Effects of Dietary Zearalenone on Oxidative Stress, Cell Apoptosis, and Tight Junction in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella)
Abstract
1. Introduction
2. Results
2.1. Growth Performance and Body Malformation of Fish
2.2. Inflammation and Histopathological Changes
2.3. Oxidative Injury, Antioxidant-Relevant Parameters, and Gene Expressions of Relevant Signalling Molecules
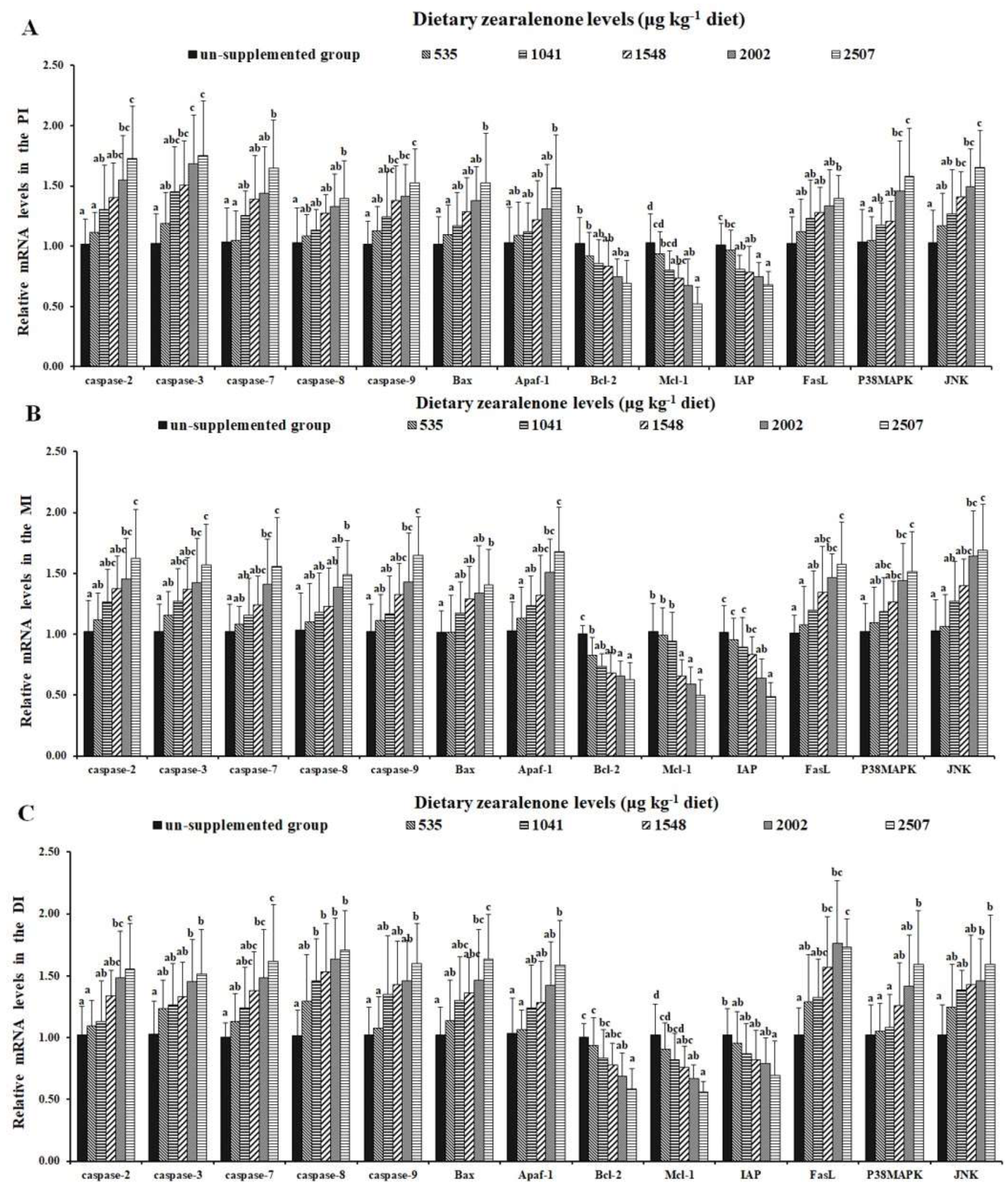
2.4. DNA Fragmentation, Apoptosis-Related Indicators, and Signalling Molecules
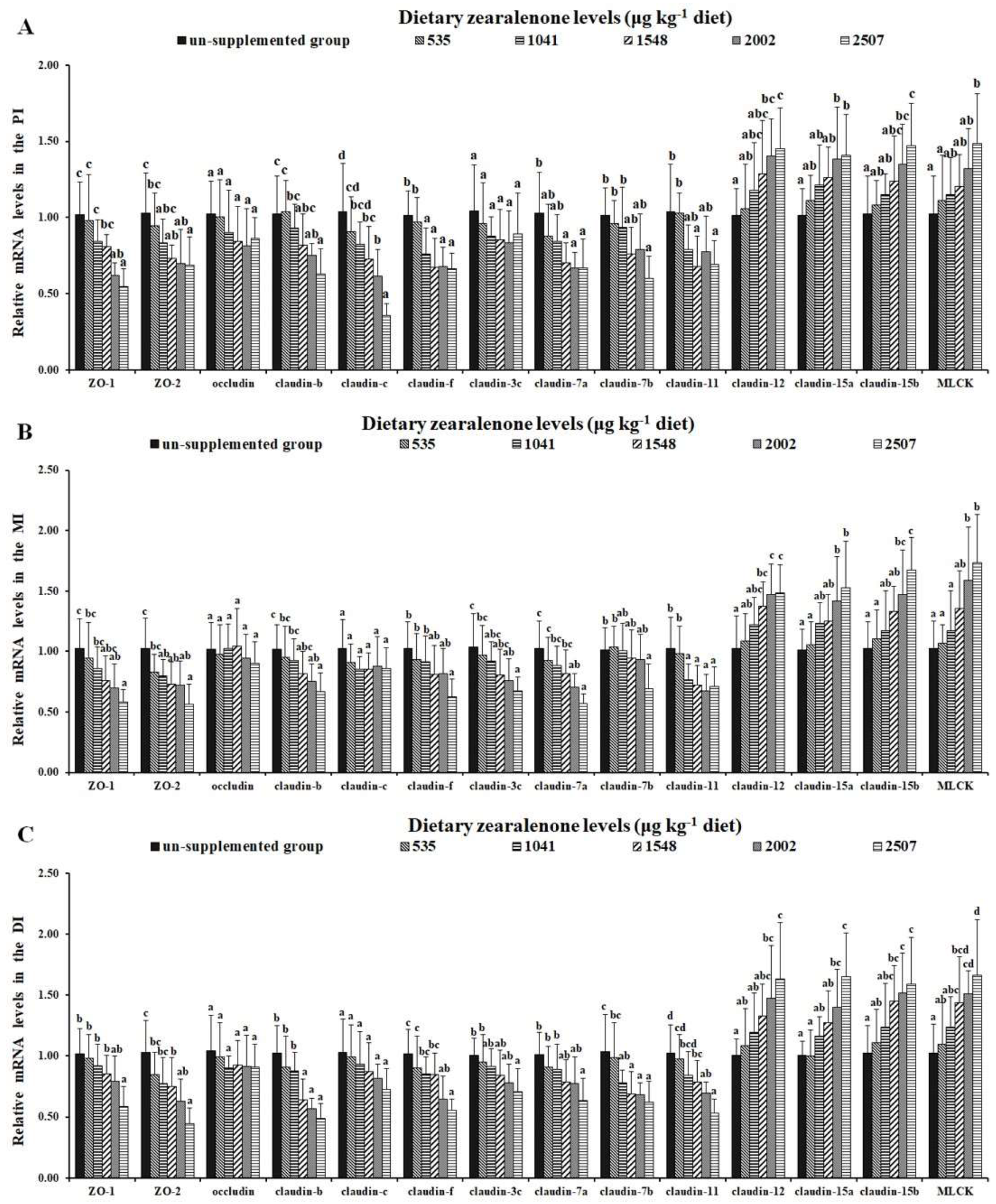
2.5. Tight Junctions and Relevant Signalling Molecules
2.6. Correlation Analyses
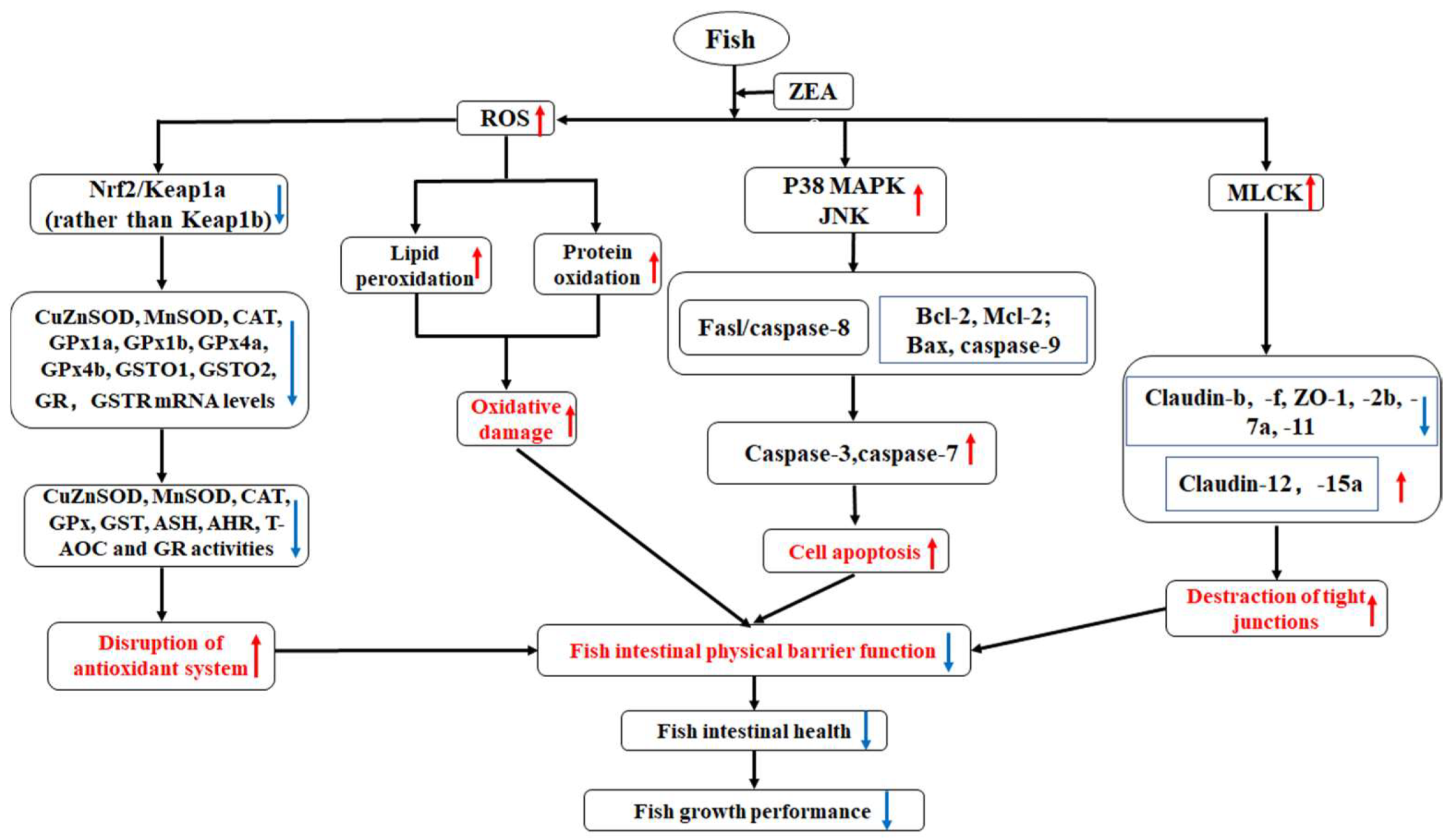
3. Discussion
3.1. Growth Performance and Body Malformation of Fish
3.2. Intestinal Histopathological Lesions Caused by ZEA and Possible Mechanisms
3.3. The Dose of NOAEL for ZEA
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Diets
5.3. Experiments and Samples Collection
5.4. Analysis of Feeding Components
5.5. Histological Examination
5.6. Biochemical Analysis
5.7. DNA Fragmentation
5.8. RT-PCR Analysis
5.9. Western Blot
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rudolf, K.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; MacDonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam. 2008, 25, 152–163. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Neagoe, I.V.; Calin, L.; Taranu, I. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food Chem. Toxicol. 2013, 58, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Solcan, C.; Pavel, G.; Floristean, V.C.; Chiriac, I.S.; Şlencu, B.G.; Solcan, G. Effect of ochratoxin A on the intestinal mucosa and mucosa-associated lymphoid tissues in broiler chickens. Acta Vet. Hung. 2015, 63, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Matejova, I.; Svobodova, Z.; Vakula, J.; Mares, J.; Modra, H. Impact of Mycotoxins on Aquaculture Fish Species: A Review. J. World Aquac. Soc. 2016, 48, 186–200. [Google Scholar] [CrossRef]
