Pharmacokinetics of Human Recombinant Anti-Botulinum Toxin Antibodies in Rats
Abstract
1. Introduction
2. Results
2.1. Safety
2.2. Pharmacokinetic Analyses NTM-1631
2.3. ADA of NTM-1631
2.4. Pharmacokinetic Analyses NTM-1632
2.5. ADA of NTM-1632
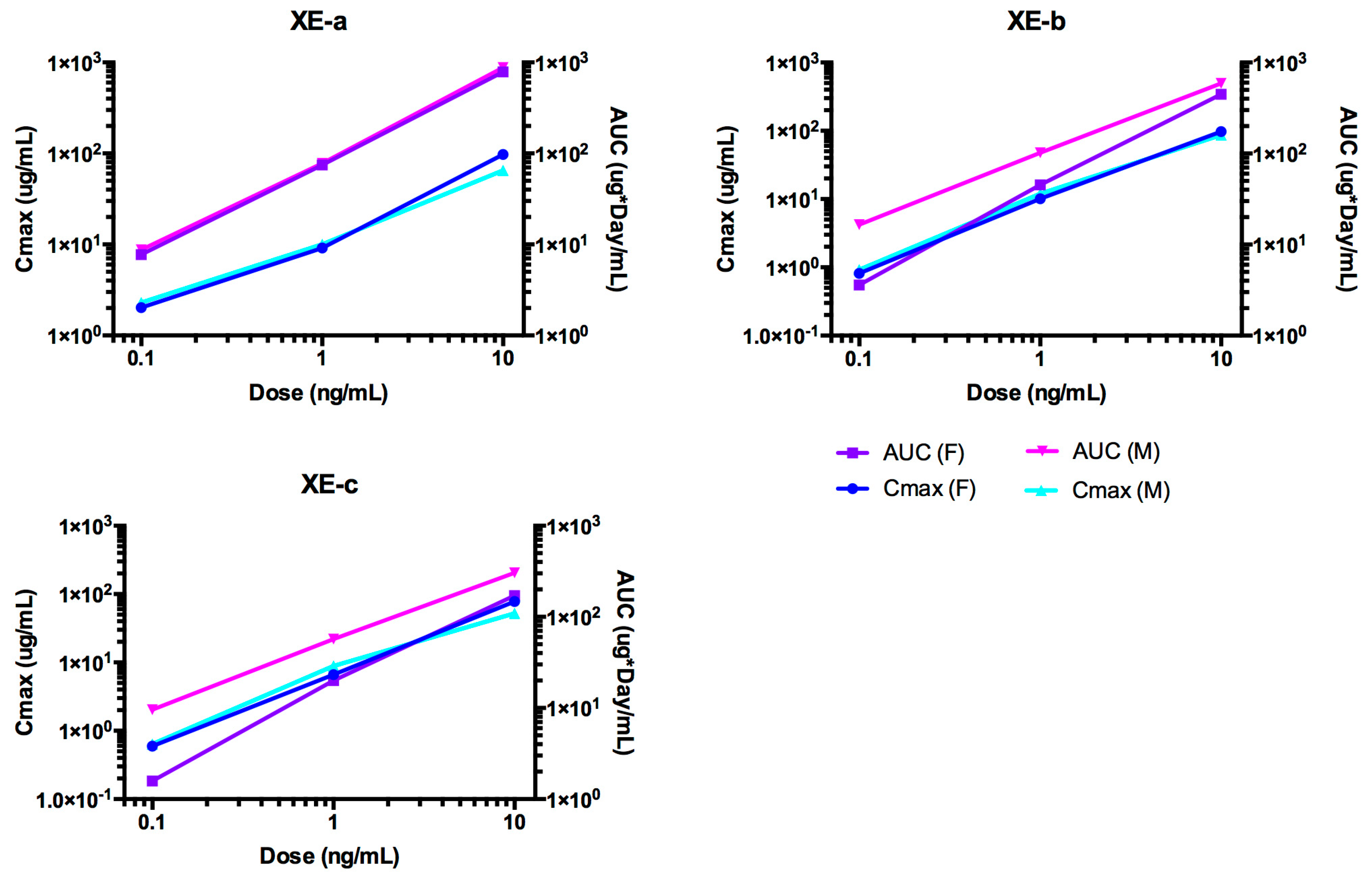
2.6. Pharmacokinetic Analyses NTM-1633
2.7. ADA of NTM-1633
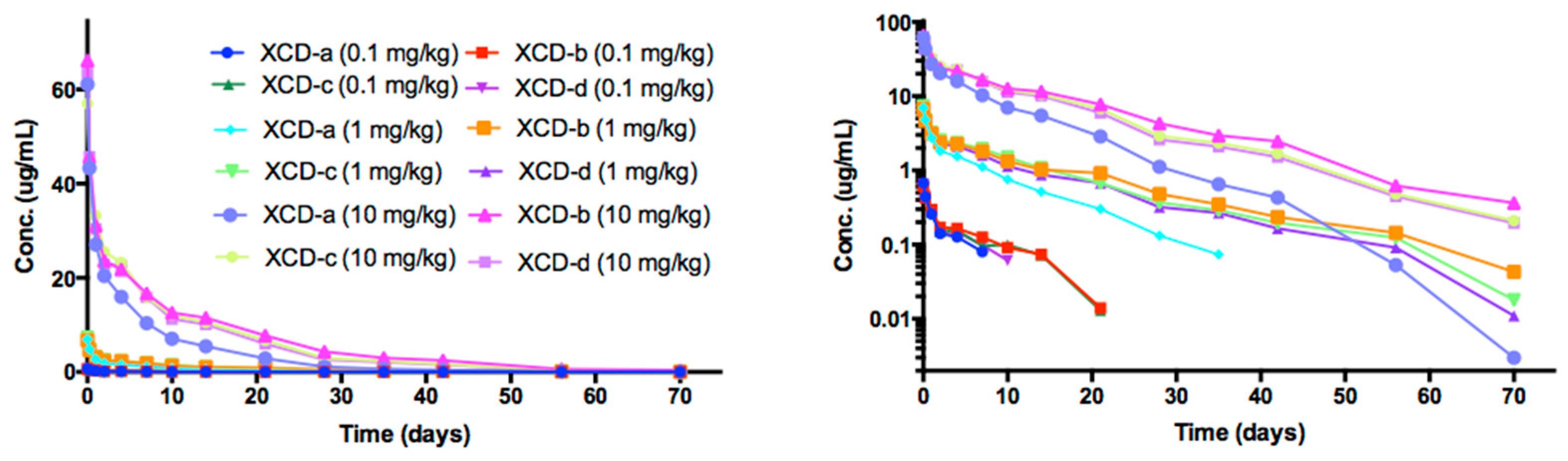
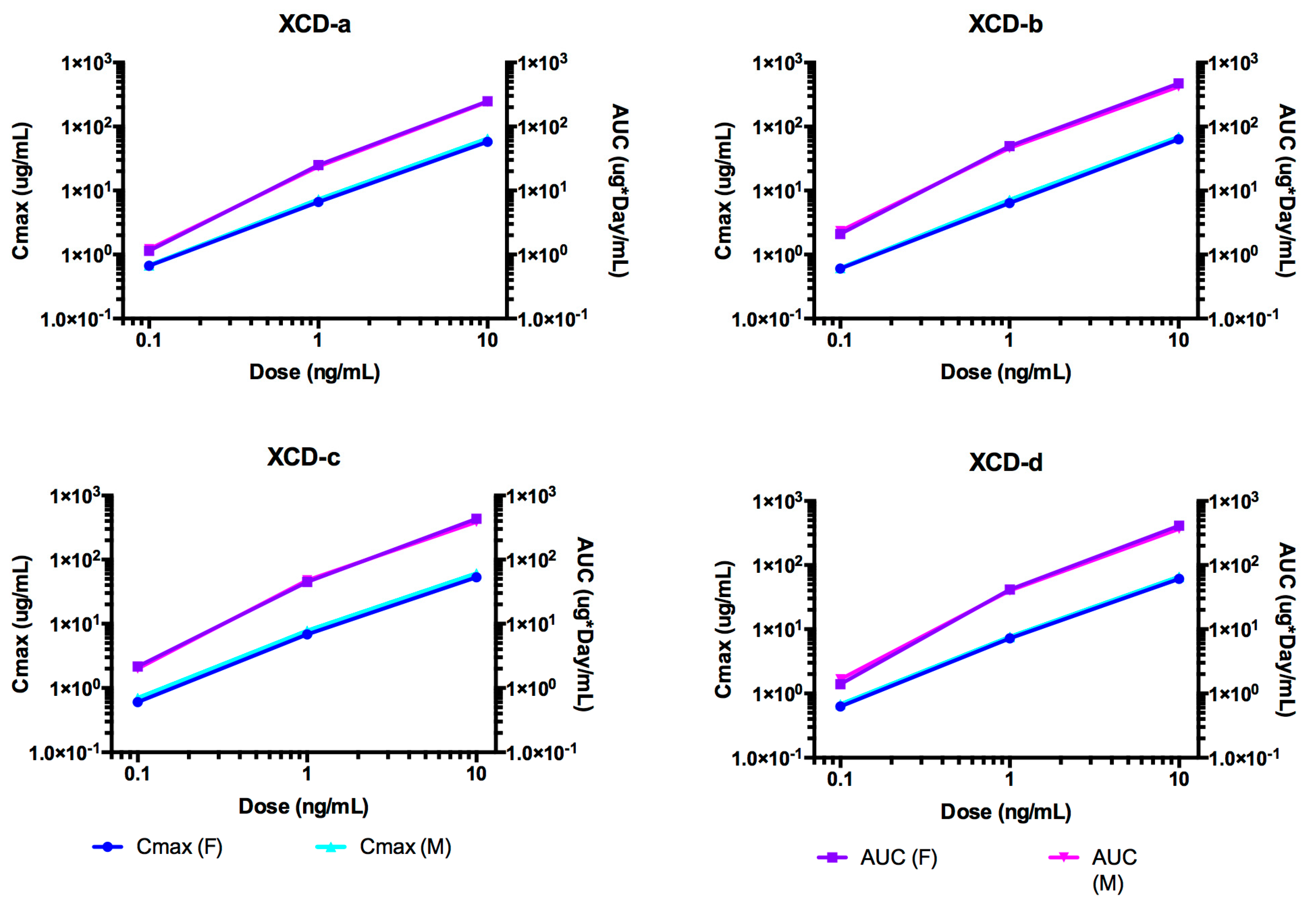
2.8. Pharmacokinetic Analyses NTM-1634
2.9. ADA of NTM-1634
2.10. Relationship of Half-Life and Clearance to pI
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of Test Articles
5.2. Animal Care
5.3. Dose Administration
5.4. Clinical Observations
5.5. Blood Sample Collection
5.6. Body Weights
5.7. Serum Concentration for Pharmacokinetic Analysis
5.8. Immunogenicity
5.9. Pharmacokinetic Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hatheway, C.L. Botulism: The Present Status of the Disease. Curr. Top. Microbiol. Immunol. 1995, 195, 55–75. [Google Scholar] [PubMed]
- Schiavo, G.; Benfenati, F.; Poulain, B.; Rossetto, O.; De Laureto, P.P.; DasGupta, B.R.; Montecucco, C. Tetanus and Botulinum-B Neurotoxins Block Neurotransmitter Release by Proteolytic Cleavage of Synaptobrevin. Nature 1992, 359, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.L. Identification of the Major Steps in Botulinum Toxin Action. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J. Botulism. Clin. Infect. Dis. 2005, 41, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Hibbs, R.G.; Weber, J.T.; Corwin, A.; Allos, B.M.; Abd El Rehim, M.S.; Sharkawy, S.E.; Sarn, J.E.; McKee, K.T., Jr. Experience with the Use of an Investigational F(ab′)2 Heptavalent Botulism Immune Globulin of Equine Origin During an Outbreak of Type E Botulism in Egypt. Clin. Infect. Dis. 