Rapid Purification of Endotoxin-Free RTX Toxins
Abstract
1. Introduction
2. Results and Discussion
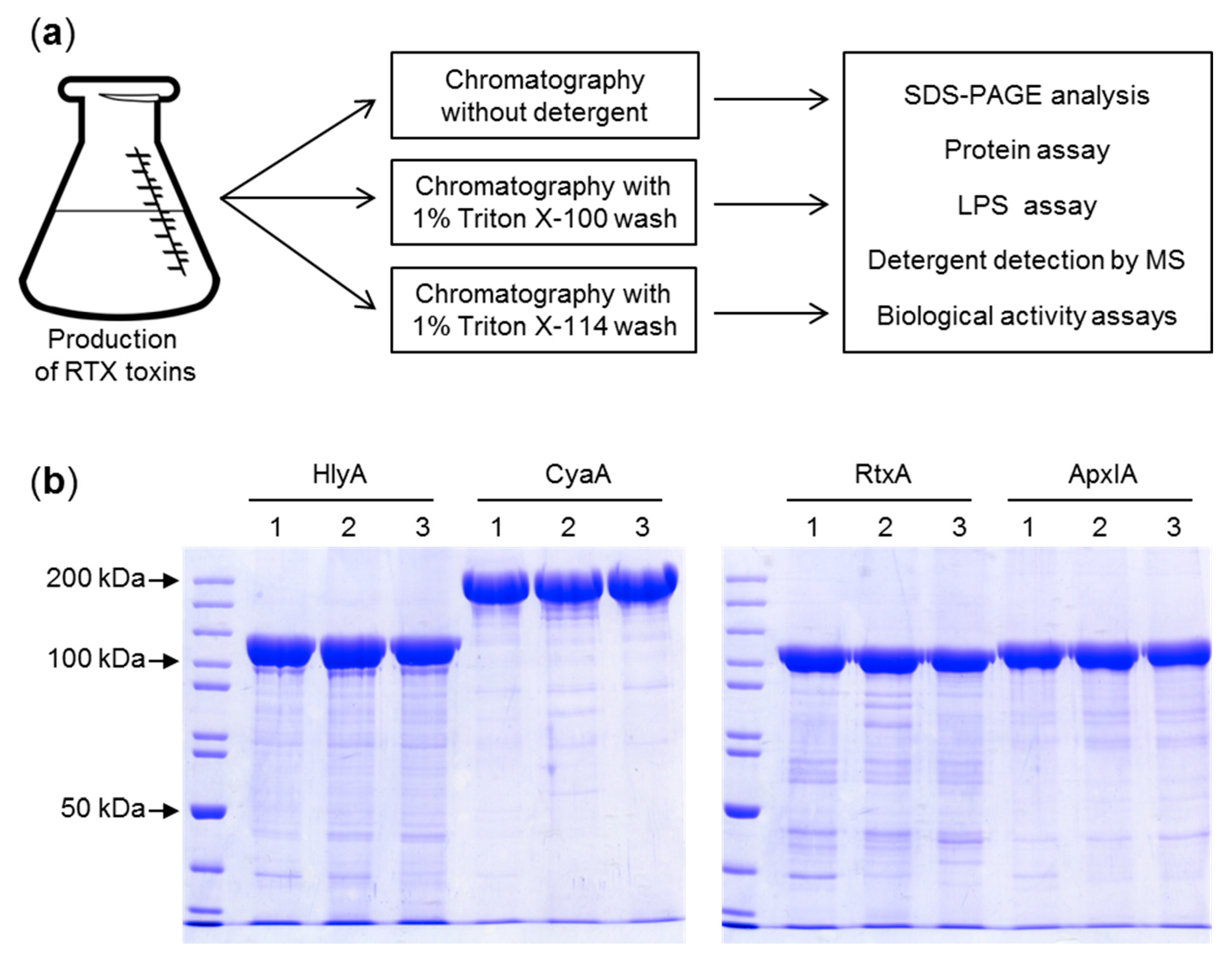
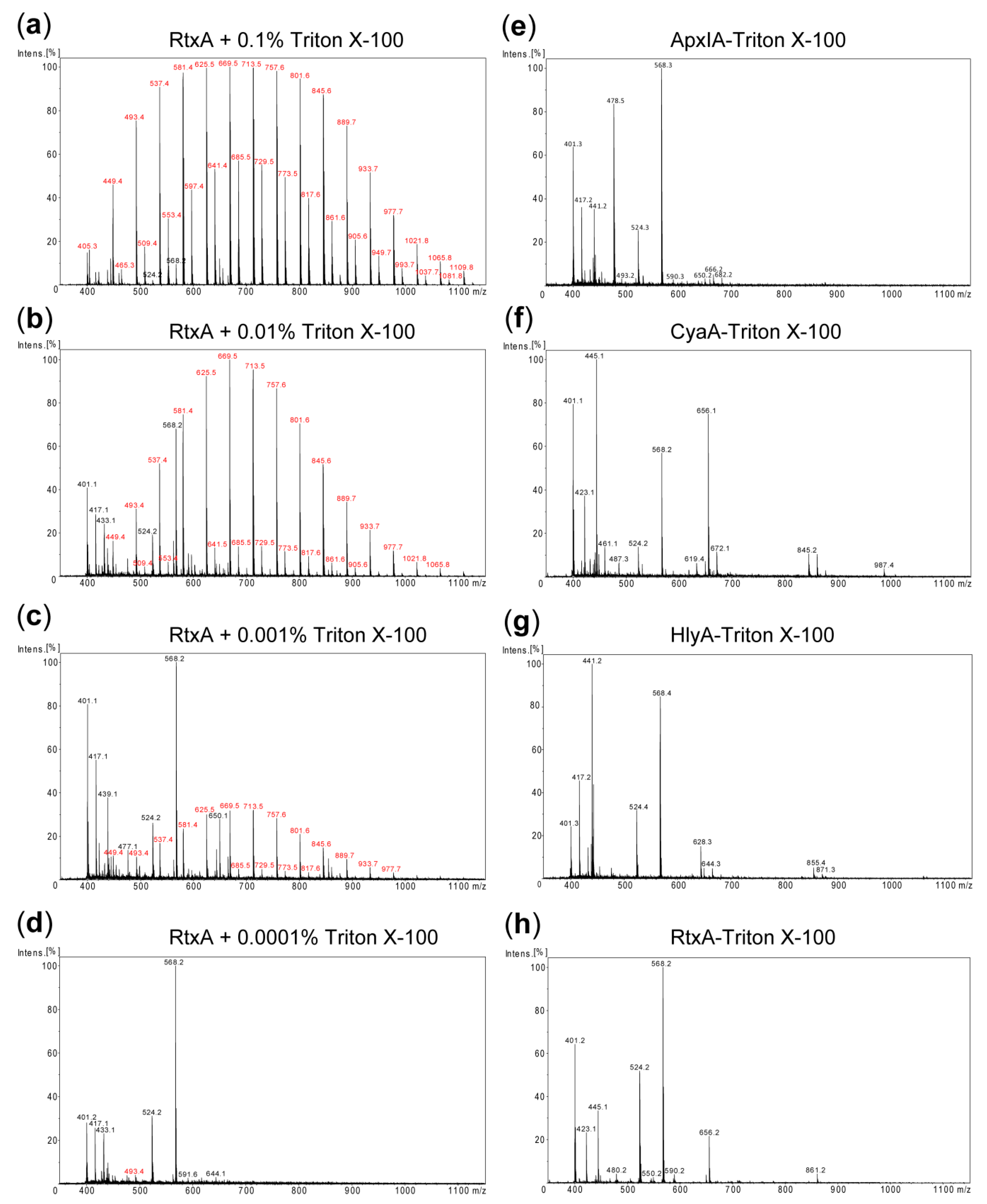
2.1. Purification of LPS-free RTX Toxins
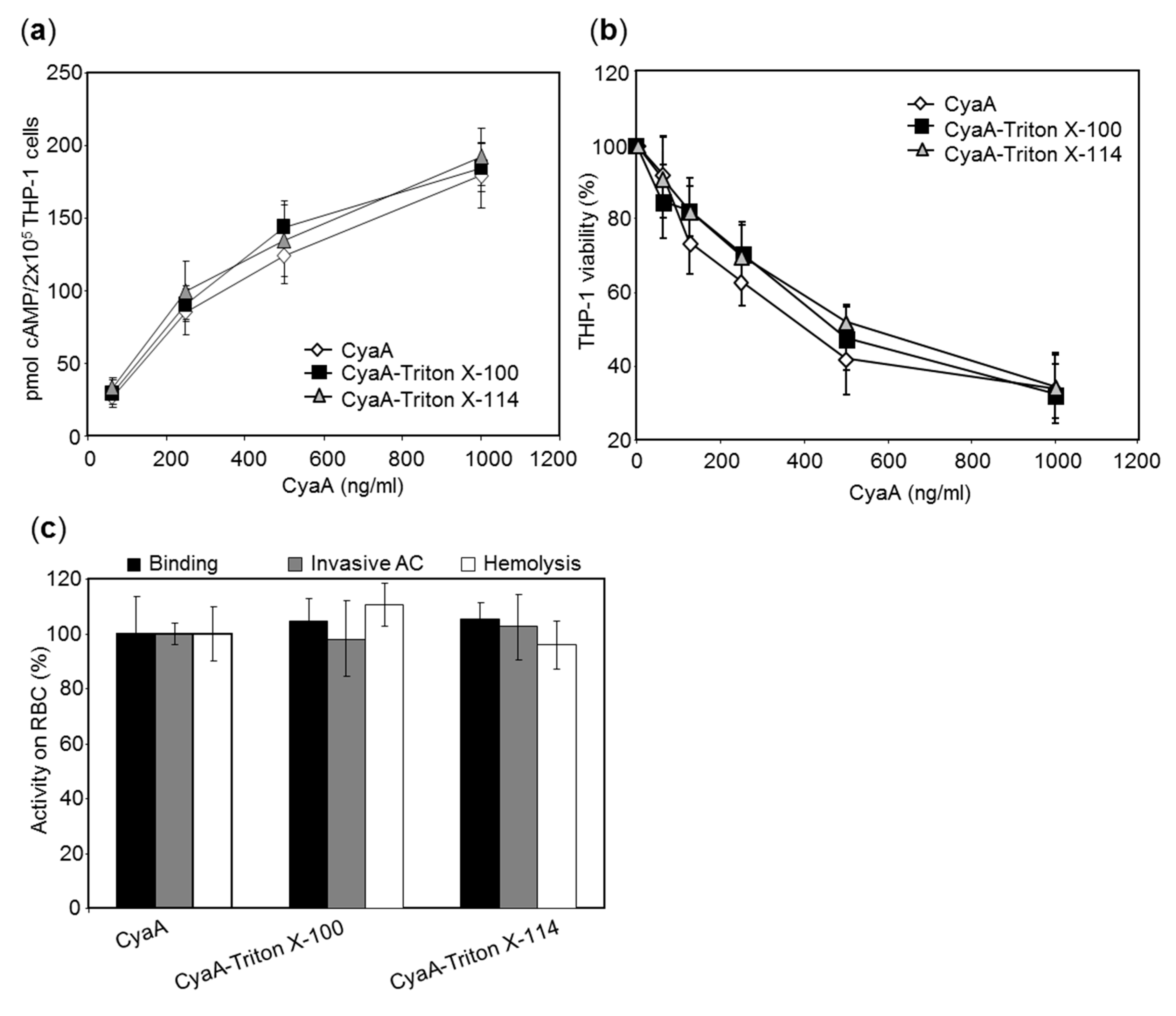
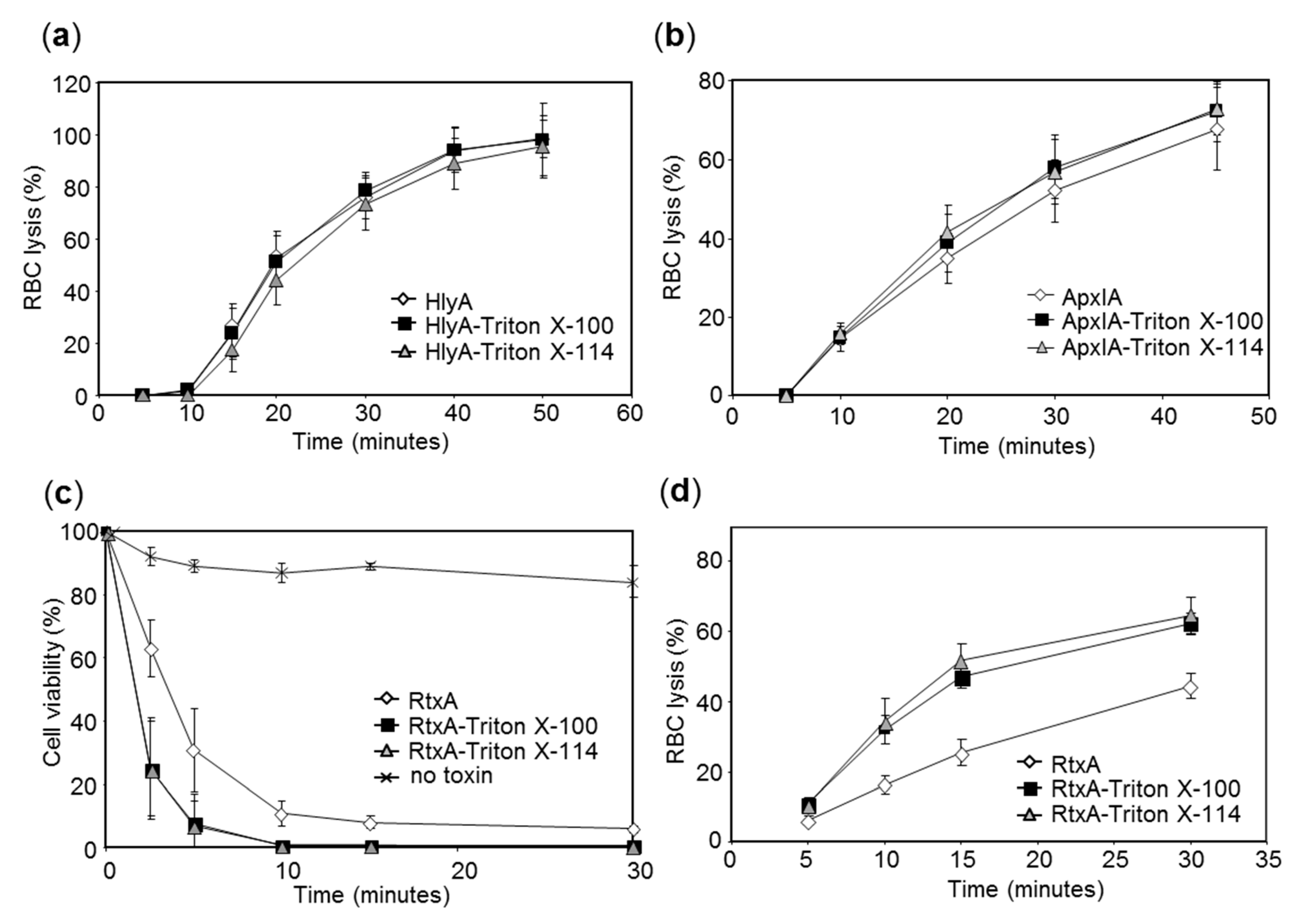
2.2. The LPS-Free RTX Toxins Are Cytotoxic on Different Cell Types
3. Conclusions
4. Materials and Methods
4.1. Expression and Isolation of RTX Toxins
4.2. Purification of CyaA
4.3. Purification of HlyA, ApxIA, and RtxA
4.4. Determination of LPS Levels and Protein Concentrations
4.5. Detection of Residual Detergent
4.6. Determination of Hemolytic Activity on Sheep Erythrocytes
4.7. Cell Binding and Invasive Activities of CyaA on Sheep Erythrocytes
4.8. Human Cell Lines
4.9. cAMP Elevation and Viability Assays on THP-1 Cells
4.10. Determination of RtxA Cytotoxicity on HLaC-78 Cells
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Linhartova, I.; Bumba, L.; Masin, J.; Basler, M.; Osicka, R.; Kamanova, J.; Prochazkova, K.; Adkins, I.; Hejnova-Holubova, J.; Sadilkova, L.; et al. RTX proteins: A highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010, 34, 1076–1112. [Google Scholar] [CrossRef]
- Goodwin, M.S.; Weiss, A.A. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 1990, 58, 3445–3447. [Google Scholar]
- Khelef, N.; Sakamoto, H.; Guiso, N. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb. Pathog. 1992, 12, 227–235. [Google Scholar] [CrossRef]