- Pietsch, C. Zearalenone (ZEN) and Its Influence on Regulation of Gene Expression in Carp (Cyprinus carpio L.) Liver Tissue. Toxins 2017, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Bouhet, S.; Oswald, I.P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2010, 51, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, P.; Jiang, W.D.; Liu, Y.; Zeng, Y.Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q. Deoxynivalenol decreased the growth performance and impaired intestinal physical barrier in juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 80, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Amany, M.K.; Hala, M.; Mohammad, M.; Abdel-Wahab, M. Pathological studies on effects of aflatoxin on Oreochromis niloticus with application of different trials of control. Egypt. J. Comp. Pathol. Clin. Pathol 2009, 22, 175–193. [Google Scholar]
- Poston, H.A.; Coffin, J.L.; Combs, G.F. Biological effects of dietary T-2 toxin on rainbow trout, Salmo gairdneri. Aquat. Toxicol. 1982, 2, 79–88. [Google Scholar] [CrossRef]
- Hooft, J.M.; Elmor, A.E.H.I.; Encarnação, P.; Bureau, D.P. Rainbow trout (Oncorhynchus mykiss) is extremely sensitive to the feed-borne Fusarium mycotoxin deoxynivalenol (DON). Aquaculture 2011, 311, 224–232. [Google Scholar] [CrossRef]
- Bouaziz, C.; Dein, O.S.E.; Golli, E.E.; Brenner, C.; Lemaire, C.; Bacha, H.; Abid-Essefi, S. Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 2008, 254, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Anater, A.; PimpãO, C.U.T.; Ferrer, E.; Luciano, F.B.; Meca, G.; Font, G.; Manyes, L. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Nisar, S.; Zia, K.M.; Jinap, S.; Malik, N. A Limited Survey of Aflatoxins and Zearalenone in Feed and Feed Ingredients from Pakistan. J. Food Prot. 2016, 79. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.; Hanif, N.Q.; Tahira, I.; Sultana, N.; Sultana, K.; Ayub, N. Natural occurrence of aflatoxins, zearalenone and trichothecenes in maize grown in Pakistan. Pak. J. Bot 2012, 44, 231–236. [Google Scholar]