1996, 23, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Jalava, K.; Selby, K.; Pihlajasaari, A.; Kolho, E.; Dahlsten, E.; Forss, N.; Backlund, T.; Korkeala, H.; Honkanen-Buzalski, T.; Hulkko, T.; et al. Two Cases of Food-Borne Botulism in Finland Caused by Conserved Olives, October 2011. Eurosurveillance 2011, 16, 20034. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Li, Y.; Song, C.; Sun, Y.; Wei, Y.; Xu, Z.; Yang, A.; Xu, Z.; Yang, K.; et al. High Sensitivity ELISA for Detection of Botulinum Neurotoxin Serotype F. Hybridoma 2012, 31, 233–239. [Google Scholar] [CrossRef]
- Pingeon, J.M.; Vanbockstael, C.; Popoff, M.R.; King, L.A.; Deschamps, B.; Pradel, G.; Dupont, H.; Spanjaard, A.; Houdard, A.; Mazuet, C.; et al. Two Outbreaks of Botulism Associated with Consumption of Green Olive Paste, France, September 2011. Eurosurveillance 2011, 16, 20035. [Google Scholar] [CrossRef]
- Rosen, O.; Ozeri, E.; Barnea, A.; David, A.B.; Zichel, R. Development of an Innovative in Vitro Potency Assay for Anti-Botulinum Antitoxins. Toxins 2016, 8, 276. [Google Scholar] [CrossRef]
- Demarchi, J.; Mourgues, C.; Orio, J.; Prevot, A.R. Existence Du Botulisme De Type D. Bull. Acad. Nat. Med. 1958, 142, 580–582. [Google Scholar]
- Prevot, A.R.; Terrasse, J.; Daumail, J.; Cavaroc, M.; Riol, J.; Sillioc, R. Existence En France Du Botulisme Humain De Type C. Bull. Acad. Med. (Paris) 1955, 139, 355–358. [Google Scholar]
- Oguma, K.; Yokota, K.; Hayashi, S.; Takeshi, K.; Kumagai, M.; Itoh, N.; Tachi, N.; Chiba, S. Infant Botulism Due to Clostridium Botulinum Type C Toxin. Lancet 1990, 336, 1449–1450. [Google Scholar] [CrossRef]
- Sonnabend, O.; Sonnabend, W.; Heinzle, R.; Sigrist, T.; Dirnhofer, R.; Krech, U. Isolation of Clostridium Botulinum Type G and Identification of Type G Botulinal Toxin in Humans: Report of Five Sudden Unexpected Deaths. J. Infect. Dis. 1981, 143, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://emergency.cdc.gov/agent/agentlist-category.asp (accessed on 18 April 2019).
- Bowman, S.R. Iraqi Chemical and Biological Weapons (CBW) Capabilities. 1998. Available online: https://www.globalsecurity.org/wmd/library/report/crs/98-129.pdf (accessed on 5 June 2019).
- United Nations Security Council. Tenth Report of the Executive Chairman of the Special Commission Established by the Secretary-General Pursuant to Paragraph 9(b)(I) of Security Council Resolution 687 (1991) and Paragraph 3 of Resolution 699 (1991) on the Activities of the Special Commission; United Nations Security Council: New York, NY, USA, 1995. [Google Scholar]
- Nowakowski, A.; Wang, C.; Powers, D.B.; Amersdorfer, P.; Smith, T.J.; Montgomery, V.A.; Sheridan, R.; Blake, R.; Smith, L.A.; Marks, J.D. Potent Neutralization of Botulinum Neurotoxin by Recombinant Oligoclonal Antibody. Proc. Natl. Acad. Sci. USA 2002, 99, 11346–11350. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum Toxin as a Biological Weapon: Medical and Public Health Management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Gunn, R.A. Hypersensitivity Reactions Associated with Botulinal Antitoxin. Am. J. Med. 1980, 69, 567–570. [Google Scholar] [CrossRef]
- Arnon, S.S.; Schechter, R.; Maslanka, S.E.; Jewell, N.P.; Hatheway, C.L. Human Botulism Immune Globulin for the Treatment of Infant Botulism. New Engl. J. Med. 2006, 354, 462–471. [Google Scholar] [CrossRef]
- Cangene Corp. BAT® [Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G)—(Equine)] Sterile Solution for Injection. Available online: https://www.fda.gov/downloads/.../UCM345147.pdf (accessed on 11 October 2017).
- Amersdorfer, P.; Wong, C.; Chen, S.; Smith, T.; Deshpande, S.; Sheridan, R.; Finnern, R.; Marks, J.D. Molecular Characterization of Murine Humoral Immune Response to Botulinum Neurotoxin Type A Binding Domain as Assessed by Using Phage Antibody Libraries. Infect. Immun. 1997, 65, 3743–3752. [Google Scholar]
- Amersdorfer, P.; Wong, C.; Smith, T.; Chen, S.; Deshpande, S.; Sheridan, R.; Marks, J.D. Genetic and Immunological Comparison of Anti-Botulinum Type A Antibodies from Immune and Non-Immune Human Phage Libraries. Vaccine 2002, 20, 1640–1648. [Google Scholar] [CrossRef]
- Nayak, S.U.; Griffiss, J.M.; McKenzie, R.; Fuchs, E.J.; Jurao, R.A.; An, A.T.; Ahene, A.; Tomic, M.; Hendrix, C.W.; Zenilman, J.M. Safety and Pharmacokinetics of XOMA 3AB, a Novel Mixture of Three Monoclonal Antibodies Against Botulinum Toxin A. Antimicrob. Agents Chemother. 2014, 58, 5047–5053. [Google Scholar] [CrossRef]
- Lou, J.; Geren, I.; Garcia-Rodriguez, C.; Forsyth, C.M.; Wen, W.; Knopp, K.; Brown, J.; Smith, T.; Smith, L.A.; Marks, J.D. Affinity Maturation of Human Botulinum Neurotoxin Antibodies by Light Chain Shuffling Via Yeast Mating. Protein Eng. Des. Sel. PEDS 2010, 23, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Pless, D.D.; Torres, E.R.; Reinke, E.K.; Bavari, S. High-Affinity, Protective Antibodies to the Binding Domain of Botulinum Neurotoxin Type A. Infect. Immun. 2001, 69, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, M.; Silberg, M.A.; Conrad, F.; Bettencourt, J.; To, R.; Huang, C.; Ma, J.; Meyer, K.; Shimizu, R.; et al. Domain-Based Assays of Individual Antibody Concentrations in an Oligoclonal Combination Targeting a Single Protein. Anal. Biochem. 2012, 421, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Garcia-Rodriguez, C.; Manzanarez, G.; Silberg, M.; Conrad, F.; Bettencourt, J.; Pan, X.; Breece, T.; To, R.; Li, M. Engineered Domain-Based Assays to Identify Individual Antibodies in Oligoclonal Combinations Targeting the Same Protein. Anal. Biochem. 2012, 430, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Tsunoda, H.; Tachibana, T.; Maeda, A.; Mimoto, F.; Moriyama, C.; Nanami, M.; Sekimori, Y.; Nabuchi, Y.; Aso, Y. Reduced Elimination of IgG Antibodies by Engineering the Variable Region. Protein Eng. Des. Sel. 2010, 23, 385–392. [Google Scholar] [CrossRef]
- Lacy, D.B.; Stevens, R.C. Sequence Homology and Structural Analysis of the Clostridial Neurotoxins. J. Mol. Biol. 1999, 291, 1091–1104. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Notice of CDC’s Discontinuation of Investigational Pentavalent (ABCDE) Botulinum Toxoid Vaccine for Workers at Risk for Occupational Exposure to Botulinum Toxins. MMWR. Morb. Mortal. Wkly. Rep. 2011, 60, 1454–1455. [Google Scholar]
- Tomic, M.T.; Espinoza, Y.; Martinez, Z.; Pham, K.; Cobb, R.R.; Snow, D.M.; Earnhart, C.G.; Pals, T.; Syar, E.S.; Niemuth, N. Monoclonal Antibody Combinations Prevent Serotype A and Serotype B Inhalational Botulism in a Guinea Pig Model. Toxins 2019, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Dong, J.; Lou, J.; Wen, W.; Conrad, F.; Geren, I.N.; Garcia-Rodriguez, C.; Smith, T.J.; Smith, L.A.; Ho, M.; et al. Monoclonal Antibodies that Inhibit the Proteolytic Activity of Botulinum Neurotoxin Serotype/B. Toxins 2015, 7, 3405–3423. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, C.; Razai, A.; Geren, I.N.; Lou, J.; Conrad, F.; Wen, W.H.; Farr-Jones, S.; Smith, T.J.; Brown, J.L.; Skerry, J.C.; et al. A Three Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotype E Subtypes. Toxins 2018, 10, 105. [Google Scholar] [CrossRef]
- Fan, Y.; Garcia-Rodriguez, C.; Lou, J.; Wen, W.; Conrad, F.; Zhai, W.; Smith, T.J.; Smith, L.A.; Marks, J.D. A Three Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotype F Subtypes. PLoS ONE 2017, 12, e0174187. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Barash, J.R.; Lou, J.; Conrad, F.; Marks, J.D.; Arnon, S.S. Immunological Characterization and Neutralizing Ability of Monoclonal Antibodies Directed Against Botulinum Neurotoxin Type H. J. Infect. Dis. 2016, 213, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.W.; Stanker, L.H.; Lou, J.; Marks, J.D.; Henderson, T.D., 2nd. Antibody Protection Against Botulinum Neurotoxin Intoxication in Mice. Infect. Immun. 2009, 77, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.L.; Underwood, W.; Anthony, R.; Cartner, S.; Corey, D.; Grandin, T.; Greenacre, C.; Gwaltney-Bran, S.; McCrackin, M.; Meyer, R. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition; American Veterinary Medical Association Schaumburg: Schaumburg, IL, USA, 2013. [Google Scholar]

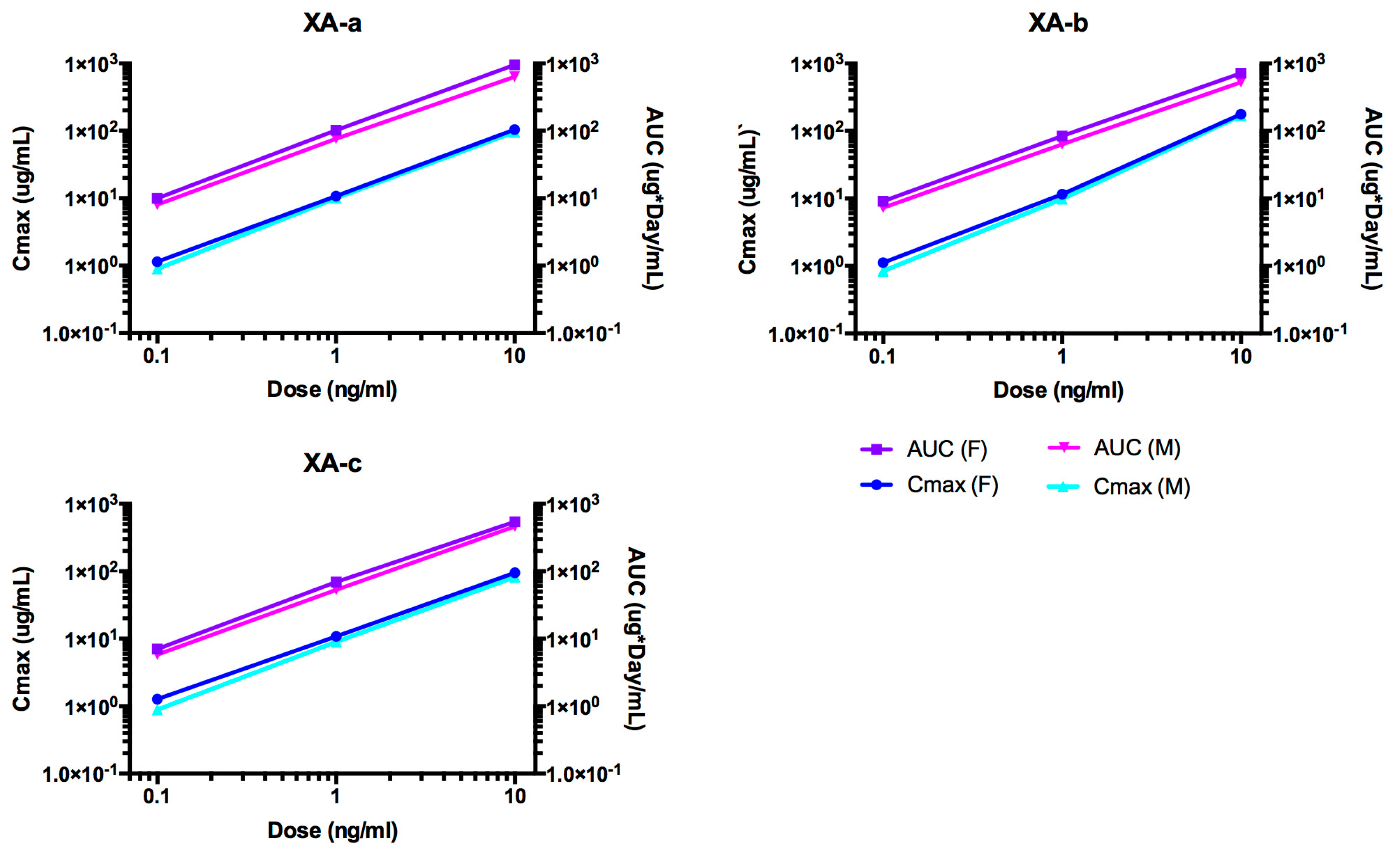
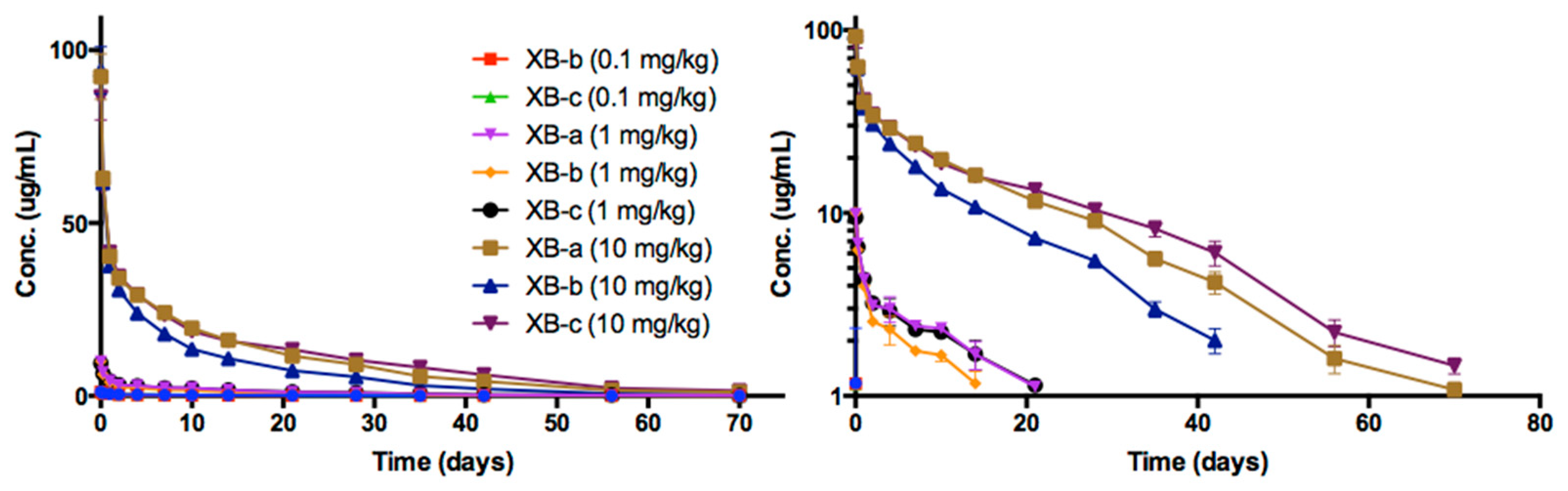
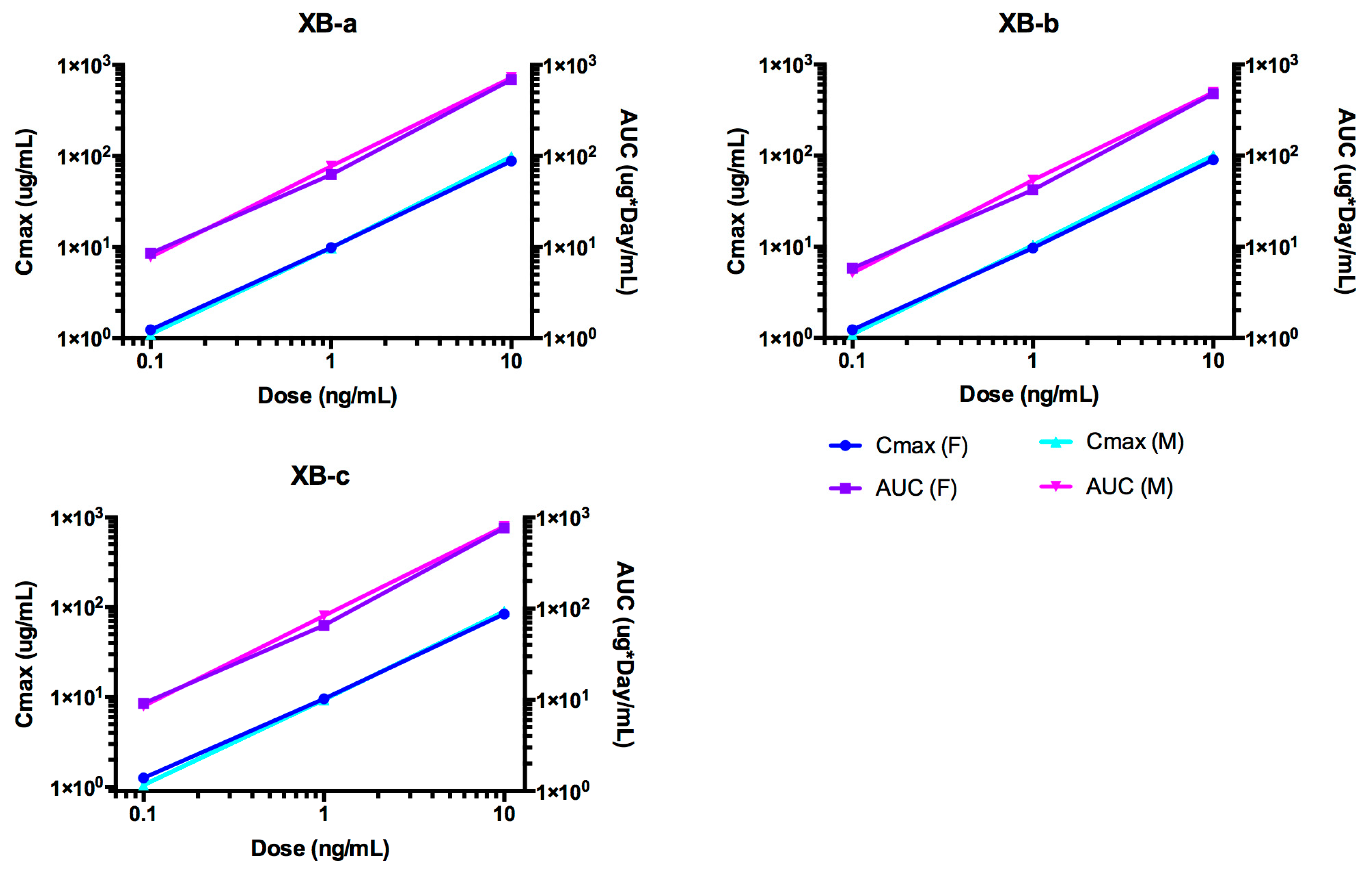
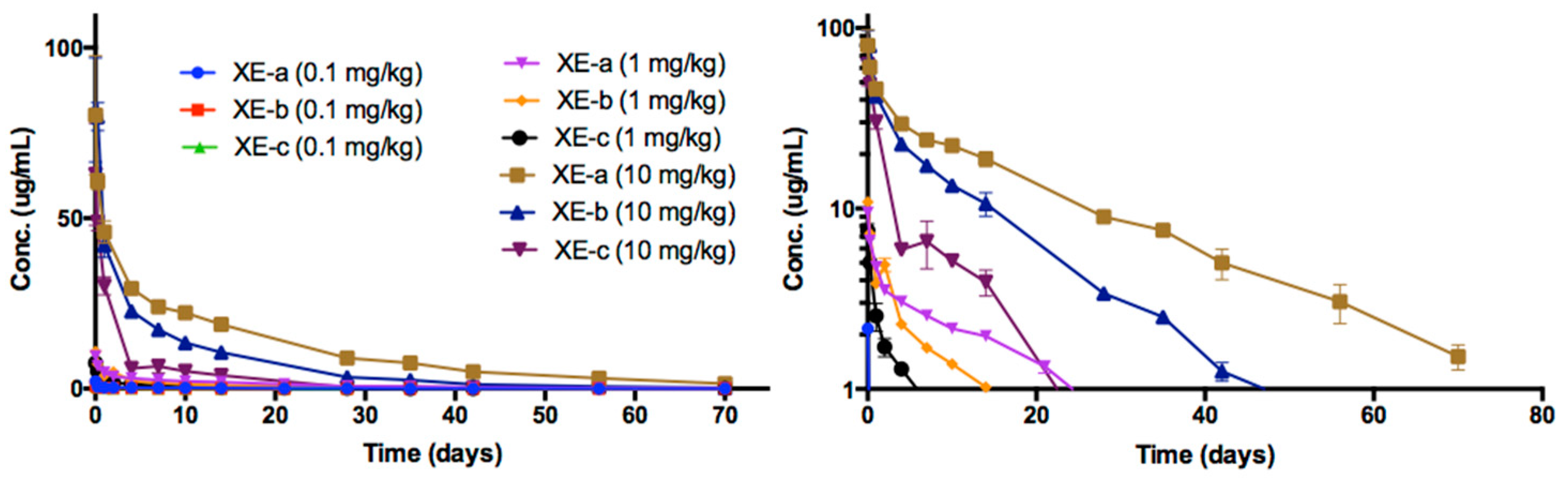
| Antibody | Dose mg/kg | Sex | N | T1/2 day | Cmax/Dose kg × µg /mL/mg | AUC∞/Dose Day × kg × µg /mL/mg | CL mL/day/kg | Vc mL/kg |
|---|---|---|---|---|---|---|---|---|
| XA-a | 0.1 | M | 14 | 17.23 | 8.957 | 85.6955 | 3.85 | 95.75 |
| 0.1 | F | 15 | 15.56 | 11.4517 | 104.6601 | 3.15 | 70.79 | |
| 1 | M | 7 | 16.97 | 10.00825 | 63.3717 | 4.15 | 101.62 | |
| 1 | F | 9 | 15.26 | 10.79059 | 97.5364 | 3.16 | 69.58 | |
| 10 | M | 5 | 15.26 | 9.568051 | 62.5584 | 4.86 | 107.07 | |
| 10 | F | 10 | 14.61 | 10.454225 | 87.4375 | 3.37 | 71.04 | |
| Mean | 15.82 | 10.18 | 83.5433 | 3.76 | 85.98 | |||
| SE | 0.43 | 0.36 | 7.09 | 0.27 | 7.09 | |||
| XA-b | 0.1 | M | 14 | 15.82 | 8.445 | 76.6225 | 4.33 | 98.88 |
| 0.1 | F | 15 | 14.66 | 11.1411 | 94.1734 | 3.5 | 74.11 | |
| 1 | M | 7 | 14.74 | 9.18426 | 60.5433 | 5.03 | 106.89 | |
| 1 | F | 9 | 13.4 | 9.92303 | 84.5505 | 3.86 | 74.56 | |
| 10 | M | 5 | 14.45 | 16.9725 | 57.6311 | 5.97 | 124.38 | |
| 10 | F | 10 | 13.78 | 16.8571 | 68.8351 | 4.5 | 89.5 | |
| Mean | 14.48 | 12.0872 | 73.7260 | 4.53 | 94.72 | |||
| SE | 0.34 | 1.57 | 5.77 | 0.36 | 5.88 | |||
| XA-c | 0.1 | M | 14 | 16.17 | 8.87910 | 60.6509 | 5.39 | 125.66 |
| 0.1 | F | 15 | 15.33 | 12.73490 | 73.7408 | 4.48 | 98.95 | |
| 1 | M | 7 | 14.39 | 8.71959 | 50.8748 | 6.02 | 124.88 | |
| 1 | F | 9 | 12.36 | 11.69086 | 71.4821 | 4.69 | 83.64 | |
| 10 | M | 5 | 14.52 | 8.23345 | 44.0113 | 6.84 | 143.27 | |
| 10 | F | 10 | 13.68 | 9.17088 | 54.0151 | 5.88 | 116.08 | |
| Mean | 14.41 | 9.90480 | 59.13 | 5.55 | 115.41 | |||
| SE | 0.54 | 0.69 | 4.38 | 0.33 | 7.91 |
| Antibody | Dose mg/kg | Sex | T1/2 Day | Cmax/Dose kg × µg /mL/mg | AUC∞/Dose Day × kg × µg /mL/mg | CL mL/kg/Day | Vc mL/kg |
|---|---|---|---|---|---|---|---|
| XB-a | 0.1 | M | 17.9 | 11.1 | 77.8 | 4.29 | 33.5 |
| 0.1 | F | 17.5 | 12.4 | 86 | 3.88 | 30.5 | |
| 1 | M | 16 | 9.83 | 77.6 | 4.3 | 35.3 | |
| 1 | F | 15.1 | 9.92 | 62.4 | 5.35 | 37.5 | |
| 10 | M | 14.7 | 9.865 | 72.8 | 4.59 | 36.4 | |
| 10 | F | 13.6 | 8.82 | 68.79 | 4.85 | 41.2 | |
| Mean | 15.9 | 10.32 | 74.23 | 4.54 | 35.73 | ||
| SE | 1 | 1.25 | 8.18 | 0.51 | 3.63 | ||
| XB-b | 0.1 | M | 9.51 | 11 | 51.5 | 6.48 | 33.7 |
| 0.1 | F | 20.3 | 12.3 | 58.1 | 5.75 | 30.6 | |
| 1 | M | 13.3 | 10.4 | 53.8 | 6.2 | 35.9 | |
| 1 | F | 14.2 | 9.71 | 42.1 | 7.94 | 38 | |
| 10 | M | 11.7 | 10.035 | 49.49 | 6.75 | 36.6 | |
| 10 | F | 11.4 | 9.013 | 47.76 | 6.98 | 40.1 | |
| Mean | 13.40 | 10.41 | 50.46 | 6.68 | 35.82 | ||
| SE | 3.75 | 1.14 | 5.45 | 0.75 | 3.33 | ||
| XB-c | 0.1 | M | 19.6 | 10.5 | 79.3 | 4.21 | 34.9 |
| 0.1 | F | 18.7 | 12.6 | 91.5 | 3.65 | 29.9 | |
| 1 | M | 16.8 | 9.35 | 80.4 | 4.15 | 37.3 | |
| 1 | F | 16.7 | 9.59 | 65.4 | 5.11 | 39.1 | |
| 10 | M | 13.7 | 8.995 | 79.16 | 4.22 | 40 | |
| 10 | F | 13.3 | 8.427 | 76.62 | 4.35 | 43.1 | |
| Mean | 16.47 | 9.91 | 78.73 | 4.28 | 37.38 | ||
| SE | 2.56 | 1.49 | 8.35 | 0.47 | 4.57 |
| Antibody | Dose mg/kg | T1/2 day | Cmax/Dose kg × µg /mL/mg | AUC∞/Dose Day × kg × µg /mL/mg | CL mL/kg/day | Vc mL/kg |
|---|---|---|---|---|---|---|
| XE-a | 0.1 | 21.7 | 21.6 | 81.8 | 4.09 | 22.8 |
| 1 | 13.9 | 9.5 | 75.2 | 4.43 | 38.2 | |
| 10 | 16.3 | 8.02 | 80.9 | 4.12 | 44.3 | |
| Mean | 17.3 | 13.04 | 79.3 | 4.21 | 35.1 | |
| SE | 2.3 | 4.3 | 2.1 | 0.11 | 6.4 | |
| XE-b | 0.1 | 30.1 | 8.71 | 40.9 | 8.18 | 43.4 |
| 1 | 10.7 | 10.8 | 45.9 | 7.26 | 32.7 | |
| 10 | 11.8 | 8.18 | 45.7 | 7.29 | 38.1 | |
| Mean | 17.5 | 9.23 | 44.17 | 7.58 | 38.1 | |
| SE | 6.3 | 0.8 | 1.63 | 0.3 | 3.1 | |
| XE-c | 0.1 | 8.19 | 6.15 | 18.7 | 17.9 | 54.4 |
| 1 | 22.5 | 7.52 | 20.8 | 16 | 47.1 | |
| 10 | 9.91 | 6.28 | 17.2 | 19.4 | 54.8 | |
| Mean | 13.5 | 6.65 | 18.9 | 17.8 | 52.1 | |
| SE | 4.5 | 0.44 | 1.04 | 1 | 2.5 |
| Antibody | Dose mg/kg | T1/2 day | Cmax/Dose µg × kg/mL/mg | AUC∞/Dose Day × kg × µg /mL/mg | CL mL/day/kg | Vc mL/kg |
|---|---|---|---|---|---|---|
| 0.1 | 5.19 | 6.75 | 19.2 | 53.4 | 373 | |
| XCD-a | 1 | 7.58 | 6.96 | 25.5 | 39.2 | 429 |
| 10 | 7.47 | 6.16 | 25.4 | 39.4 | 425 | |
| Mean | 6.747 | 6.62 | 23.37 | 44.00 | 409.00 | |
| SE | 1.349 | 0.41 | 3.61 | 8.14 | 31.24 | |
| 0.1 | 7.57 | 6.10 | 29.6 | 34.6 | 370 | |
| XCD-b | 1 | 11.6 | 6.77 | 49.7 | 20.2 | 338 |
| 10 | 11.1 | 6.56 | 49.0 | 20.4 | 328 | |
| Mean | 10.090 | 6.48 | 42.77 | 25.07 | 345.33 | |
| SE | 2.197 | 0.34 | 11.41 | 8.26 | 21.94 | |
| 0.1 | 10.8 | 6.52 | 32.1 | 32.7 | 461 | |
| XCD-c | 1 | 10.1 | 7.34 | 47.5 | 21.1 | 307 |
| 10 | 10.2 | 5.71 | 44.5 | 22.5 | 331 | |
| Mean | 10.367 | 6.52 | 41.37 | 25.43 | 366.33 | |
| SE | 0.379 | 0.82 | 8.16 | 6.33 | 82.86 | |
| 0.1 | 7.3 | 6.55 | 24.5 | 45.8 | 386 | |
| XCD-d | 1 | 9.6 | 7.42 | 41.7 | 24.0 | 335 |
| 10 | 9.9 | 6.31 | 42.2 | 23.7 | 338 | |
| mean | 8.933 | 6.76 | 36.13 | 31.17 | 353 | |
| SE | 1.422 | 0.58 | 10.08 | 12.67 | 28.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, Y.; Wong, D.; Ahene, A.; Der, K.; Martinez, Z.; Pham, J.; Cobb, R.R.; Farr-Jones, S.; Marks, J.D.; Tomic, M.T. Pharmacokinetics of Human Recombinant Anti-Botulinum Toxin Antibodies in Rats. Toxins 2019, 11, 345. https://doi.org/10.3390/toxins11060345
Espinoza Y, Wong D, Ahene A, Der K, Martinez Z, Pham J, Cobb RR, Farr-Jones S, Marks JD, Tomic MT. Pharmacokinetics of Human Recombinant Anti-Botulinum Toxin Antibodies in Rats. Toxins. 2019; 11(6):345. https://doi.org/10.3390/toxins11060345
Chicago/Turabian StyleEspinoza, Yero, David Wong, Ago Ahene, Kenneth Der, Zachary Martinez, John Pham, Ronald R. Cobb, Shauna Farr-Jones, James. D. Marks, and Milan T. Tomic. 2019. "Pharmacokinetics of Human Recombinant Anti-Botulinum Toxin Antibodies in Rats" Toxins 11, no. 6: 345. https://doi.org/10.3390/toxins11060345
APA StyleEspinoza, Y., Wong, D., Ahene, A., Der, K., Martinez, Z., Pham, J., Cobb, R. R., Farr-Jones, S., Marks, J. D., & Tomic, M. T. (2019). Pharmacokinetics of Human Recombinant Anti-Botulinum Toxin Antibodies in Rats. Toxins, 11(6), 345. https://doi.org/10.3390/toxins11060345






