Abstract
Bacillus thuringiensis (Bt) is a Gram-positive soil bacteria that infects invertebrates, predominantly of Arthropoda phylum. Due to its immense host range Bt has become a leading producer of biopesticides applied both in biotechnology and agriculture. Cytotoxic effect of Bt, as well as its host specificity, are commonly attributed either to proteinaceous crystal parasporal toxins (Cry and Cyt) produced by bacteria in a stationary phase or to soluble toxins of Vip and Sip families secreted by vegetative cells. At the same time, numerous non-toxin virulence factors of Bt have been discovered, including metalloproteases, chitinases, aminopolyol antibiotics and nucleotide-mimicking moieties. These agents act at each stage of the B. thuringiensis invasion and contribute to cytotoxic properties of Bt strains enhancing toxin activity, ensuring host immune response evasion and participating in extracellular matrix degeneration. In this review we attempt to classify Bt virulence factors unrelated to major groups of protein toxins and discuss their putative role in the establishment of Bt specificity to various groups of insects.
Keywords:
Bacillus thuringiensis; Bt; virulence; specificity; toxin; insect; metalloprotease; chitinase; host; pathogen Key Contribution:
This review summarizes rapidly accumulating data on the Bacillus thuringiensis virulence factors unrelated to major groups of protein toxins and discusses their involvement in the control of the host-pathogen specificity.
1. Introduction
Gram-positive sporulating bacterium Bacillus thuringuensis (Bt) of phylum Firmicutes belongs to Bacillus cereus (Bc) group which includes an opportunistic pathogen B. cereus itself, as well as an obligatory pathogen B. anthracis [1]. In contrast to the two latter species, Bt does not affect vertebrates, though it shows toxicity to several mammalian cell lines [2], but rather, is referred to as an insect pathogen infecting their hosts on larval stages. However, its real host spectrum appears to comprise a much broader range of arthropods as well as nematodes of order Rhabditida, fungi, protozoans and terrestrial gastropods [3,4,5,6]. Because of its striking insecticidal activity and wide range of affected species, Bt is widely used either as a biopesticide [7] or as a source of resistance determinants for transgenic crops [8].
Though they can exist as free-living vegetative cells, Bt are usually isolated from their environment in the form of spores [9]. Once they enter the host’s organism, the spores use their immense arsenal of virulence factors to transfer from digestive organs to circulating fluids, such as blood or haemolymph, where they transit to the vegetative stage, propagate and disseminate within the host’s organism. After the host dies of a resulting septicemia, the bacteria dwelling in its cadaver propagate until they exhaust all consumable organics and then transit to sporulation. Such ecological strategy involving exploitation of both living host and its remnants is known as necromeny and can be viewed as a specific form of symbiotic interactions [10].
Insecticidal activity of Bt is usually attributed to the proteinaceous toxins produced at various stages of the bacterial life cycle. Vegetative Bt cells secrete soluble toxins comprising Vip (vegetative insecticidal proteins) and Sip (secreted insecticidal proteins) protein families. The Vip family includes four subfamilies: the Vip1 and Vip2 subfamilies comprise heterodimeric toxins, which inhibit actin polymerization and tend to affect insects of Coleoptera and Hemiptera orders [11]; the Vip3 subfamily members are putative pore-formers specific to lepidopteran hosts [12], and the last subfamily includes a sole protein Vip4 in which both mode of action and target range are still unknown [11]. The only known Sip protein, Sip1Aa, demonstrates toxicity against coleopteran larvae [13].
On the transition to sporulation, Bt shift to the production of insoluble δ-endotoxins. These toxins associate with auxiliary proteins to form crystal aggregates known as parasporal bodies, which are then released from the exosporium. δ-endotoxins include two families of non-selective pore-forming proteins, namely Cry (crystal) and Cyt (cytotoxic) [14], and demonstrate a wide range of affected hosts, including insects of Coleoptera, Lepidoptera, Diptera, Hymenoptera, Hemiptera and Orthoptera orders, as well as phytopathogenic nematodes and terrestrial gastropods. For most of the known δ-endotoxins, however, no suitable natural targets have been discovered so far, though some of these cryptic toxins show toxicity against species, which are unlikely to be encountered by Bt in natural conditions, such as parasitic nematodes [15] and trematodes [16] and a flagellar protist Trichomonas vaginalis [5]. Regardless of their structure and mode of action, to fulfill their cytotoxic properties all Bt toxins need to bind specific receptors exposed on membranes of host midgut cells. Besides, several toxins, for example, the members of Cry family, are secreted in the form of inactive protoxins requiring alkaline proteolysis mediated by the host’s digestive enzymes for activation [14].
At the same time, apart from the proteinaceous toxins, several other molecules produced by Bt appear to be crucial for virulence establishment and successful infection. Some of these factors show a cytotoxic effect on their own while others act as regulators of major toxins’ activity. In the present work, we focus on three classes of proteinaceous virulence factors standing apart from the canonical Bt toxins (that is, chitinases, zinc metalloproteases and cytolysins) and two groups of low-weight moieties (aminopolyol antibiotics and β-exotoxins). Here, we provide a comprehensive review of rapidly accumulating data on the virulence factors of Bt unrelated to major groups of protein toxins and discuss their impact on virulence and pathogenesis to elucidate their role in Bt host-specificity.
2. Proteinaceous Virulence factors of Bt
2.1. Bt Chitinases
The first barrier, which Bt spores ingested by insects need to overcome, is typically presented by a peritrophic membrane constituting a dense film consisting of chitin fibrils cross-linked by chitin-binding proteins called peritrophins [17]. This structure isolates apical surface of midgut epitheliocytes from the ingested nutriments thus providing protection from both mechanical damage and pathogen absorption. Depending on their content and biogenesis, peritrophic structures fall into two distinct types. Type I membranes are temporary structures formed directly around food lumps being digested. In their turn, type II membranes persist being attached to the midgut walls in its anterior section. While type I peritrophic membranes are common for the species of the orders Blattodea, Orthoptera, Coleoptera, Hymenoptera and Diptera, occurrence of type II is restricted within orders Diptera and Lepidoptera. The sanguivorous members of order Diptera, such as black flies (Diptera: Simuliidae) and mosquitoes (Diptera: Culicidae) can switch from type II to type I during metamorphosis due to transition from solid diet of larvae to the liquid feeding of mature species [18,19]. Besides solid peritrophic membranes, several insects produce a semi-liquid peritrophic gel devoid of chitin component which can sustain a gel-like structure due to a specific peritrophin and mucin content [17]. Such strategy is exploited by bean weevils (Coleoptera: Bruchidae) who feed upon legume seeds known to store lectins [20]. In species of orders Hemiptera and Thysanoptera, the peritrophic compartment is represented by a glycoprotein structure known as the perimicrovillar membrane [21]. Despite these examples of the elimination of the chitin component from peritrophic structures, complete absence of peritrophic structure rarely occurs in insects and is usually related to a low-weight molecular diet, as in the case of several hymenopteran and lepidopteran species [22].
A widely used way to penetrate these chitin-based membranes is the production of chitinases, hydrolytic enzymes capable of digesting chitins, a group of N-acetyl D-glucosamine polymers, via hydrolysis of β-(1→4)-glycoside bonds [23]. Based on their mode of action, chitinases are categorized in endochitinases, which split chitin by random internal sites resulting in the formation of di-acetylchitobiose, chitotriose, and chitotetraose, and exochitinases cleaving short chitooligosaccharides from molecule termini [24]. Chitinase substrate affinity is provided by the presence of special chitin-binding modules, or CBMs. These modules are marked by impressive structural variety, but in all cases, their chitin-binding affinity is associated with the conservative tryptophan residues [25]. In terms of glycoside hydrolase (GH) structural classification chitinases belong to three families, of which families GH18 and GH19 appear to be the largest ones and comprise both endo- and exochitinases (according to Carbohydrate-active Enzymes Database (CAZy) on 2019) [26]. Proteins belonging to these two families have been encountered in all major groups of living organisms as well as several groups of viruses [24,27].
The presence of the chitinase-encoding genes in bacterial genomes, including those of Bacillus isolates, allows them to assimilate chitin effectively and even use it as a sole carbon source [28,29]. Chitinases found in Bt belong to the GH18 family [24]. Though most of them, such as ChiA, ChiB and ChiC, act in endochitinolytic manner, occurrences of exochitinases have also been reported [30,31]. Chitinase-producing Bt strains often possess fungicidal activity [4] and are capable of inhibiting growth of the phytopathogenic fungi which makes them a promising antimycotic agent for agricultural needs [32,33]. Unlike their fungicidal properties, the role of chitinases in virulence of insecticidal strains of Bt has long been confined to the facilitation of the cytotoxicity of crystal toxins [34]. Nonetheless, several insect endoparasites such as protists of genera Plasmodium [35] and Leishmania [36], nematode Brugia malayi [37] and baculoviruses [38], use chitinases to digest the peritrophic membrane enveloping the lumps of ingested substrates once they get in the midgut. Since Bt infect their hosts mostly, and apparently exclusively, through the digestive tract, it seems plausible that they might exploit their chitinase repertoire in a similar manner.