- Pietsch, C.; Kersten, S.; Burkhardt-Holm, P.; Valenta, H.; Dänicke, S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins 2013, 5, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kanora, A.; Maes, D. The role of mycotoxins in pig reproduction: A review. Vet. Med. 2010, 54, 565–576. [Google Scholar] [CrossRef]
- Oliver, W.T.; Miles, J.R.; Diaz, D.E.; Dibner, J.J.; Rottinghaus, G.E.; Harrell, R.J. Zearalenone enhances reproductive tract development, but does not alter skeletal muscle signaling in prepubertal gilts. Anim. Feed Sci. Technol. 2012, 174, 79–85. [Google Scholar] [CrossRef]
- Johns, S.M.; Denslow, N.D.; Kane, M.D.; Watanabe, K.H.; Orlando, E.F.; Sepúlveda, M.S. Effects of estrogens and antiestrogens on gene expression of fathead minnow (Pimephales promelas) early life stages. Environ. Toxicol. 2011, 26, 195–206. [Google Scholar] [CrossRef]
- Schwartz, P.; Thorpe, K.L.; Bucheli, T.D.; Wettstein, F.E.; Burkhardt-Holm, P. Short-term exposure to the environmentally relevant estrogenic mycotoxin zearalenone impairs reproduction in fish. Sci. Total Environ. 2010, 409, 326–333. [Google Scholar] [CrossRef]
- Pietsch, C.; Junge, R.; Burkhardt-Holm, P. Immunomodulation by Zearalenone in Carp (Cyprinus carpio L.). BioMed Res. Int. 2015, 2015, 420702. [Google Scholar] [CrossRef]
- Döll, S.; Valenta, H.; Baardsen, G.; Möller, P.; Koppe, W.; Stubhaug, I.; Dänicke, S. Effects of increasing concentrations of deoxynivalenol, zearalenone and ochratoxin A in diets for Atlantic salmon (Salmo salar) on performance, health and toxin residues. In Proceedings of the Abstracts of the 33rd Mycotoxin Workshop, Freising, Germany, 30 May–1 June 2011. [Google Scholar]
- Woźny, M.; Dobosz, S.; Obremski, K.; Hliwa, P.; Gomułka, P.; Łakomiak, A.; Różyński, R.; Zalewski, T.; Brzuzan, P. Feed-borne exposure to zearalenone leads to advanced ovarian development and limited histopathological changes in the liver of premarket size rainbow trout. Aquaculture 2015, 448, 71–81. [Google Scholar] [CrossRef]