- Kamanova, J.; Kofronova, O.; Masin, J.; Genth, H.; Vojtova, J.; Linhartova, I.; Benada, O.; Just, I.; Sebo, P. Adenylate cyclase toxin subverts phagocyte function by RhoA inhibition and unproductive ruffling. J. Immunol. 2008, 181, 5587–5597. [Google Scholar] [CrossRef]
- Novak, J.; Cerny, O.; Osickova, A.; Linhartova, I.; Masin, J.; Bumba, L.; Sebo, P.; Osicka, R. Structure-function relationships underlying the capacity of Bordetella adenylate cyclase toxin to disarm host phagocytes. Toxins 2017, 9, 300. [Google Scholar] [CrossRef]
- Ristow, L.C.; Welch, R.A. Hemolysin of uropathogenic Escherichia coli: A cloak or a dagger? Biochim. ET Biophys. Acta 2016, 1858, 538–545. [Google Scholar] [CrossRef]
- Kehl-Fie, T.E.; St Geme, J.W., 3rd. Identification and characterization of an RTX toxin in the emerging pathogen Kingella kingae. J. Bacteriol. 2007, 189, 430–436. [Google Scholar] [CrossRef]
- Maier, E.; Reinhard, N.; Benz, R.; Frey, J. Channel-forming activity and channel size of the RTX toxins ApxI, ApxII, and ApxIII of Actinobacillus pleuropneumoniae. Infect. Immun. 1996, 64, 4415–4423. [Google Scholar]
- Masin, J.; Fiser, R.; Linhartova, I.; Osicka, R.; Bumba, L.; Hewlett, E.L.; Benz, R.; Sebo, P. Differences in purinergic amplification of osmotic cell lysis by the pore-forming RTX toxins Bordetella pertussis CyaA and Actinobacillus pleuropneumoniae ApxIA: The role of pore size. Infect. Immun. 2013, 81, 4571–4582. [Google Scholar] [CrossRef]