At present, the role of chitinases in Bt virulence is mainly perceived through the hydrolysis of the peritrophic membranes. In most cases, chitinases act synergistically with crystal toxins and other Bt virulence factors. For instance, a joint inoculation of Cry-producing Bt ser. israelensis and avirulent chitinase producers bolsters the effect of crystal toxins on Spodoptera exigua (Lepidoptera: Noctuidae) by 2.35 times [39]. In other experiments the presence of external chitinases doubled the toxicity of Cry4Aa, Cry4Ba, Cry11Aa and Cyt1Aa against Aedes aegyptii (Diptera: Culicidae) [40] and enhanced the toxicity of CryIC against S. littoralis by six times [41]. Also, enhancement of Cry4Ba toxicity against its natural targets A. aegyptii and Culex quinquefasciatus (Diptera: Culicidae) in the presence of chitin-binding agent calcofluor white may serve as an indirect evidence for the cumulative effect of Cry and chitinases or any other chitin-binding moieties [42]. Notably, there is at least one example of analogous synergy of chitinases and unidentified Vip toxin [30] which suggests that not only the Cry family but also other major classes of Bt toxins benefit in their cytotoxicity on the chitinase production background.
The diversity of insect peritrophic structures suggests that chitinases may be involved in the control of the host-specificity of Bt. For instance, CBM-containing chitinases mostly split crystal chitin, also known as α-chitin, while specificity of other chitinase families may differ [43]. Besides canonical chitinases, a unique CBM2-containing chitinase BthChi74 was shown to bind not only crystal chitin but also cellulose [44]. Apart from their substrate affinity, the CBM structure may be affected by the temperature and pH values, since these two factors in the midgut compartment differ between insect species depending on their phylogenetic position, diet, ontogenesis stage and gut microflora diversity [45,46].
Finally, it is worth mentioning that several species of bacterial entomopathogens, such as orthopteran pathogens of Sanguibacter genus [47] and Gram-negative bacterium Yersinia entomophaga known to infect Costelytra zealandica (Coleoptera: Scarabaeidae) [48], exploit chitinases as their main virulence factors. Considering that chitinase-producing Bt strains have been tested mainly on the insect species sharing common structure of peritrophic matrix, it is possible that the role of chitinases in Bt virulence and host specificity needs additional clarification and may be utterly underestimated.
2.2. Bt Metalloproteases
After penetrating the chitin-rich peritrophic membrane, Bt cells need to overcome several barriers enriched with different protein factors, such as a mucin layer, a basal lamina and cadherins. To achieve this aim, Bt utilize many different metalloproteases. By definition, these proteins represent enzymes capable of hydrolyzing peptide bond in the presence of one or more metal ions, usually those of zinc (II) [49]. One of the existing classifications divides all known metalloproteases into two subclasses depending on the presence of one or two metal ions in the catalytic site, which subsequently diverge into tribes, clans and, finally, distinct families [50]. Despite their structural diversity, monometallic metalloproteases share an ordered single-displacement mechanism of hydrolysis catalyzed by a metal ion and a neutral or basic residue within the active center, and operate in the common milieu, such as pH = 6.5 [50].
Based on the structure of metal-binding motifs, monometallic zinc metalloproteases are divided into five tribes, of which the zincin tribe distinguished by the HEXXH consensus metal-binding motif is the largest one [51]. In turn, zincins split into five clans according to an active residue of a loop adjacent to a metal-binding motif, namely metzincins, gluzincinz, aspzincins, S2P-zincins and FtsH-like metalloproteases. Finally, notation suggested by the MEROPS database (https://www.ebi.ac.uk/merops/index.shtml) divides all known metalloproteases into 103 families with each family name comprising of a letter M standing for ‘metalloprotease’ and a respective family number [52]. Due to their structural and functional variability metalloproteases serve as virulence factors in both Gram-positive and Gram-negative bacteria as well as pathogenic fungi and protists [49]. Their insecticidal properties alongside with other proteases have been reviewed previously in [53]. According to MEROPS, 30 metalloprotease families have been encountered in Bt including the most important M60, M6, M9 and M73. Here we summarize data on these metalloprotease families known to impact pathogenicity of Bt at different stages of infection.
2.2.1. Enhancin-Like Metalloproteases
Enhancin is a zinc metalloprotease of Trichoplusia ni granulovirus (TnGV) marked with M60 family metalloprotease domain [54]. In viruses, enhancins serve mostly to digest the peritrophic matrix in order to facilitate virion transition to haemocoel [55]. Among the components of the peritrophic matrix, invertebrate intestintal mucin (IIM) appears to be the primary target of enhancin-mediated proteolysis. This protein was first discovered in T. ni (Lepidoptera: Noctuidae) larvae, though its orthologues are present in the species outside Lepidoptera order, such as T. castaneum (Coleoptera: Tenebrionidae) [56]. Viral enhancins were shown to positively affect Bt virulence against at least six lepidopteran species: T. ni, H. zea, H. virescens, S. exigua, Chrysodeixis includens and Anticarsia gemmatalis (Lepidoptera: Noctuidae) [57]. Since then, genes encoding enhancin-like proteins have been found in several bacterial genomes, while in B. cereus and B. thuringiensis these genes were shown to belong to PlcR (Phospholipase C Regulator) regulon, whose activity is triggered in the end of the phase of exponential growth and is strongly mitigated during the stationary stage shift due to the general sporulation regulator Spo0A [58,59,60]. These genes are called bel (for Bacillus enhancin-like).
The first bel-encoded protein discovered, mpbE (metalloprotease Bacillus enhancin), from B. cereus strain ATCC14579 failed to show any virulence reinforcement in G. mellonella larvae assays [60]. However, the Bel protein isolated from strains YBT1520 and BMB171 in 2009 was found to directly influence Bt virulence against H. armigera: the knockout of the bel gene led to rise of Bt semilethal dose by 5.8 times, while the addition of Bel to a purified Cry1Ac preparation bolstered its lethality from 34.2 to 74.4% [61]. Bel shares 20–30% sequence identity with TnGV enhancins and operates in the similar manner hydrolyzing IIM. For now, at least one more Bt enhancin-like protein has been described [62]; although it shares 23–41% identity with viral enhancins, it, however, did not show any toxicity to S. exigua and T. ni larvae in respective assays.
2.2.2. InhA Metalloproteases
Another example of PlcR-regulated Bt metalloproteases is an InhA group of family M6 [63] represented by three proteins with similar functions [64]. All three proteins participate in numerous processes associated with different stages of Bt infection. For instance, InhA1 plays an ambiguous role in Bt infection. On the one hand, it was shown that InhA1 is capable of hydrolyzing cecropins and attacins, antimicrobial peptides playing the key role in the insect humoral immune response [65]. Beyond that, the exosporal localization of InhA1 molecules in those Bc species virulent to vertebrates allows them to escape phagocytosis by macrophages by cleaving membrane-associated proteins [66]. This function can be extrapolated on Bt InhA1 proteins since insects were clearly shown to possess macrophage-like professional phagocytic cells [67]. On the other hand, the proteolytic activity of InhA1 resolves in the destruction of host extracellular matrix, including basal lamina of midgut epithelium, which is essential for haemolymph infesting [65]. InhA2 and InhA3 bear similar functions to those of InhA1 and share 66% and 72% sequence identity with it, respectively [64]. However, in contrast to InhA1, InhA2 does not affect Bt virulence in the absence of other PlcR regulon products [68]. In its turn, InhA3 is incapable of cecropin proteolysis, though its general proteolytic properties are preserved [64].
In the haemocoel, InhA1 demonstrates a strong toxic effect, which can be explained by non-specific proteolysis of haemolymph components [69]. Cytotoxic effect of InhA1 was first demonstrated in oral infection assays of Spodoptera littoralis (Lepidoptera: Noctuidae) larvae [70]. This effect is manifested through detachment of epitheliocytes from basal lamina due to the lysis of its main components (such as type IV collagen, laminin and fibronectin) as well as partial cell lysis. The same research provided evidence for the cumulative effect of InhA1 Bt crystal toxins. Similar enhancement of cytotoxicity was shown for joint application of Cry1C and InhA2 against Galleria mellonella (Lepidoptera: Pyralidae) larvae [71].
2.2.3. Other Bt Metalloproteases and Their Association with Biofilm Formation
Recently a new Bt metalloprotease, ColB, has been discovered [72]. ColB belongs to M9, or collagenase, family, which comprises numerous bacteria virulence factors [73]. Presence of ColB drastically enhances the cytolytic activity of several Cry toxins, including Cry5Ba, Cry55Aa and Cry6Aa, against H. armigera and nematodes Caenorhabditis elegans. ColB acts through proteolysis of both E-cadherins comprising epithelial intercellular contacts and collagens of basal lamina, resulting in degradation of midgut epithelium [74]. Thus, ColB mediates Bt transition from digestive tract to host haemocoel and subsequent spreading throughout the host body. Similar to that of ColB is the effect of Bmp1 M4-family protease, which also belongs to PlcR regulon, even though its involvement in Bt virulence has been shown for nematicidal strains only [75].