- Woźny, M.; Obremski, K.; Hliwa, P.; Gomułka, P.; Różyński, R.; Wojtacha, P.; Florczyk, M.; Segner, H.; Brzuzan, P. Feed contamination with zearalenone promotes growth but affects the immune system of rainbow trout. Fish & Shellfish Immunol. 2019, 84, 680–694. [Google Scholar] [CrossRef]
- Pietsch, C.; Junge, R. Physiological responses of carp (Cyprinus carpio L.) to dietary exposure to zearalenone (ZEN). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 188, 52–59. [Google Scholar] [CrossRef]
- Yu, J.Y.; Zheng, Z.H.; Son, Y.O.; Shi, X.; Jang, Y.O.; Lee, J.C. Mycotoxin zearalenone induces AIF- and ROS-mediated cell death through p53- and MAPK-dependent signaling pathways in RAW264.7 macrophages. Toxicol. In Vitro 2011, 25, 1654–1663. [Google Scholar] [CrossRef]
- Li, Z.; Hui, Y.; Chengzhi, G.; Yin, L.; Sijun, D.; Yang, Y.; Qiang, W.; Lixin, W.; Zuping, H. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway. J. Cell. Physiol. 2012, 227, 1814–1820. [Google Scholar] [CrossRef]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.Q.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N. Deficiency of dietary niacin impaired intestinal mucosal immune function via regulating intestinal NF-κB, Nrf2 and MLCK signaling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 49, 177–193. [Google Scholar] [CrossRef]
- Mann, G.E.; Rowlands, D.J.; Li, F.Y.L.; Patricia, D.W.; Siow, R.C.M. Activation of endothelial nitric oxide synthase by dietary isoflavones: Role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 2007, 75, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Gavaia, P.J.; Dinis, M.T.; Cancela, M.L. Osteological development and abnormalities of the vertebral column and caudal skeleton in larval and juvenile stages of hatchery-reared Senegal sole (Solea senegalensis). Aquaculture 2002, 211, 305–323. [Google Scholar] [CrossRef]