- Bumba, L.; Masin, J.; Macek, P.; Wald, T.; Motlova, L.; Bibova, I.; Klimova, N.; Bednarova, L.; Veverka, V.; Kachala, M.; et al. Calcium-driven folding of RTX domain beta-rolls ratchets translocation of RTX proteins through type I secretion ducts. Mol. Cell 2016, 62, 47–62. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Bache, C.; Hoonakker, M.; Hendriksen, C.; Buchheit, K.H.; Spreitzer, I.; Montag, T. Workshop on animal free detection of pertussis toxin in vaccines--alternatives to the histamine sensitisation test. Biol. J. Int. Assoc. Biol. Stand. 2012, 40, 309–311. [Google Scholar] [CrossRef]
- Gao, B.; Tsan, M.F. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J. Biol. Chem. 2003, 278, 22523–22529. [Google Scholar] [CrossRef]
- Tsan, M.F.; Gao, B. Endogenous ligands of toll-like receptors. J. Leukoc. Biol. 2004, 76, 514–519. [Google Scholar] [CrossRef]
- Villarino Romero, R.; Hasan, S.; Fae, K.; Holubova, J.; Geurtsen, J.; Schwarzer, M.; Wiertsema, S.; Osicka, R.; Poolman, J.; Sebo, P. Bordetella pertussis filamentous hemagglutinin itself does not trigger anti-inflammatory interleukin-10 production by human dendritic cells. Int. J. Med. Microbiol. IJMM 2016, 306, 38–47. [Google Scholar] [CrossRef]
- Wakelin, S.J.; Sabroe, I.; Gregory, C.D.; Poxton, I.R.; Forsythe, J.L.; Garden, O.J.; Howie, S.E. “Dirty little secrets”—Endotoxin contamination of recombinant proteins. Immunol. Lett. 2006, 106, 1–7. [Google Scholar] [CrossRef]
- Magalhaes, P.O.; Lopes, A.M.; Mazzola, P.G.; Rangel-Yagui, C.; Penna, T.C.; Pessoa, A., Jr. Methods of endotoxin removal from biological preparations: A review. J. Pharm. Pharm. Sci. 2007, 10, 388–404. [Google Scholar]
- Aida, Y.; Pabst, M.J. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 1990, 132, 191–195. [Google Scholar] [CrossRef]
- Cavallaro, A.S.; Mahony, D.; Commins, M.; Mahony, T.J.; Mitter, N. Endotoxin-free purification for the isolation of bovine viral diarrhoea virus E2 protein from insoluble inclusion body aggregates. Microb. Cell Factories 2011, 10, 57. [Google Scholar] [CrossRef]
- Liu, S.; Tobias, R.; McClure, S.; Styba, G.; Shi, Q.; Jackowski, G. Removal of endotoxin from recombinant protein preparations. Clin. Biochem. 1997, 30, 455–463. [Google Scholar] [CrossRef]
- Reichelt, P.; Schwarz, C.; Donzeau, M. Single step protocol to purify recombinant proteins with low endotoxin contents. Protein Expr. Purif. 2006, 46, 483–488. [Google Scholar] [CrossRef]
- Orr, B.; Douce, G.; Baillie, S.; Parton, R.; Coote, J. Adjuvant effects of adenylate cyclase toxin of Bordetella pertussis after intranasal immunisation of mice. Vaccine 2007, 25, 64–71. [Google Scholar] [CrossRef]
- Franken, K.L.; Hiemstra, H.S.; van Meijgaarden, K.E.; Subronto, Y.; den Hartigh, J.; Ottenhoff, T.H.; Drijfhout, J.W. Purification of his-tagged proteins by immobilized chelate affinity chromatography: The benefits from the use of organic solvent. Protein Expr. Purif. 2000, 18, 95–99. [Google Scholar] [CrossRef]
- Mascarell, L.; Fayolle, C.; Bauche, C.; Ladant, D.; Leclerc, C. Induction of neutralizing antibodies and Th1-polarized and CD4-independent CD8+ T-cell responses following delivery of human immunodeficiency virus type 1 Tat protein by recombinant adenylate cyclase of Bordetella pertussis. J. Virol. 2005, 79, 9872–9884. [Google Scholar] [CrossRef]
- Dunne, A.; Ross, P.J.; Pospisilova, E.; Masin, J.; Meaney, A.; Sutton, C.E.; Iwakura, Y.; Tschopp, J.; Sebo, P.; Mills, K.H. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J. Immunol. 2010, 185, 1711–1719. [Google Scholar] [CrossRef]
- Prior, S.; Corbel, M.J.; Xing, D.K. Development of an approach for the laboratory toxicological evaluation of Bordetella pertussis adenylate cyclase genetic toxoid constructs as multipurpose vaccines. Hum. Vaccines 2005, 1, 151–159. [Google Scholar] [CrossRef]
- Nasrin, A.; Hassan, M.; Ye, P. Inhibition of notch signaling pathway using gamma-secretase inhibitor delivered by a low dose of Triton-x100 in cultured oral cancer cells. Biochem. Biophys. Res. Commun. 2018, 495, 2118–2124. [Google Scholar] [CrossRef]
- Guermonprez, P.; Khelef, N.; Blouin, E.; Rieu, P.; Ricciardi-Castagnoli, P.; Guiso, N.; Ladant, D.; Leclerc, C. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J. Exp. Med. 2001, 193, 1035–1044. [Google Scholar] [CrossRef]
- Osicka, R.; Osickova, A.; Hasan, S.; Bumba, L.; Cerny, J.; Sebo, P. Bordetella adenylate cyclase toxin is a unique ligand of the integrin complement receptor 3. eLife 2015, 4, e10766. [Google Scholar] [CrossRef]
- Osickova, A.; Balashova, N.; Masin, J.; Sulc, M.; Roderova, J.; Wald, T.; Brown, A.C.; Koufos, E.; Chang, E.H.; Giannakakis, A.; et al. Cytotoxic activity of Kingella kingae RtxA toxin depends on post-translational acylation of lysine residues and cholesterol binding. Emerg. Microbes Infect. 2018, 7, 178. [Google Scholar] [CrossRef]
- Osicka, R.; Osickova, A.; Basar, T.; Guermonprez, P.; Rojas, M.; Leclerc, C.; Sebo, P. Delivery of CD8(+) T-cell epitopes into major histocompatibility complex class I antigen presentation pathway by Bordetella pertussis adenylate cyclase: Delineation of cell invasive structures and permissive insertion sites. Infect. Immun. 2000, 68, 247–256. [Google Scholar]
- Bellalou, J.; Sakamoto, H.; Ladant, D.; Geoffroy, C.; Ullmann, A. Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect. Immun. 1990, 58, 3242–3247. [Google Scholar]
- Ladant, D. Interaction of Bordetella pertussis adenylate cyclase with calmodulin. Identification of two separated calmodulin-binding domains. J. Biol. Chem. 1988, 263, 2612–2618. [Google Scholar]
- Masin, J.; Osickova, A.; Sukova, A.; Fiser, R.; Halada, P.; Bumba, L.; Linhartova, I.; Osicka, R.; Sebo, P. Negatively charged residues of the segment linking the enzyme and cytolysin moieties restrict the membrane-permeabilizing capacity of adenylate cyclase toxin. Sci. Rep. 2016, 6, 29137. [Google Scholar] [CrossRef]
- Zenner, H.P.; Lehner, W.; Herrmann, I.F. Establishment of carcinoma cell lines from larynx and submandibular gland. Arch. Oto-Rhino-Laryngol. 1979, 225, 269–277. [Google Scholar] [CrossRef]
- Karimova, G.; Pidoux, J.; Ullmann, A.; Ladant, D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 5752–5756. [Google Scholar] [CrossRef]
| RTX Toxin 1 | Without Detergent Wash | 1% Triton X-100 Wash | 1% Triton X-114 Wash | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Conc. 2 (mg/mL) | LPS 3 (EU/mg) | Total 4 (mg) | Conc. 2 (mg/mL) | LPS 3 (EU/mg) | Total 4 (mg) | Conc. 2 (mg/mL) | LPS 3 (EU/mg) | Total 4 (mg) | |
| CyaA | 1.3 | >4 × 105 | 6 | 1.0 | 12 | 6 | 1.2 | 8 | 6 |
| RtxA | 2.0 | >1 × 106 | 8 | 2.1 | 115 | 8 | 2.0 | 25 | 8 |
| HlyA | 1.4 | >1 × 106 | 4 | 1.3 | 95 | 4 | 1.4 | 15 | 4 |
| ApxIA | 1.9 | >1 × 106 | 8 | 2.1 | 85 | 8 | 1.6 | 15 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanek, O.; Masin, J.; Osicka, R.; Jurnecka, D.; Osickova, A.; Sebo, P. Rapid Purification of Endotoxin-Free RTX Toxins. Toxins 2019, 11, 336. https://doi.org/10.3390/toxins11060336
Stanek O, Masin J, Osicka R, Jurnecka D, Osickova A, Sebo P. Rapid Purification of Endotoxin-Free RTX Toxins. Toxins. 2019; 11(6):336. https://doi.org/10.3390/toxins11060336
Chicago/Turabian StyleStanek, Ondrej, Jiri Masin, Radim Osicka, David Jurnecka, Adriana Osickova, and Peter Sebo. 2019. "Rapid Purification of Endotoxin-Free RTX Toxins" Toxins 11, no. 6: 336. https://doi.org/10.3390/toxins11060336
APA StyleStanek, O., Masin, J., Osicka, R., Jurnecka, D., Osickova, A., & Sebo, P. (2019). Rapid Purification of Endotoxin-Free RTX Toxins. Toxins, 11(6), 336. https://doi.org/10.3390/toxins11060336