A casein-cleaving membrane protease (CCMP), or camelysin (CalY), poses another example of the Bt neutral metalloproateases [76]. According to the MEROPS classification, CalY belongs to M73 metalloprotease family. Although CalY demonstrates proteolytic activity against a broad range of proteins, including casein, actin and collagen [77], it was also shown to co-participate in biofilm formation with TasA fibril-forming protein [78]. Biofilms are bacterial communities floating on the culture medium or sticking to solid surfaces with the help to strong extracellular matrix, which consists of exopolysaccharides, proteins and extracellular DNA [79]. Interestingly, the proteinaceous component of such matrix tends to form amyloids, extremely stable fibrils with highly ordered spatial structure [80,81], in many different bacteria, such as Bacillus [82] and Escherichia coli [83]. TasA fibrils were previously reported to possess amyloidogenic properties based on their structure and detergent resistance [84], but were not tested for their biological activity in amyloid-like state, thus remaining the status of TasA as a functional amyloid dubious [85]. Biofilm formation is an important stage of attachment to the host tissues for many pathogens [86]. CalY plays the key role in both processes, in the adhesion to insect cells and in the biofilm formation, which makes it a very important virulence factor in Bt [78]. Interestingly, the evolutionary conserved M60 zinc metalloprotease domain found in both Gram-positive and Gram-negative bacteria [87], was found to be amyloidogenic as well [88,89] also providing a link between bacterial metalloprotease virulence factors and amyloid formation.
Apart from the involvement in the biofilm formation, CalY was shown to increase the cytolytic properties of Cyt1Aa (from 40 to 70%) and Cyt2Ba (from 6% to 50%) protoxins in rabbit erythrocyte assays [90]. Though a native proteolytic activation of Cry toxins by host proteases is realized at the alkaline values of pH, in the presence of CalY toxins they turn into active state at pH = 6.5. Thus, the synergistic effect of crystal toxins and metalloproteases might lie not only in a similar influence on epithelial components, but also in the formation of host-independent mechanism of the protoxin activation [90,91].
2.2.4. Role of Metalloproteases in Processing of Cry-Toxins
Judging by an example of CalY, it seems possible that Bt metalloproteases can hydrolyze not only host proteins but also bacterium’s own toxins. Such host-independent toxin proteolysis has already been found in other bacteria, for instance, in Vibiro cholera, which produces metalloproteases activating hemolysins [92]. Though Bt toxin can be activated by trypsic enzymes of various origin, cases of alteration of toxin’s host range by application of gut juice from different hosts have been reported [93]. The most intriguing examples come from the hemipterans who exploit membrane-bound cysteine proteases rather than serine proteases for protein digestion. For instance, in the pea aphid Acyrthosiphon pisum native gut proteases fail to cleave Cry3Aa sufficiently, which resolves in partial activation of the toxin [94]. At the same time, pretreatment by serine proteases restored toxicity of Cry3A, Cry4A and Cry11A in the same species [95]. This suggests that impaired proteolysis might be a reason for low susceptibility of hemipterans to Bt toxins though it might be affected by many other factors [96]. The assumption of Bt metalloprotease-mediated toxin processing in this case is further underpinned by the lower rates of pH in the aphid anterior midgut which are quite close to optimal for metalloprotease activity [97]. Thus, metalloproteases may act, not only against host proteins, but also provide host-independent systems for toxins activation.
2.3. Cytolysins
Formerly known as B. sphaericus, Lysinibacillus sphaericus, or Ls, is another entomopathogenic representative of Firmicutes phylum [98]. Ls is mostly known to infect mosquitoes (Diptera: Culicidae) by means of production of proteinaceous toxins, such as non-canonical Cry toxins Cry48Aa1/Cry49Aa1 and binary toxins BinA/BinB [14] Mtx vegetative toxins [99] and several specific cytolysins such as sphaericolysin [100]. A related toxin named alveolysin, was initially discovered as a virulence factor of Paenibacillus alvei (previously B. alvei), another species close to the Bacillus genus [101].
Both sphaericolysin and alveolysin belong to the thiol-activated cytolysin (TACY) family of cytolysins, a class of toxins, which act through formation of Na+-conductive olygomeric pores with subsequent cell swelling and lysis [102,103]. These cytolysins often serve as virulence factors of Gram-positive bacteria [102], while their occurrence in Gram-negative bacteria seems rare [104]. Since TACYs exploit membrane cholesterol as a main receptor, it is likely that these toxins may embrace a wide range of the affected species [102]. Indeed, sphaericolysin was shown to affect two such phylogenetically distant species as Blattella germanica (Blattodea: Ectobiidae) and Spodoptera litura (Lepidoptera: Noctuidae) [100]. In Bt, several cytolysins discovered so far bear a close structural resemblance to the Ls toxins, which suggests a similar mode of action and the range of affected hosts [14]. This idea is supported by observation of pathogenicity of several Bt strains to species of order Blattodea despite the absence of known Blattodea-specific Bt toxins [100,105].
3. Non-Proteinaceous Virulence Factors of Bt
3.1. Zwittermycin A
Produced by several strains of B. thuringiensis and B. subtilis, zwittermycin A (ZwA) is the only known antibiotic moiety of aminopolyol nature [106]. Structurally ZwA constitutes a peptide decorated with accessory amino and hydroxyl groups and shows resemblance to poliketide antibiotics. The biosynthesis of zwittermycin A is controlled by nine open reading frames forming a 16 kb gene cluster; proteins encoded within these frames form a unitary macromolecular complex which operates all stages of the antibiotic synthesis including the extraribosomal formation of peptide bonds [107]. Inactivation of ZwA, which forms the main mechanism of Bt autoresistance to its own antibiotic, is mediated by acetylase encoded by the zmaR gene associated with the synthetic cluster [108]. ZwA affects a wide spectrum of bacterial species as well as some eukaryotes including phytopathogenic fungi and oomycetes [109]. However, its mode of action remains elusive. Though resistant strains of E. coli are known to possess mutations in rpoB and rpoC genes encoding RNA polymerase core subunits, no direct effect of ZwA on transcription or any other stage of nucleic acid metabolism has been shown so far [110].
Despite the lack of obvious toxicity for eukaryotes, ZwA somehow appears to enhance Bt virulence. In particular, the synergistic effect of ZwA and main virulence determinants was demonstrated for lepidopteran-specific strains belonging to kurstaki serovar. The increase of ZwA production by site-specific mutagenesis enhanced the insecticidal effect of the respective strain against S. exigua and H. armigera (Lepidoptera: Noctuidae) by 115.4 and 25.9%, respectively [111]. Alternatively, a cooperative inoculation of Bt and avirulent B. cereus ZwA-producing strain resulted in the burst of Bt pathogenicity against Lymantria dispar (Lepidoptera: Erebidae) [112]. Such synergy between zwittermycin A and proteinaceous toxins is likely to be related to the eradicating effect of ZwA on midgut microbiota. According to one of the hypotheses present, these bacteria can influence pH rate in the midgut, which in its turn alleviates toxin solubility and proteolysis [113,114].
3.2. β-Exotoxins
Low weight toxins produced by some of the Bt strains vegetative cells are commonly referred to as β-exotoxins [115]. Due to preservance of their biocidal properties after being exposed to high temperature β-exotoxins are sometimes called thermostable Bt exotoxins which emphasizes their difference from thermolabile proteinaceous exotoxins. The most widespread moiety of this class is thuringiensin, or Thu, also called type I β-exotoxin [116]. Thuringiensin constitutes an adenosine analog whose 5′-hydroxile is conjugated with a phosphorylated diglucuronic acid residue. Type II β-exotoxin, having been discovered later, presumably constitutes a similar analog of uridine [117]. Thu biosynthesis is operated by thu cluster consisting of eleven genes which is usually located on large plasmids [118]. It is noteworthy that both the ability of Thu production and amount of toxin produced correlate with the presence of particular cry genes and serovar identity of the strain [119,120].
Cytotoxic activity of thuringiensin is likely to be a consequence of its structural similarity to ATP, since it is capable of binding to ATP-binding sites of RNA polymerase causing transcription inhibition [121,122]. The morphological effect of Thu on larval midgut epithelium was demonstrated on dipteran Culex sitiens and includes microvilli reduction and fragmentation of cellular membranous compartments such as granular ER and Golgi apparatus [123]. Because of the conservative structure of eukaryotic RNA polymerase functional sites β-exotoxins affect an outstandingly wide range of insect hosts from different orders. To date, insecticidal effect of thuriniensin has been shown for Anastrepha ludens, A. obliqua and A. serpentina (Diptera: Tephritidae) [124], C. sitiens (Diptera: Culicidae) [123], Lasioderma serricorne (Coleoptera: Anobiidae) [125], H. armigera, H. zea, S. exigua, Heliothis virescens, T. ni (Lepidoptera: Noctuidae) [116,126], Estigmene acrea (Lepidoptera: Erebidae), Pectinophora gossypiella (Lepidoptera: Gelechiidae) [126], Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) [127], Anthonomus grandis (Coleoptera: Curculionidae) [103], Lygus hesperus (Hemiptera: Miridae) [128]; some sources also state thuringiensin toxicity for Orthoptera and Neuroptera [116]. Apart from insects, Thu is toxic to mites of the Tetranychidae family [129] and nematodes of order Rhabditida [130]. In addition, thuringiensin is potentially harmful for mammals as it was shown to cause inflammatory processes leading to lung tissue damage because of adenylate cyclase activation [131].
4. Discussion
Taken together, apart from the major proteinaceous toxins B. thuringiensis produces a vast range of molecules affecting its pathogenesis which can be perceived as virulence factors. Moreover, these molecules not only enhance toxicity formed by crystal toxins but also exploit principally different mechanisms of pathogenesis that are summarized in Table 1 and Figure 1. These factors can be divided into two major groups. The first group contains proteinaceous factors showing certain host specificity, while the second group is formed by low weight moieties, which, vice versa, affect a wide range of organisms in a non-specific way.

Table 1.
The virulence factors of Bt unrelated to major protein toxins.
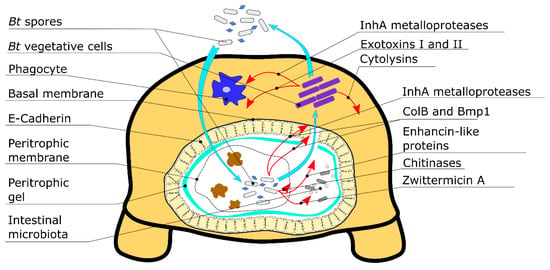
Figure 1.
Bt minor virulence factors produced on different stages of its life cycle and their respective targets. The figure depicts a schematic cross-section of an insect larva body; denoted are the Bt virulence factors and their modes of actions.
Transition from the digestive tract to host body cavities, such as haemocoel in the case of insect hosts, is the key stage of Bt infection (Figure 1). At this stage numerous virulence factors, such as Cry toxins, chitinases and metalloproteases are resolved to penetrate midgut walls. Since all of these factors show, at least to some degree, specificity in their hosts affected, this transition may be the most principal step to define bacterium’s host range, and minor virulence factors here may be viewed as a mean of its enlargement. Another crucial function of minor virulence factors is the alleviation of the host’s immune response which takes place mostly in the haemocoel. This process recruits both specific determinants, such as InhA metalloproteases, and unspecific factors defined by low diversity and functional degeneracy (Figure 1). That is, thuringiensin is shown to be toxic for several phylogenetically distant insect orders as well as several other groups of invertebrates and even vertebrates [121] (Table 1). Since the structure of β-exotoxins potentially inhibits all the ATP-dependent enzymes, such a wide range of vulnerable species does not seem to be surprising. A similar broad range of susceptible species can be expected from other low molecular inhibitors; for instance, trans-aconitate, a Krebs cycle inhibiting agent newly discovered in Bt, performs in a non-specific manner and affects both nematodes and the brown planthopper Nilaparvata lugens (Hemiptera: Delphacidae) [132].
To estimate the spreading of the minor virulence factors across known Bt strains we searched for several of the discussed proteins in genome annotations available at NCBI Assembly (downloaded on 20.04.2019). Of 511 genome assemblies, we chose 468 that had annotations which comprised more than 4500 genes, and then discarded one annotation which did not contain any functional information on its entries. The results are presented in Table 2.

Table 2.
Distribution of proteinacious virulence factors among 467 sequenced strains.
Unsurprisingly, chitinases seem to be present in all the analyzed strains except for one, thus suggesting that chitinases are essential for efficient Bt infection. This idea is sustained by the study of chi71A knockouts in Bt ser. pakistani which exhibited a dramatic loss of toxicity against A. aegyptii [133]. The only chitinase-less strain encompassed by our survey is HD73 belonging to kurstaki serovar, a standard crystaliferous strain notable for its modest chitinolytic activity and often used in recombinant chitinase genes assay [134]. Thus, chitinases in the broad sense pose as crucial and apparently non-specific virulence factors. To clarify their true role in host specificity, a thorough analysis of relations between specific chitinase groups and host range of a possessing strains is required as these proteins might differ in their optimal milieu. For instance, a recently discovered chitinase ChiA74 from Bt serovar kenyae strain LBIT-82 demonstrates an unusual bimodal distribution of pH optimum [135]. Such a mode of action might be a preadaptation to different environments and, thus, broaden a host range of a possessing strain.
The role of metalloproteases in determining host-specificity seems to be very diverse, because they act at different stages of pathogenesis. Proteolysis of mucin layer facilitates the penetration of peritrophic structures and epithelium exposure implying functional similarity between enhancin-like metalloproteases and Bt chitinases. The selective effect of the enhancin-like enzymes tied exclusively to lepidopteran hosts suggests differences in proteinaceous content of peritrophic membrane to be one of the specificity factors of Bt relating their metalloprotease repertoire [136]. In this light, a wide distribution of enhancins and enhancin-related metalloproteases whose genes are encountered in 59.6% of the analyzed strains seems quite bewildering. Because of the lack of information on host specificity or even serovar attribution for most of the strains deposited on NCBI Assembly verification of correlation between enhancin occurrence and anti-lepidopteran potency falls from the scope of this review. Nevertheless, of eight strains explicitly attributed to kurstaki serovar known for its high toxicity against Lepidoptera and Diptera [3], seven possess enhancin genes, while none were found in four strains identified as serovar israelensis specimens, which is known to act against dipteran species [137]. On the contrary, Bt matrix metalloproteases and collagenases demonstrate low specificity between hosts, presumably because of common structure of basal lamina; for instance, ColB metalloprotease equally contributes to infection of such phylogenetically distant hosts as insects and nematodes [72]. This idea is further supported by wide distribution of collagenase genes in Bt (Table 2).
Among Bt metalloproteases, InhA proteins appear to serve most various and complex functions. Apart from extracellular matrix lysis, these proteins are involved in the evasion of the humoral and, most likely, cellular immune response from the host organism. Differences in proteolytic affinity between InhA1 and InhA3 paralogues may be explained either by structural differences between metalloproteases themselves or by variety of antimicrobial peptides produced by insects. inhA genes are present in 59 strains in our brief survey. Interestingly, six of the eight discussed kurstaki strains possess these genes. An uneven distribution of different metalloproteases might by associated with the host specificity of Bt strains, and a more detailed assessment of their role in the establishment of host specificity including a parallel consideration of proteinaceous toxins, minor factors and the known host range of the analyzed strains might shed light on their role. At the same time, such analysis seems to be very difficult due to lacking metadata for some Bt strains.
In connection with metalloproteases, a probable role of amyloid fibril formation in Bt virulence and host-specificity seems to be very interesting. CalY, which was discovered as metalloprotease [77], was shown to form amyloid-like fibrils [78,82]. In Assembly-provided strains, calY is found in 12.6% of the strains, and in at least seven cases it is accompanied by tasA, a gene encoding another fibril-forming protein in Bt. Utilizing amyloid protein as a structural element of biofilm matrix is a very conservative feature shared by many different bacterial species [138]. Thus, one may expect that amyloid formation in biofilms involving co-polymerization of different virulence factors such as metalloproteases represents a general non-specific way of bacterial pathogenesis. Such amyloid formation by proteinaceous virulence factors could be important to provide their survival in the aggressive internal environment of the host body.
Recently, it was shown, that M60-like metalloprotease of E. coli, YghJ, forms amyloid fibrils [88,89]. YghJ is a metalloprotease involved in mucin degradation in mammalian intestine and belongs to the same family as enhancin-like enzymes, which act very host-specific. So, the role of amyloidogenesis in host specificity of Bt remains unclear.
Compared to other aforementioned factors, zwittermicin A shows a truly unique mode of virulence modulation. Despite its insecticidal effect has not yet been shown on other hosts rather than lepidopterans, one might suggest that similar ZwA-mediated suppression of native midgut microbiota may take place in Bt infection of various insects [98,99]. This, however, raises a question whether ZwA is a bona fide virulence factor rather than an allelopatic agent used to compete for ecological niches, both environmental and endogenous, with other microorganisms. If the latter is true, ZwA should be put in one row with other Bt antibiotics, such as, for example, peptides of bacteriocin group [139].
To conclude, the acquired data on the molecular mechanisms underlying the mode of action and specificity of the Bt virulence factors unrelated to major classes of protein toxins demonstrate their unequivocal role during Bt infection, state that proteinaceous factors have a greater impact on specificity of such interactions than low-weight non-protein molecules bearing a more generalized effect, and suggest involvement of functional protein aggregation in host-pathogen interactions.
Author Contributions
Y.V.M.—Writing, original draft preparation; Y.V.M., A.A.N. and K.S.A.—Writing, review and editing; A.A.N. and K.S.A.—Project administration; A.A.N. and K.S.A.—Funding acquisition. All authors reviewed and approved the final manuscript.
Funding
The study of the roles of the Bt minor virulence factors in the host-specificity was financially supported by the Russian Science Foundation (grant No 18-76-00028). The part of this work regarding bacterial amyloids was financially supported by the Russian Science Foundation (grant No 17-16-01100).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Drobniewski, F.A. Bacillus cereus and related species. Clin. Microbiol. Rev. 1993, 6, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Ohba, M.; Mizuki, E.; Uemori, A. Parasporin, a new anticancer protein group from Bacillus thuringiensis. Anticancer Res. 2009, 29, 427–433. [Google Scholar] [PubMed]
- Zheng, J.; Gao, Q.; Liu, L.; Liu, H.; Wang, Y.; Peng, D.; Ruan, L.; Raymond, B.; Sun, M. Comparative genomics of Bacillus thuringiensis reveals a path to specialized exploitation of multiple invertebrate hosts. mBio 2017, 8, e00822-17. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: Their potential in antifungal biocontrol. J. Microbiol. 2012, 50, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Mizuki, E.; Akao, T.; Ohba, M. Antitrichomonal strains of Bacillus thuringiensis. Parasitol. Res. 2002, 88, 1090–1109. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, A.M.; Abd El-Ghany, N.M. Molluscicidal activity of Bacillus thuringiensis strains against Biomphalaria alexandrina snails. Beni Suef Univ. J. Basic Appl. Sci. 2017, 6, 391–393. [Google Scholar] [CrossRef]
- Rosas-garcía, N.M. Biopesticide Production from Bacillus thuringiensis: An Environmentally Friendly Alternative. Biotechnology 2009, 3, 28–36. [Google Scholar] [CrossRef]
- International Service for the Acquisition of Agri-Biotech Applications (ISAAA). Brief 52: Global Status of Commercialized Biotech/GM Crops: 2016; ISAAA: Ithaca, NY, USA, 2016; ISBN 978-1-892456-66-4. [Google Scholar]
- Raymond, B.; Johnston, P.R.; Nielsen-LeRoux, C.; Crickmore, N. Bacillus thuringiensis: An impotent pathogen? Trends Microbiol. 2010, 18, 189–194. [Google Scholar] [CrossRef]
- Ruan, L.; Crickmore, N.; Peng, D.; Sun, M. Are nematodes a missing link in the confounded ecology of the entomopathogen Bacillus thuringiensis? Trends Microbiol. 2015, 23, 341–346. [Google Scholar] [CrossRef]
- Chakroun, M.; Banyuls, N.; Bel, Y.; Escriche, B.; Ferré, J. Bacterial Vegetative Insecticidal Proteins (Vip) from Entomopathogenic Bacteria Microbiol. Mol. Biol. Rev. 2016, 80, 329–350. [Google Scholar] [CrossRef]
- Estruch, J.J.; Warren, G.W.; Mullins, M.A.; Nye, G.J.; Craig, J.A.; Koziel, M.G. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA 1996, 93, 5389–5394. [Google Scholar] [CrossRef] [PubMed]
- Donovan, W.P.; Engleman, J.T.; Donovan, J.C.; Baum, J.A.; Bunkers, G.J.; Chi, D.J.; Clinton, W.P.; English, L.; Heck, G.R.; Ilagan, O.M.; et al. Discovery and characterization of Sip1A: A novel secreted protein from Bacillus thuringiensis with activity against coleopteran larvae. Appl. Microbiol. Biotechnol. 2006, 72, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Aroian, R.V. Bacterial pore-forming proteins as anthelmintics. Invertebr. Neurosci. 2012, 12, 37–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendoza-Estrada, L.J.; Hernández-Velázquez, V.M.; Arenas-Sosa, I.; Flores-Pérez, F.I.; Morales-Montor, J.; Peña-Chora, G. Anthelmintic Effect of Bacillus thuringiensis Strains against the Gill Fish Trematode Centrocestus formosanus. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. Physiol. 2001, 47, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Mahowald, A.; Jacobs-Lorena, M. Peritrophic matrix of the black fly Simulium vittatum: Formation, structure, and analysis of its protein components. J. Exp. Zool. 1994, 268, 269–281. [Google Scholar] [CrossRef]
- Venancio, T.M.; Cristofoletti, P.T.; Ferreira, C.; Verjovski-Almeida, S.; Terra, W.R. The Aedes aegypti larval transcriptome: A comparative perspective with emphasis on trypsins and the domain structure of peritrophins. Insect Mol. Biol. 2009, 18, 33–44. [Google Scholar] [CrossRef]
- Sales, M.P.; Gomes, V.M.; Fernandas, K.V.S.; Xavier-Filho, J. Chitin-binding proteins from cowpea (Vigna unguiculata) seeds. Braz. J. Med. Biol. Res. 1996, 29, 319–326. [Google Scholar] [CrossRef]
- Gutiérrez-Cabrera, A.E.; Córdoba-Aguilar, A.; Zenteno, E.; Lowenberger, C.; Espinoza, B. Origin, evolution and function of the hemipteran perimicrovillar membrane with emphasis on Reduviidae that transmit Chagas disease. Bull. Entomol. Res. 2016, 106, 279–291. [Google Scholar] [CrossRef]
- Waniek, P.J. The digestive system of human lice: Current advances and potential applications. Physiol. Entomol. 2009, 34, 203–210. [Google Scholar] [CrossRef]
- Sahai, A.S.; Manocha, M.S. Chitinases of fungi and plants: Their involvement in morphogenesis and host-parasite interaction. FEMS Microbiol. Rev. 1993, 11, 317–338. [Google Scholar] [CrossRef]
- Rathore, A.S.; Gupta, R.D. Chitinases from Bacteria to Human: Properties, Applications, and Future Perspectives. Enzym. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Vaaje-Kolstad, G.; Horn, S.J.; Sørlie, M.; Eijsink, V.G.H. The chitinolytic machinery of Serratia marcescens—A model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J. 2013, 280, 3028–3049. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, 490–495. [Google Scholar] [CrossRef]
- Berini, F.; Katz, C.; Gruzdev, N.; Casartelli, M.; Tettamanti, G.; Marinelli, F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018, 36, 818–838. [Google Scholar] [CrossRef]
- Wang, S.L.; Shih, I.L.; Liang, T.W.; Wang, C.H. Purification and characterization of two antifungal chitinases extracellularly produced by Bacillus amyloliquefaciens V656 in a shrimp and crab shell powder medium. J. Agric. Food Chem. 2002, 50, 2241–2248. [Google Scholar] [CrossRef]
- Chang, W.T.; Chen, M.L.; Wang, S.L. An antifungal chitinase produced by Bacillus subtilis using chitin waste as a carbon source. World J. Microbiol. Biotechnol. 2010, 26, 945–950. [Google Scholar] [CrossRef]
- Arora, N.; Ahmad, T.; Rajagopal, R.; Bhatnagar, R.K. A constitutively expressed 36 kDa exochitinase from Bacillus thuringiensis HD-1. Biochem. Biophys. Res. Commun. 2003, 307, 620–625. [Google Scholar] [CrossRef]
- Morales de la Vega, L.; Barboza-Corona, J.E.; Aguilar-Uscanga, M.G.; Ramírez-Lepe, M. Purification and characterization of an exochitinase from Bacillus thuringiensis subsp. aizawai and its action against phytopathogenic fungi. Can. J. Microbiol. 2006, 52, 651–657. [Google Scholar] [CrossRef]
- De la Fuente-Salcido, N.M.; Casados-Vázquez, L.E.; García-Pérez, A.P.; Barboza-Pérez, U.E.; Bideshi, D.K.; Salcedo-Hernández, R.; García-Almendarez, B.E.; Barboza-Corona, J.E. The endochitinase ChiA Btt of Bacillus thuringiensis subsp. tenebrionis DSM-2803 and its potential use to control the phytopathogen Colletotrichum gloeosporioides. MicrobiologyOpen 2016, 5, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Hollensteiner, J.; Wemheuer, F.; Harting, R.; Kolarzyk, A.M.; Diaz Valerio, S.M.; Poehlein, A.; Brzuszkiewicz, E.B.; Nesemann, K.; Braus-Stromeyer, S.A.; Braus, G.H.; et al. Bacillus thuringiensis and Bacillus weihenstephanensis inhibit the growth of phytopathogenic Verticillium species. Front. Microbiol. 2017, 7, 2171. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.L.D.A.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Cabib, E.; Miller, L.H. Malaria parasite chitinase and penetration of the mosquito peritrophic membrane. Proc. Natl. Acad. Sci. USA 1991, 88, 2807–2810. [Google Scholar] [CrossRef] [PubMed]
- Schlein, Y.; Jacobson, R.L.; Shlomai, J. Chitinase secreted by Leishmania functions in the sandfly vector. Proc. R. Soc. B Biol. Sci. 1991, 245, 121–126. [Google Scholar] [CrossRef]
- Fuhrman, J.A.; Lee, J.; Dalamagas, D. Structure and function of a family of chitinase isozymes from Brugian microfilariae. Exp. Parasitol. 1995, 80, 672–680. [Google Scholar] [CrossRef]
- Hawtin, R.E.; Zarkowska, T.; Arnold, K.; Thomas, C.J.; Gooday, G.W.; King, L.A.; Kuzio, J.A.; Possee, R.D. Liquefaction of Autographa califo;rnica nucleopolyhedrovirus-infected insects is dependent on the integrity of virus-encoded chitinase and cathepsin genes. Virology 1997, 238, 243–253. [Google Scholar] [CrossRef]
- Liu, M.; Cai, Q.X.; Liu, H.Z.; Zhang, B.H.; Yan, J.P.; Yuan, Z.M. Chitinolytic activities in Bacillus thuringiensis and their synergistic effects on larvicidal activity. J. Appl. Microbiol. 2002, 93, 374–379. [Google Scholar] [CrossRef]
- Juárez-Hernández, E.O.; Casados-Vázquez, L.E.; del Rincón-Castro, M.C.; Salcedo-Hernández, R.; Bideshi, D.K.; Barboza-Corona, J.E. Bacillus thuringiensis subsp. israelensis producing endochitinase ChiA74Δsp inclusions and its improved activity against Aedes aegypti. J. Appl. Microbiol. 2015, 119, 1692–1699. [Google Scholar] [CrossRef]
- Regev, A.; Keller, M.; Strizhov, N.; Sneh, B.; Prudovsky, E.; Chet, I.; Ginzberg, I.; Koncz-Kalman, Z.; Koncz, C.; Schell, J.; et al. Synergistic activity of a Bacillus thuringiensis δ-endotoxin and a bacterial endochitinase against Spodoptera littoralis larvae. Appl. Environ. Microbiol. 1996, 62, 3581–3586. [Google Scholar]
- Leetachewa, S.; Khomkhum, N.; Sakdee, S.; Wang, P.; Moonsom, S. Enhancement of insect susceptibility and larvicidal efficacy of Cry4Ba toxin by calcofluor. Parasites Vectors 2018, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.T.; Chao, Y.P. Chitin-binding domain based immobilization of d-hydantoinase. J. Biotechnol. 2005, 117, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Kunii, T.; Nohara, K.; Wakita, S.; Sugahara, Y.; Kawakita, M.; Oyama, F.; Sakaguchi, M. Characterization of a Bacillus thuringiensis chitinase that binds to cellulose and chitin. AMB Express 2017, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Neeraja, C.; Subramanyam, R.; Moerschbacher, B.M.; Podile, A.R. Swapping the chitin-binding domain in Bacillus chitinases improves the substrate binding affinity and conformational stability. Mol. Biosyst. 2010, 6, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.D.; Kim, Y.J.; Choi, J.H. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Zhangfu, L.; Jing, X.; Jin, H.; Ran, H.; Tao, K.; Ge, S.; Liu, K.; Liu, S. Identification of a chitinase-producing bacterium C4 and histopathologic study on locusts. Pest Manag. Sci. 2005, 61, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Busby, J.N.; Landsberg, M.J.; Simpson, R.M.; Jones, S.A.; Hankamer, B.; Hurst, M.R.; Lott, J.S. Structural analysis of Chi1 chitinase from Yen-Tc: The multisubunit insecticidal ABC toxin complex of Yersinia entomophaga. J. Mol. Biol. 2012, 415, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, S.; Shinoda, S. Microbial metalloproteases and pathogenesis. Microbes Infect. 2000, 2, 91–98. [Google Scholar] [CrossRef]
- Cerdà-Costa, N.; Gomis-Rüth, F.X. Architecture and function of metallopeptidase catalytic domains. Protein Sci. 2014, 23, 123–144. [Google Scholar] [CrossRef]
- Hooper, N.M. Families of zinc metalloproteases. FEBS Lett. 1994, 354, 1–6. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014, 40, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; Bonning, B.C. Proteases as insecticidal agents. Toxins 2010, 2, 935–953. [Google Scholar] [CrossRef] [PubMed]
- Lepore, L.S.; Roelvink, P.R.; Granados, R.R. Enhancin, the granulosis virus protein that facilitates nucleopolyhedrovirus (NPV) infections, is a metalloprotease. J. Invertebr. Pathol. 1996, 68, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Granados, R.R. An intestinal mucin is the target substrate for a baculovirus enhancin. Proc. Natl. Acad. Sci. USA 1997, 94, 6977–6982. [Google Scholar] [CrossRef]
- Agrawal, S.; Kelkenberg, M.; Begum, K.; Steinfeld, L.; Williams, C.E.; Kramer, K.J.; Beeman, R.W.; Park, Y.; Muthukrishnan, S.; Merzendorfer, H. Two essential peritrophic matrix proteins mediate matrix barrier functions in the insect midgut. Insect Biochem. Mol. Biol. 2014, 49, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Granados, R.R.; Fu, Y.; Corsaro, B.; Hughes, P.R. Enhancement of Bacillus thuringiensis toxicity to lepidopterous species with the enhancin from Trichoplusia ni granulovirus. Biol. Control 2001, 20, 153–159. [Google Scholar] [CrossRef]
- Gohar, M.; Faegri, K.; Perchat, S.; Ravnum, S.; Økstad, O.A.; Gominet, M.; Kolstø, A.-B.; Lereclus, D. The PlcR virulence regulon of Bacillus cereus. PLoS ONE 2008, 3, e2793. [Google Scholar] [CrossRef] [PubMed]
- Slamti, L.; Lemy, C.; Henry, C.; Guillot, A.; Huillet, E.; Lereclus, D. CodY regulates the activity of the virulence quorum sensor PlcR by controlling the import of the signaling peptide PapR in Bacillus thuringiensis. Front. Microbiol. 2016, 6, 1501. [Google Scholar] [CrossRef] [PubMed]
- Hajaij-Ellouze, M.; Fedhila, S.; Lereclus, D.; Nielsen-LeRoux, C. The enhancin-like metalloprotease from the Bacillus cereus group is regulated by the pleiotropic transcriptional activator PlcR but is not essential for larvicidal activity. FEMS Microbiol. Lett. 2006, 260, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, L.; Guo, W.; Zhang, X.; Peng, D.; Luo, C.; Yu, Z.; Sun, M. Bacillus thuringiensis bel protein enhances the toxicity of Cry1Ac protein to Helicoverpa armigera larvae by degrading insect intestinal mucin. Appl. Environ. Microbiol. 2009, 75, 5237–5243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guo, W.; Sun, W.; Xu, D.; Liu, D. Identification of a novel enhancin-like gene from Bacillus thuringiensis. Front. Agric. China 2011, 5, 423–429. [Google Scholar] [CrossRef]
- Chung, M.C.; Popova, T.G.; Millis, B.A.; Mukherjee, D.V.; Zhou, W.; Liotta, L.A.; Petricoin, E.F.; Chandhoke, V.; Bailey, C.; Popov, S.G. Secreted neutral metalloproteases of Bacillus anthracis as candidate pathogenic factors. J. Biol. Chem. 2006, 281, 31408–31418. [Google Scholar] [CrossRef] [PubMed]
- Guillemet, E.; Cadot, C.; Tran, S.L.; Guinebretière, M.H.; Lereclus, D.; Ramarao, N. The InhA metalloproteases of Bacillus cereus contribute concomitantly to virulence. J. Bacteriol. 2010, 192, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Dalhammar, G.; Steiner, H. Characterization of inhibitor A, a protease from Bacillus thuringiensis which degrades attacins and cecropins, two classes of antibacterial proteins in insects. Eur. J. Biochem. 1984, 139, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, N.; Lereclus, D. The InhA 1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell Microbiol. 2005, 7, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Fedhila, S.; Gohar, M.; Slamti, L.; Nel, P.; Lereclus, D. The Bacillus thuringiensis PlcR-regulated gene inhA2 is necessary, but not sufficient, for virulence. J. Bacteriol. 2003, 185, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Siden, I.; Dalhammar, G.; Telander, B.; Boman, H.G.; Somerville, H. Virulence factors in Bacillus thuringiensis: Purification and properties of a protein inhibitor of immunity in insects. J. Gen. Microbiol. 1979, 114, 45–52. [Google Scholar] [CrossRef]
- Dammak, I.; Dammak, M.; Tounsi, S. Histopathological and combinatorial effects of the metalloprotease InhA1 and Cry proteins of Bacillus thuringiensis against Spodoptera littoralis. Int. J. Biol. Macromol. 2015, 81, 759–762. [Google Scholar] [CrossRef]
- Fedhila, S.; Nel, P.; Lereclus, D. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 2002, 184, 3296–3304. [Google Scholar] [CrossRef]
- Peng, D.; Lin, J.; Huang, Q.; Zheng, W.; Liu, G.; Zheng, J.; Zhu, L.; Sun, M. A novel metalloproteinase virulence factor is involved in Bacillus thuringiensis pathogenesis in nematodes and insects. Environ. Microbiol. 2016, 18, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.S.; Correia, A.; Esteves, A.C. Bacterial collagenases—A review. Crit. Rev. Microbiol. 2016, 42, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Lin, J.; Du, H.; Bravo, A.; Soberón, M.; Sun, M.; Peng, D. Bacillus thuringiensis targets the host intestinal epithelial junctions for successful infection of Caenorhabditis elegans. Environ. Microbiol. 2019, 21, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, L.; Huang, Q.; Zheng, J.; Zhou, W.; Peng, D.; Ruan, L.; Sun, M. Bacillus thuringiensis metalloproteinase Bmp1 functions as a nematicidal virulence factor. Appl. Environ. Microbiol. 2013, 79, 460–468. [Google Scholar] [CrossRef]
- Grass, G.; Schierhorn, A.; Sorkau, E.; Müller, H.; Rücknagel, P.; Nies, D.H.; Fricke, B. Camelysin Is a Novel Surface Metalloproteinase from Bacillus cereus. Infect. Immun. 2004, 72, 219–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fricke, B.; Drößler, K.; Willhardt, I.; Schierhorn, A.; Menge, S.; Rücknagel, P. The cell envelope-bound metalloprotease (camelysin) from Bacillus cereus is a possible pathogenic factor. Biochim. Biophys. Acta Mol. Basis Dis. 2001, 1537, 132–146. [Google Scholar] [CrossRef]
- Candela, T.; Fagerlund, A.; Buisson, C.; Gilois, N.; Kolstø, A.; Økstad, O.; Aymerich, S.; Nielsen-Leroux, C.; Lereclus, D.; Gohar, M. CalY is a major virulence factor and a biofilm matrix protein. Mol. Microbiol. 2018, 111, 1416–1429. [Google Scholar] [CrossRef]
- Houry, A.; Gohar, M.; Deschamps, J.; Tischenko, E.; Aymerich, S.; Gruss, A.; Briandet, R. Bacterial swimmers that infiltrate and take over the biofilm matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 13088–13093. [Google Scholar] [CrossRef] [PubMed]
- Nizhnikov, A.A.; Antonets, K.S.; Inge-Vechtomov, S.G. Amyloids: From pathogenesis to function. Biochemistry 2015, 80, 1127–1144. [Google Scholar] [CrossRef]
- Nizhnikov, A.A.; Antonets, K.S.; Bondarev, S.A.; Inge-Vechtomov, S.G.; Derkatch, I.L. Prions, amyloids, and RNA: Pieces of a puzzle. Prion 2016, 10, 182–206. [Google Scholar] [CrossRef]
- Caro-Astorga, J.; Pérez-García, A.; de Vicente, A.; Romero, D. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front. Microbiol. 2014, 5, 745. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.; Aguilar, C.; Losick, R.; Kolter, R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2010, 107, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Erskine, E.; MacPhee, C.E.; Stanley-Wall, N.R. Functional amyloid and other Protein fibers in the biofilm matrix. J. Mol. Biol. 2018, 430, 3642–3656. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2017, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Nakjang, S.; Ndeh, D.A.; Wipat, A.; Bolam, D.N.; Hirt, R.P. A novel extracellular metallopeptidase domain shared by animal Host-Associated mutualistic and pathogenic microbes. PLoS ONE 2012, 7, e30287. [Google Scholar] [CrossRef] [PubMed]
- Antonets, K.S.; Volkov, K.V.; Maltseva, A.L.; Arshakian, L.M.; Galkin, A.P.; Nizhnikov, A.A. Proteomic analysis of Escherichia coli protein fractions resistant to solubilization by ionic detergents. Biochemistry 2016, 81, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Belousov, M.V.; Bondarev, S.A.; Kosolapova, A.O.; Antonets, K.S.; Sulatskaya, A.I.; Sulatsky, M.I.; Zhouravleva, G.A.; Kuznetsova, I.M.; Turoverov, K.K.; Nizhnikov, A.A. M60-like metalloprotease domain of the Escherichia coli YghJ protein forms amyloid fibrils. PLoS ONE 2018, 13, e0191317. [Google Scholar] [CrossRef] [PubMed]
- Nisnevitch, M.; Sigawi, S.; Cahan, R.; Nitzan, Y. Isolation, characterization and biological role of camelysin from Bacillus thuringiensis subsp. israelensis. Curr. Microbiol. 2010, 61, 176–183. [Google Scholar] [CrossRef]
- Nisnevitch, M.; Cohen, S.; Ben-Dov, E.; Zaritsky, A.; Sofer, Y.; Cahan, R. Cyt2Ba of Bacillus thuringiensis israelensis: Activation by putative endogenous protease. Biochem. Biophys. Res. Commun. 2006, 344, 99–105. [Google Scholar] [CrossRef]
- Nagamune, K.; Yamamoto, K.; Naka, A.; Matsuyama, J.; Miwatani, T.; Honda, T. In vitro proteolytic processing and activation of the recombinant precursor of El Tor cytolysin/hemolysin (pro-HlyA) of Vibrio cholerae by soluble hemagglutinin/protease of V. cholerae, trypsin, and other proteases. Infect. Immun. 1996, 64, 4655–4658. [Google Scholar] [PubMed]
- Haider, M.Z.; Ward, E.S.; Ellar, D.J. Cloning and heterologous expression of an insecticidal delta-endotoxin gene from Bacillus thuringiensis var. aizawai ICI toxic to both lepidoptera and diptera. Gene 1987, 52, 285–290. [Google Scholar] [CrossRef]
- Li, H.; Chougule, N.P.; Bonning, B.C. Interaction of the Bacillus thuringiensis delta endotoxins Cry1Ac and Cry3Aa with the gut of the pea aphid, Acyrthosiphon pisum (Harris). J. Invertebr. Pathol. 2011, 107, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Porcar, M.; Grenier, A.M.; Federici, B.; Rahbé, Y. Effects of Bacillus thuringiensis δ-endotoxins on the pea aphid (Acyrthosiphon pisum). Appl. Environ. Microbiol. 2009, 75, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Shao, E.; Liu, S.; Lin, L.; Guan, X. Proteolytic processing of Bacillus thuringiensis toxin Cry1Ab in rice brown planthopper, Nilaparvata lugens (Stål). J. Invertebr. Pathol. 2013, 114, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Deraison, C.; Darboux, I.; Duportets, L.; Gorojankina, T.; Rahbé, Y.; Jouanin, L. Cloning and characterization of a gut-specific cathepsin L from the aphid Aphis gossypii. Insect Mol. Biol. 2004, 13, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Bideshi, D.K.; Federici, B.A. Properties and applied use of the mosquitocidal bacterium, Bacillus sphaericus. J. Asia Pac. Entomol. 2010, 13, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.-F. Bacillus sphaericus Toxins: Molecular Biology and Mode of Action. Annu. Rev. Entomol. 1996, 41, 451–472. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Nakashima, K.; Ishida, C.; Kawamura, T.; Matsuda, K. Cloning, functional characterization, and mode of action of a novel insecticidal pore-forming toxin, sphaericolysin, produced by Bacillus sphaericus. Appl. Environ. Microbiol. 2007, 73, 3404–3411. [Google Scholar] [CrossRef]
- Geoffroy, C.; Mengaud, J.; Alouf, J.E.; Cossart, P. Alveolysin, the thiol-activated toxin of Bacillus alvei, is homologous to listeriolysin O, perfringolysin O, pneumolysin, and streptolysin O and contains a single cysteine. J. Bacteriol. 1990, 172, 7301–7305. [Google Scholar] [CrossRef]
- Billington, S.J.; Jost, B.H.; Songer, J.G. Thiol-activated cytolysins: Structure, function and role in pathogenesis. FEMS Microbiol. Lett. 2000, 182, 197–205. [Google Scholar] [CrossRef]
- Skals, M.; Praetorius, H.A. Mechanisms of cytolysin-induced cell damage—A role for auto- and paracrine signalling. Acta Physiol. 2013, 209, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Le, H.M.; Sieber, J.R.; Bruxvoort, C.; McInerney, M.J.; Tweten, R.K. Identification and characterization of the first cholesterol-dependent cytolysins from gram-negative bacteria. Infect. Immun. 2013, 81, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Porcar, M.; Navarro, L.; Jiménez-Peydró, R. Pathogenicity of intrathoracically administrated Bacillus thuringiensis spores in Blatta orientalis. J. Invertebr. Pathol. 2006, 93, 63–66. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Silo-Suh, L.A.; Handelsman, J.; Clardy, J. Zwittermicin A, an antifungal and plant protection agent from Bacillus cereus. Tetrahedron Lett. 1994, 35, 2499–2502. [Google Scholar] [CrossRef]
- Kevany, B.M.; Rasko, D.A.; Thomas, M.G. Characterization of the complete zwittermicin biosynthesis gene cluster from Bacillus cereus. Appl. Environ. Microbiol. 2009, 75, 1144–1155. [Google Scholar] [CrossRef]
- Luo, Y.; Ruan, L.F.; Zhao, C.M.; Wang, C.X.; Peng, D.H.; Sun, M. Validation of the intact zwittermicin a biosynthetic gene cluster and discovery of a complementary resistance mechanism in Bacillus thuringiensis. Antimicrob. Agents Chemother. 2011, 55, 4161–4169. [Google Scholar] [CrossRef]
- Zhou, Y.; Choi, Y.L.; Sun, M.; Yu, Z. Novel roles of Bacillus thuringiensis to control plant diseases. Appl. Microbiol. Biotechnol. 2008, 55, 4161–4169. [Google Scholar] [CrossRef]
- Stabb, E.V.; Handelsman, J. Genetic analysis of zwittermicin A resistance in Escherichia coli: Effects on membrane potential and RNA polymerase. Mol. Microbiol. 1998, 27, 311–322. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Z.; Siddiqui, Z.A.; Gong, Y.; Yu, Z.; Chen, S. Efficient screening and breeding of Bacillus thuringiensis subsp. kurstaki for high toxicity against Spodoptera exigua and Heliothis armigera. J. Ind. Microbiol. Biotechnol. 2009, 36, 815–820. [Google Scholar] [CrossRef]
- Broderick, N.A.; Goodman, R.M.; Raffa, K.F.; Handelsman, J. Synergy between zwittermicin A and Bacillus thuringiensis subsp. kurstaki against gypsy moth (Lepidoptera: Lymantriidae). Biol. Control. 2000, 29, 101–107. [Google Scholar] [CrossRef]
- Broderick, N.A.; Goodman, R.M.; Handelsman, J. Effect of host diet and insect source on synergy of gypsy moth (Lepidoptera: Lymantriidae) mortality to Bacillus thuringiensis subsp. kurstaki by zwittermicin A. Environ. Entomol. 2003, 32, 387–391. [Google Scholar] [CrossRef]
- Tagliavia, M.; Messina, E.; Manachini, B.; Cappello, S.; Quatrini, P. The gut microbiota of larvae of Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae). BMC Microbiol. 2014, 14, 136. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ruan, L.F.; Hu, Z.F.; Peng, D.H.; Cao, S.Y.; Yu, Z.N.; Liu, Y.; Zheng, J.S.; Sun, M. Genome-wide screening reveals the genetic determinants of an antibiotic insecticide in Bacillus thuringiensis. J. Biol. Chem. 2010, 285, 39191–39200. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ruan, L.; Peng, D.; Li, L.; Sun, M.; Yu, Z. Thuringiensin: A thermostable secondary metabolite from Bacillus thuringiensis with insecticidal activity against a wide range of insects. Toxins 2014, 6, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Levinson, B.L.; Kasyan, K.J.; Chiu, S.S.; Currier, T.C.; González, J.M. Identification of β-exotoxin production, plasmids encoding β-exotoxin, and a new exotoxin in Bacillus thuringiensis by using high-performance liquid chromatography. J. Bacteriol. 1990, 172, 3172–3179. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Yin, W.; Shao, X.; Zheng, H.; Li, M.; Zhao, Y.; Sun, M.; Wang, S.; Yu, Z. Complete genome sequence of Bacillus thuringiensis subsp chinensis strain CT-43. J. Bacteriol. 2011, 193, 3407–3408. [Google Scholar] [CrossRef] [PubMed]
- Espinasse, S.; Gohar, M.; Chaufaux, J.; Buisson, C.; Perchat, S.; Sanchis, V. Correspondence of high levels of beta-exotoxin I and the presence of cry1B in Bacillus thuringiensis. Appl. Environ. Microbiol. 2002, 68, 4182–4186. [Google Scholar] [CrossRef]
- Hernández, C.S.; Martínez, C.; Porcar, M.; Caballero, P.; Ferré, J. Correlation between serovars of Bacillus thuringiensis and type I β-exotoxin production. J. Invertebr. Pathol. 2003, 82, 57–62. [Google Scholar] [CrossRef]
- Beebee, T.; Korner, A.; Bond, R.P. Differential inhibition of mammalian ribonucleic acidpolymerases by an exotoxin from Bacillus thuringiensis. The direct observation of nucleoplasmic ribonucleic acid polymerase activity in intact nuclei. Biochem. J. 1972, 127, 619–634. [Google Scholar] [CrossRef]
- Gohar, M.; Perchat, S. Sample preparation for β-exotoxin determination in Bacillus thuringiensis cultures by reversed-phase high-performance liquid chromatography. Anal. Biochem. 2001, 298, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.; Zizka, Z. Effect of Bacillus thuringiensis beta exotoxin on ultrastructures of midgut cells of Culex sitiens. Cytobios 1994, 77, 19–27. [Google Scholar]
- Toledo, J.; Liedo, P.; Williams, T.; Ibarra, J. Toxicity of Bacillus thuringiensis β-exotoxin to three species of fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 1999, 92, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Kasaishi, Y.; Harada, H.; Ichimatsu, T.; Saitoh, H.; Mizuki, E.; Ohba, M. Assessment of the efficacy of Japanese Bacillus thuringiensis isolates against the cigarette beetle, Lasioderma serricorne (Coleoptera: Anobiidae). J. Invertebr. Pathol. 2002, 81, 122–126. [Google Scholar] [CrossRef]
- Ignoffo, C.M.; Gregory, B. Effects of Bacillus thuringiensis β-exotoxin on Larval Maturation, Adult Longevity, Fecundity, and Egg Viability in Several Species of Lepidoptera. Environ. Entomol. 1972, 1, 269–272. [Google Scholar] [CrossRef]
- Burgerjon, A.; Biache, G.; Cals, P. Teratology of the Colorado potato beetle, Leptinotarsa decemlineata, as provoked by larval administration of the thermostable toxin of Bacillus thuringiensis. J. Invertebr. Pathol. 1969, 14, 274–278. [Google Scholar] [CrossRef]
- Tanigoshi, L.K.; Mayer, D.F.; Babcock, J.M.; Lundenl, J.D. Efficacy of the β-Exotoxin of Bacillus thuringiensis to Lygus hesperus (Heteroptera: Miridae): Laboratory and Field Responses. J. Econ. Entomol. 1990, 83, 2200–2206. [Google Scholar] [CrossRef]
- Royalty, R.N.; Hall, F.R.; Taylor, R.A.J. Effects of thuringiensin on Tetranychus urticae (Acari: Tetranychidae) mortality, fecundity, and feeding. J. Econ. Entomol. 1990, 83, 792–798. [Google Scholar] [CrossRef]
- Iatsenko, I.; Nikolov, A.; Sommer, R.J. Identification of distinct Bacillus thuringiensis 4A4 nematicidal factors using the model nematodes Pristionchus pacificus and Caenorhabditis elegans. Toxins 2014, 6, 2050–2063. [Google Scholar] [CrossRef]
- Tsai, S.F.; Yang, C.; Liu, B.L.; Hwang, J.S.; Ho, S.P. Role of oxidative stress in thuringiensin-induced pulmonary toxicity. Toxicol. Appl. Pharmacol. 2006, 216, 347–353. [Google Scholar] [CrossRef]
- Du, C.; Cao, S.; Shi, X.; Nie, X.; Zheng, J.; Deng, Y.; Ruan, L.; Peng, D.; Sun, M. Genetic and biochemical characterization of a gene operon for trans-aconitic acid, a novel nematicide from Bacillus thuringiensis. J. Biol. Chem. 2017, 292, 3517–3530. [Google Scholar] [CrossRef] [PubMed]
- Thamthiankul, S.; Suan-Ngay, S.; Tantimavanich, S.; Panbangred, W. Chitinase from Bacillus thuringiensis subsp. pakistani. Appl. Microbiol. Biotechnol. 2001, 56, 395–401. [Google Scholar] [CrossRef] [PubMed]
- González-Ponce, K.S.; Casados-Vázquez, L.E.; Salcedo-Hernández, R.; Bideshi, D.K.; del Rincón-Castro, M.C.; Barboza-Corona, J.E. Recombinant Bacillus thuringiensis subsp. kurstaki HD73 strain that synthesizes Cry1Ac and chimeric ChiA74∆sp chitinase inclusions. Arch. Microbiol. 2017, 199, 627–633. [Google Scholar] [CrossRef]
- Barboza-Corona, J.E.; Nieto-Mazzocco, E.; Velázquez-Robledo, R.; Salcedo-Hernandez, R.; Bautista, M.; Jiménez, B.; Ibarra, J.E. Cloning, sequencing, and expression of the chitinase gene chiA74 from Bacillus thuringiensis. Appl. Environ. Microbiol. 2003, 69, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.S.; Granados, R.R. Peritrophic membrane structure and formation of larval Trichoplusia ni with an investigation on the secretion patterns of a PM mucin. Tissue Cell. 1999, 31, 202–211. [Google Scholar] [CrossRef]
- Gillis, A.; Fayad, N.; Makart, L.; Bolotin, A.; Sorokin, A.; Kallassy, M.; Mahillon, J. Role of plasmid plasticity and mobile genetic elements in the entomopathogen Bacillus thuringiensis serovar israelensis. FEMS Microbiol. Rev. 2018, 42, 829–856. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.L.; Gichana, E.; Zhou, Y.; Chapman, M.R. Bacterial amyloids. Methods Mol. Biol. 2018, 1779, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Marroquín, E.L.; Galán-Wong, L.J.; Moreno-Medina, V.R.; Reyes-López, M.Á.; Pereyra-Alférez, B. Bacteriocins synthesized by Bacillus thuringiensis: Generalities and potential applications. Rev. Med. Microbiol. 2016, 27, 95–101. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).