- Bakos, K.; Kovács, R.; Staszny, Á.; Sipos, D.K.; Urbányi, B.; Müller, F.; Csenki, Z.; Kovács, B. Developmental toxicity and estrogenic potency of zearalenone in zebrafish (Danio rerio). Aquat. Toxicol. 2013, 136, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, C.; Kersten, S.; Valenta, H.; Dänicke, S.; Schulz, C.; Burkhardt-Holm, P.; Junge, R. Effects of Dietary Exposure to Zearalenone (ZEN) on Carp (Cyprinus carpio L.). Toxins 2015, 7, 3465–3480. [Google Scholar] [CrossRef] [PubMed]
- Teixidó, E.; Piqué, E.; Gonzalez-Linares, J.; Gonzalez-Linares, J.; Gómez-Catalán, J. Developmental effects and genotoxicity of 10 water disinfection by-products in zebrafish. J. Water Health 2015, 13, 54–56. [Google Scholar] [CrossRef]
- Li, S.A.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A. Dietary myo-inositol deficiency decreased the growth performances and impaired intestinal physical barrier function partly relating to nrf2, jnk, e2f4 and mlck signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 67, 475–492. [Google Scholar] [CrossRef]
- Wang, K.Z.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q.; Feng, L. Gossypol reduced the intestinal amino acid absorption capacity of young grass carp (Ctenopharyngodon idella). Aquaculture 2018, 492, 46–58. [Google Scholar] [CrossRef]
- Tashjian, D.H.; Teh, S.J.; Sogomonyan, A.; Hung, S.S.O. Bioaccumulation and chronic toxicity of dietary l-selenomethionine in juvenile white sturgeon (Acipenser transmontanus). Aquat. Toxicol. 2006, 79, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Bracarense, A.-P.F.; Lucioli, J.; Grenier, B.; Pacheco, G.D.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jiang, W.D.; Jiang, J.; Zhao, J.; Liu, Y.; Zhang, Y.A.; Zhou, X.Q.; Lin, F. Dietary choline deficiency and excess induced intestinal inflammation and alteration of intestinal tight junction protein transcription potentially by modulating NF-κB, STAT and p38 MAPK signaling molecules in juvenile Jian carp. Fish Shellfish Immunol. 2016, 58, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Hu, H.Y.; Xie, X.; Sakoda, A.; Sagehashi, M.; Li, F.M. Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2009, 91, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Çimen, M.Y.B. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef]
- Han-Ming, S.; Zheng-Gang, L. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006, 40, 928–939. [Google Scholar] [CrossRef]
- Xia, L.; Chen, S.; Dahms, H.U.; Ying, X.; Peng, X. Cadmium induced oxidative damage and apoptosis in the hepatopancreas of Meretrix Meretrix. Ecotoxicology 2016, 25, 959–969. [Google Scholar] [CrossRef]
- Song, J.H.; Fujimoto, K.; Miyazawa, T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid–containing oils. J. Nutr. 2000, 130, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Analysis of Fatty Acid Composition of Five Freshwater Fishes in China. J. Fish. China 1991, 15, 169–171. [Google Scholar]
- Böhm, M.; Schultz, S.; Koussoroplis, A.-M.; Kainz, M.J. Tissue-specific fatty acids response to different diets in common carp (Cyprinus carpio L.). PLoS ONE 2014, 9, e94759. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Levada-Pires, A.C.; Rossoni, L.V.; Curi, R.; Pithon-Curi, T.C. Effects of aerobic exercise training on antioxidant enzyme activities and mrna levels in soleus muscle from young and aged rats. Mech. Ageing Dev. 2007, 128, 267–275. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, K. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.S.; Jun, M.; Kong, A.N. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid. Redox Signal. 2006, 8, 99–106. [Google Scholar] [CrossRef]
- Porter, A.G.; Jnicke, R.U.; Kong, A.N. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Jae, Y.C.; Hyun, C.; Sang, H. Podophyllotoxin acetate enhances γ-ionizing radiation-induced apoptotic cell death by stimulating the ROS/p38/caspase pathway. Biomed. Pharmacother. 2015, 70, 111–118. [Google Scholar] [CrossRef]
- Freund, A.; