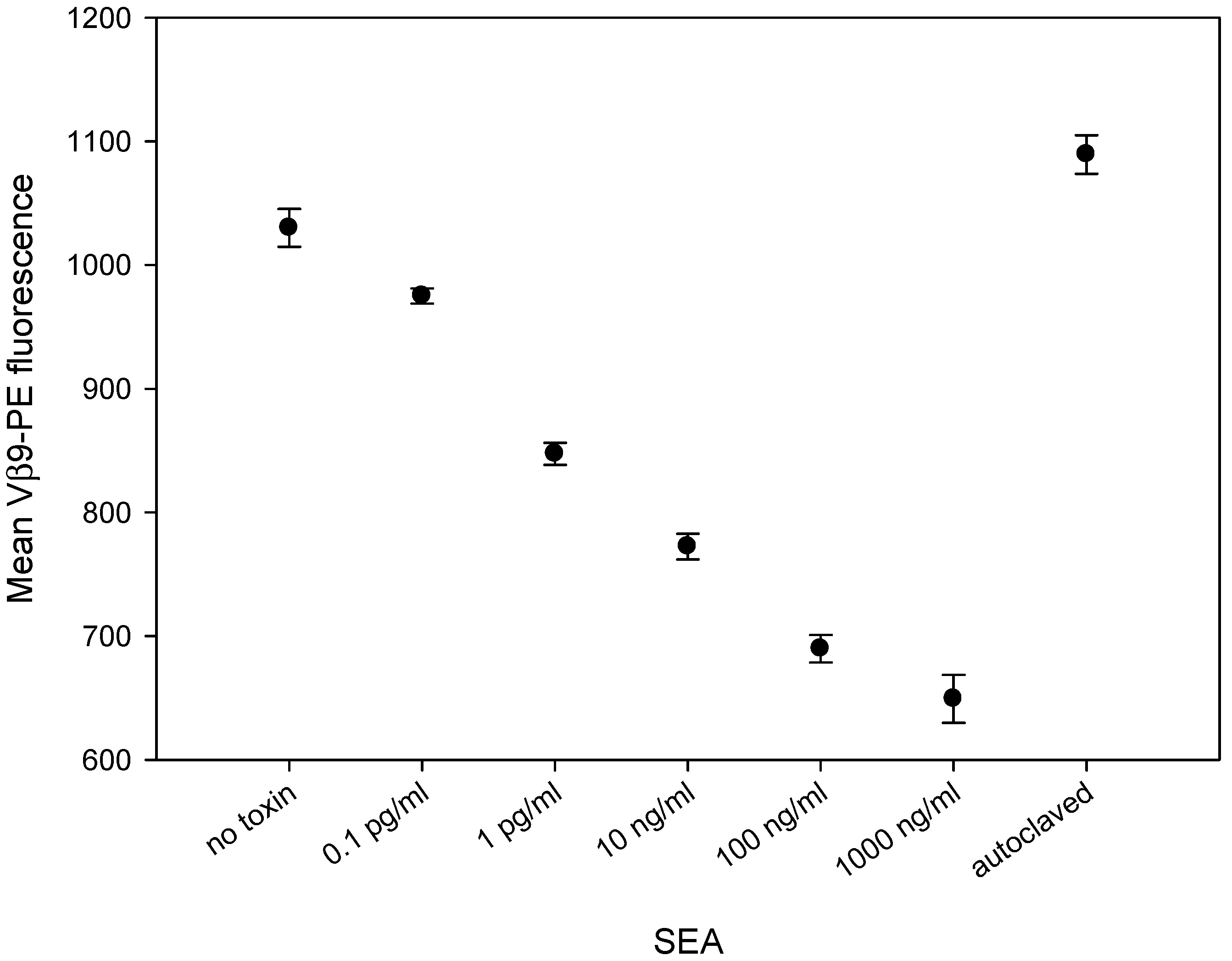
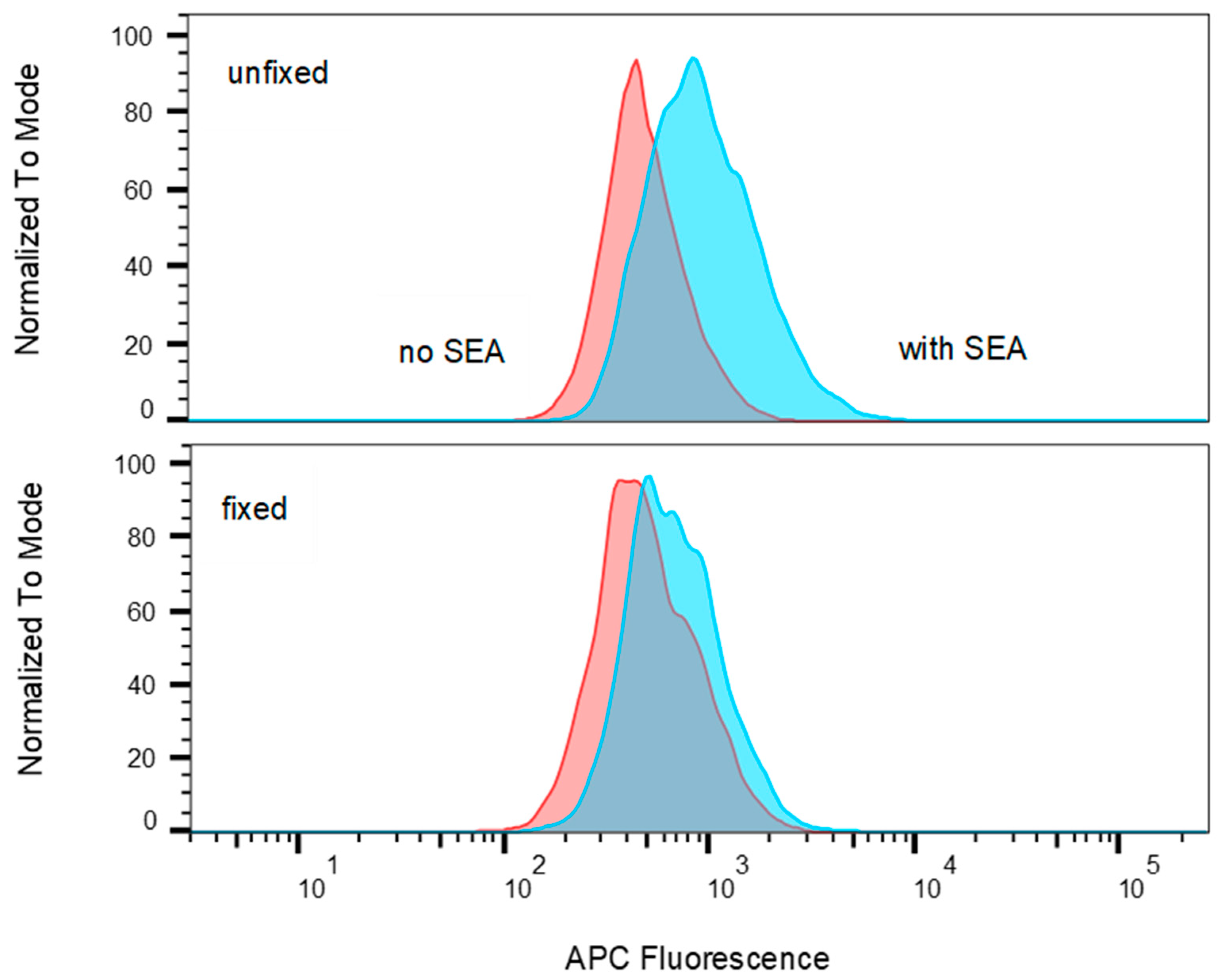
2.3. SEA Reduces TCR Vβ9 Protein Expression in a Dose Dependent Manner
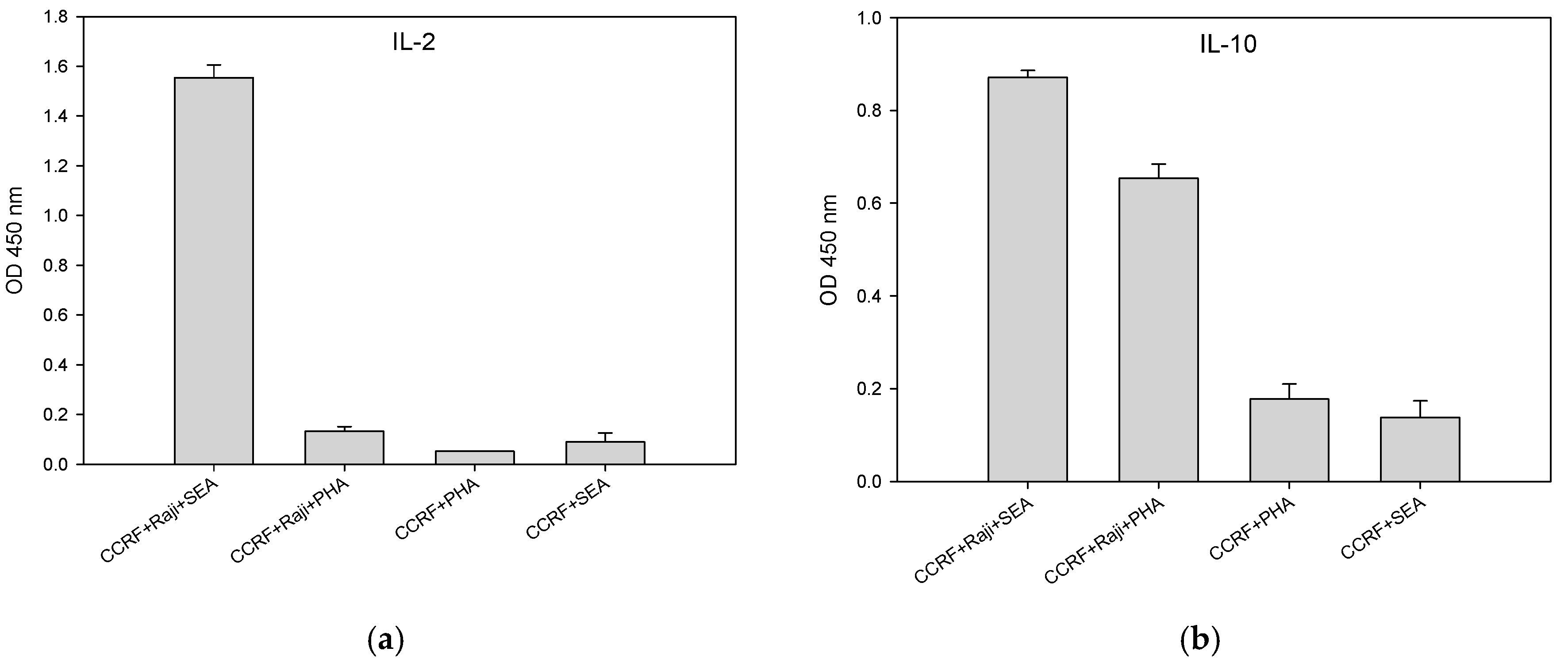
2.4. Direct Activation of T Cell by PHA, But Not with SEA
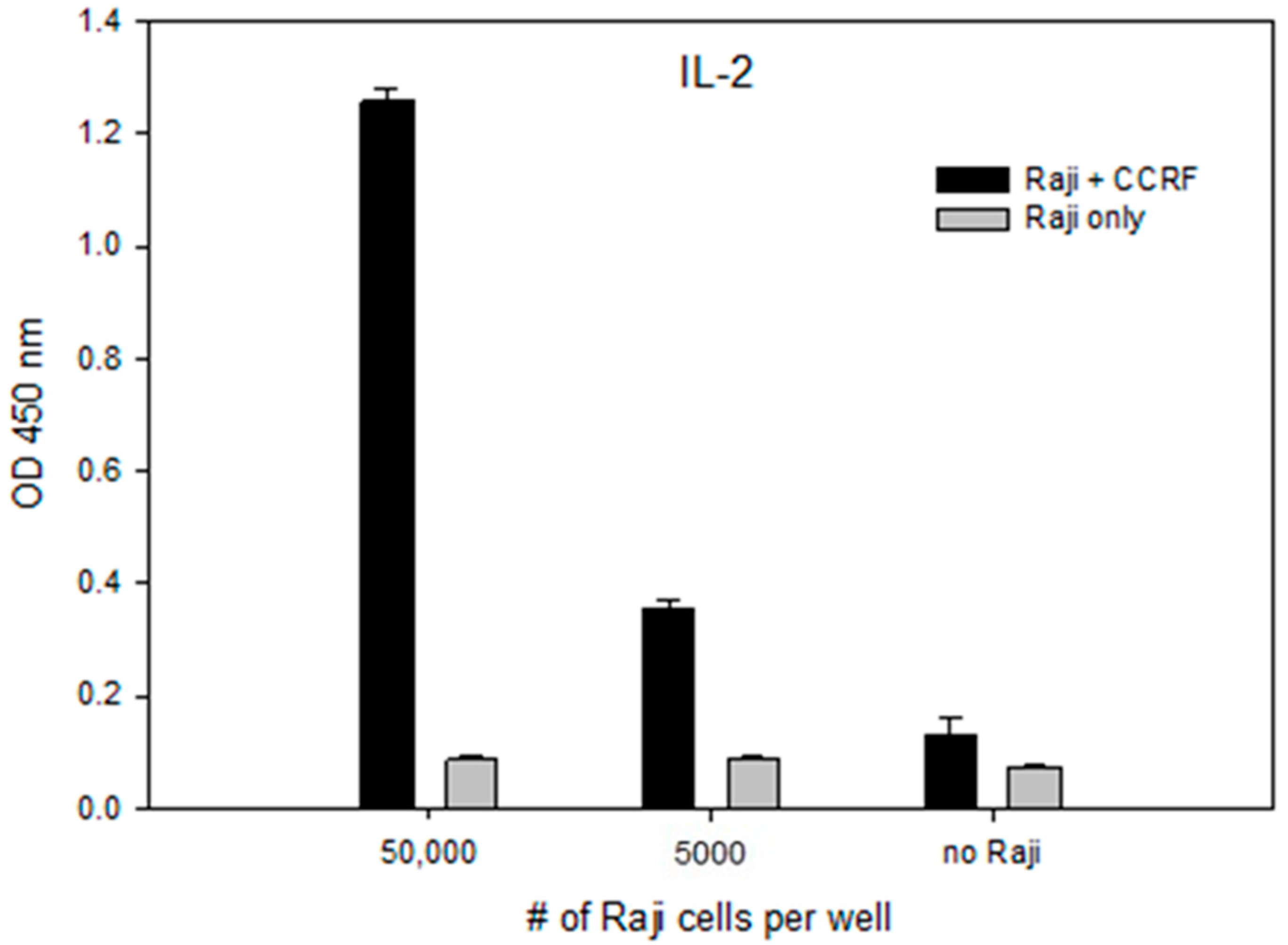
2.5. Accessory Cells Are Essential for Activation of CCRF-CEM T-Cell With SEA
2.6. SEA and PHA Stimulated Interleukin 10 (IL-10) Secretion by CCRF-CEM T-Cells is Accessory Cell Dependent
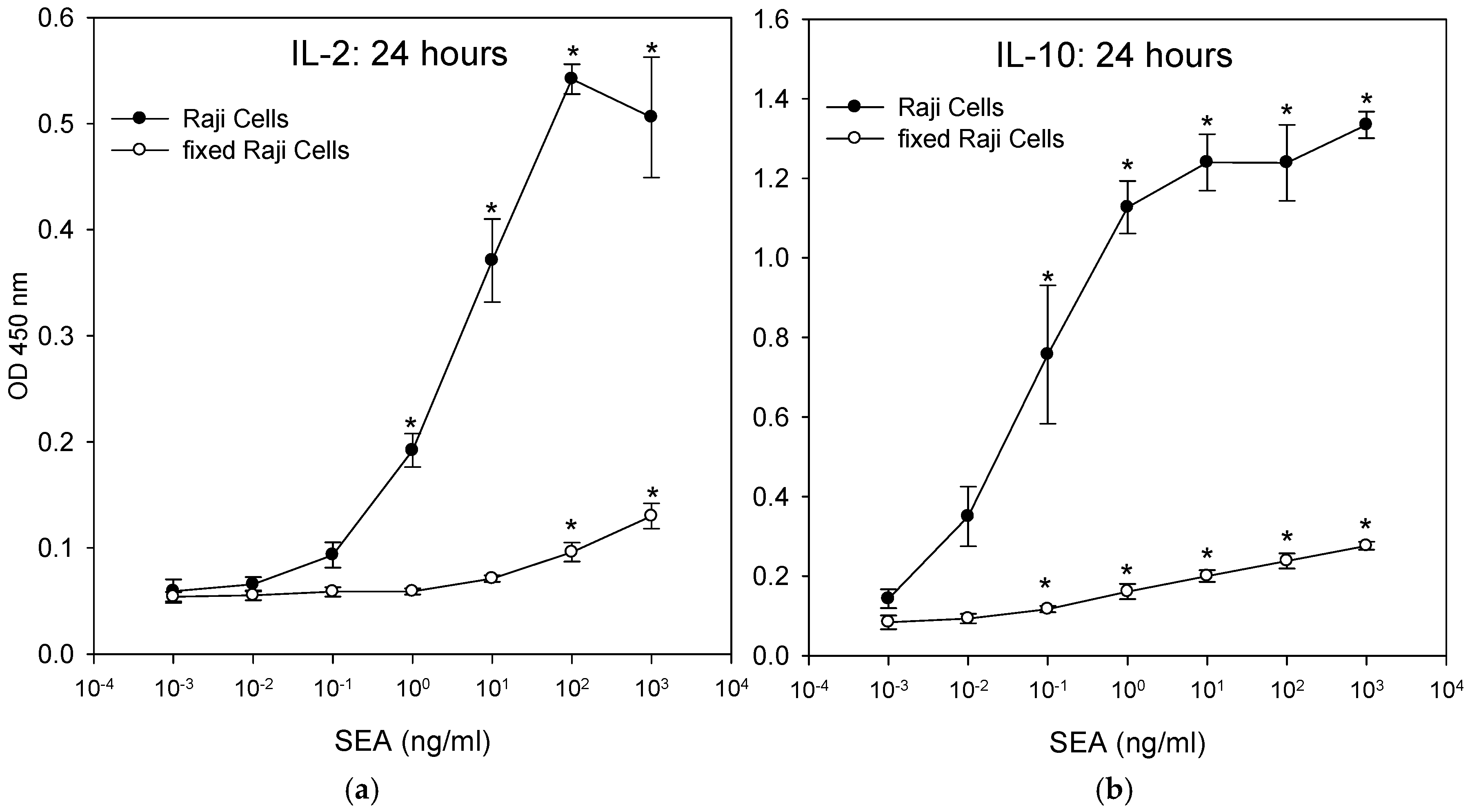
2.7. T-Cells that Were Incubated with Fixed Accessory Cells Without any Metabolic Function Secreted Considerably Lower Levels of Cytokine
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cells and Cell Culture
4.3. Assay Method
4.4. Quantitative Determinations of Active SEA by IL-2 or IL-10 Protein Secretion
4.5. Flow Cytometry
4.6. Paraformaldehyde Fixation
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States—Unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.; Hernlem, B. Sensitive, Rapid, Quantitative and in Vitro Method for the Detection of Biologically Active Staphylococcal Enterotoxin Type E. Toxins (Basel) 2016, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.; Hernlem, B.J. Interleukin 2 Secretion by T Cells for Detection of Biologically Active Staphylococcal Enterotoxin Type E. J. Food Prot. 2017, 9, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.M. In vitro cell-based assay for activity analysis of staphylococcal enterotoxin A in food. FEMS Immunol. Med. Microbiol. 2009, 56, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Hernlem, B.J. CD154 as a potential early molecular biomarker for rapid quantification analysis of active Staphylococcus enterotoxin A. FEMS Immunol. Med. Microbiol. 2012, 64, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Hernlem, B.J. Quantitative analysis of staphylococcus enterotoxin A by differential expression of IFN-gamma in splenocyte and CD4(+) T-cells. Sensors (Basel) 2014, 14, 8869–8876. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Hernlem, B. TNF as biomarker for rapid quantification of active Staphylococcus enterotoxin A in food. Sensors (Basel) 2012, 12, 5978–5985. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Omoe, K.; Sashinami, H.; Shinagawa, K.; Nakane, A. Immunization with a nontoxic mutant of staphylococcal enterotoxin A, SEAD227A, protects against enterotoxin-induced emesis in house musk shrews. J. Infect. Dis. 2009, 199, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.; Cao, Y.; Xiao, F.; Zhang, J.; Li, H.; Hu, F. Staphylococcus aureus enterotoxin C2 mutants: Biological activity assay in vitro. J. Ind. Microbiol. Biotechnol. 2008, 35, 975–980. [Google Scholar] [CrossRef]
- Czop, J.K.; Bergdoll, M.S. Staphylococcal enterotoxin synthesis during the exponential, transitional, and stationary growth phases. Infect. Immun. 1974, 9, 229–235. [Google Scholar]
- Asao, T.; Kumeda, Y.; Kawai, T.; Shibata, T.; Oda, H.; Haruki, K.; Nakazawa, H.; Kozaki, S. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: Estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol. Infect. 2003, 130, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tamate, N.; Yamaguchi, K.; Makino, S. Mass outbreak of food poisoning disease caused by small amounts of staphylococcal enterotoxins A and H. Appl. Environ. Microbiol. 2005, 71, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
- Casman, E.P.; Bennett, R.W. Detection of Staphylococcal Enterotoxin in Food. Appl. Microbiol. 1965, 13, 181–189. [Google Scholar] [PubMed]
- Wieneke, A.A.; Roberts, D.; Gilbert, R.J. Staphylococcal food poisoning in the United Kingdom, 1969–1990. Epidemiol. Infect. 1993, 110, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Evenson, M.L.; Hinds, M.W.; Bernstein, R.S.; Bergdoll, M.S. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int. J. Food Microbiol. 1988, 7, 311–316. [Google Scholar] [CrossRef]
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar] [PubMed]
- Bennett, R.W. Staphylococcal enterotoxin and its rapid identification in foods by enzyme-linked immunosorbent assay-based methodology. J. Food Prot. 2005, 68, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Bergdoll, M.S. Monkey feeding test for staphylococcal enterotoxin. Methods Enzymol. 1988, 165, 324–333. [Google Scholar] [PubMed]
- Thomas, D.; Dauwalder, O.; Brun, V.; Badiou, C.; Ferry, T.; Etienne, J.; Vandenesch, F.; Lina, G. Staphylococcus aureus superantigens elicit redundant and extensive human Vbeta patterns. Infect. Immun. 2009, 77, 2043–2050. [Google Scholar] [CrossRef]
- Bayles, K.W.; Iandolo, J.J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J. Bacteriol. 1989, 171, 4799–4806. [Google Scholar] [CrossRef]
- Iandolo, J.J. Genetic analysis of extracellular toxins of Staphylococcus aureus. Annu. Rev. Microbiol. 1989, 43, 375–402. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal enterotoxins. Toxins (Basel) 2010, 2, 2177–2197. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yan, Y.; Chen, X.; Lin, C.; Li, Y. SEA antagonizes the imatinib-meditated inhibitory effects on T cell activation via the TCR signaling pathway. BioMed Res. Int. 2014, 2014, 682010. [Google Scholar] [CrossRef] [PubMed]
- Dellabona, P.; Peccoud, J.; Kappler, J.; Marrack, P.; Benoist, C.; Mathis, D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell 1990, 62, 1115–1121. [Google Scholar] [CrossRef]
- Fischer, H.; Dohlsten, M.; Lindvall, M.; Sjogren, H.O.; Carlsson, R. Binding of staphylococcal enterotoxin A to HLA-DR on B cell lines. J. Immunol. 1989, 142, 3151–3157. [Google Scholar] [PubMed]
- Fraser, J.D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature 1989, 339, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Scholl, P.; Diez, A.; Mourad, W.; Parsonnet, J.; Geha, R.S.; Chatila, T. Toxic shock syndrome toxin 1 binds to major histocompatibility complex class II molecules. Proc. Natl. Acad. Sci. USA 1989, 86, 4210–4214. [Google Scholar] [CrossRef]
- Kappler, J.; Kotzin, B.; Herron, L.; Gelfand, E.W.; Bigler, R.D.; Boylston, A.; Carrel, S.; Posnett, D.N.; Choi, Y.; Marrack, P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science 1989, 244, 811–813. [Google Scholar] [CrossRef]
- White, J.; Herman, A.; Pullen, A.M.; Kubo, R.; Kappler, J.W.; Marrack, P. The V beta-specific superantigen staphylococcal enterotoxin B: Stimulation of mature T cells and clonal deletion in neonatal mice. Cell 1989, 56, 27–35. [Google Scholar] [CrossRef]
- Bavari, S.; Hunt, R.E.; Ulrich, R.G. Divergence of human and nonhuman primate lymphocyte responses to bacterial superantigens. Clin. Immunol. Immunopathol. 1995, 76, 248–254. [Google Scholar] [CrossRef]
- Hufnagle, W.O.; Tremaine, M.T.; Betley, M.J. The carboxyl-terminal region of staphylococcal enterotoxin type A is required for a fully active molecule. Infect. Immun. 1991, 59, 2126–2134. [Google Scholar] [PubMed]
- Kasper, K.J.; Xi, W.; Rahman, A.K.; Nooh, M.M.; Kotb, M.; Sundberg, E.J.; Madrenas, J.; McCormick, J.K. Molecular requirements for MHC class II alpha-chain engagement and allelic discrimination by the bacterial superantigen streptococcal pyrogenic exotoxin C. J. Immunol. 2008, 181, 3384–3392. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.; He, X.; Hernlem, B. TCR-Vβ8 as Alternative to Animal Testing for Quantifying Active SEE. J. Environ. Anal. Toxicol. 2017, 7, 1–6. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasooly, R.; Do, P.; He, X.; Hernlem, B. T cell Receptor Vβ9 in Method for Rapidly Quantifying Active Staphylococcal Enterotoxin Type-A without Live Animals. Toxins 2019, 11, 399. https://doi.org/10.3390/toxins11070399
Rasooly R, Do P, He X, Hernlem B. T cell Receptor Vβ9 in Method for Rapidly Quantifying Active Staphylococcal Enterotoxin Type-A without Live Animals. Toxins. 2019; 11(7):399. https://doi.org/10.3390/toxins11070399
Chicago/Turabian StyleRasooly, Reuven, Paula Do, Xiaohua He, and Bradley Hernlem. 2019. "T cell Receptor Vβ9 in Method for Rapidly Quantifying Active Staphylococcal Enterotoxin Type-A without Live Animals" Toxins 11, no. 7: 399. https://doi.org/10.3390/toxins11070399
APA StyleRasooly, R., Do, P., He, X., & Hernlem, B. (2019). T cell Receptor Vβ9 in Method for Rapidly Quantifying Active Staphylococcal Enterotoxin Type-A without Live Animals. Toxins, 11(7), 399. https://doi.org/10.3390/toxins11070399





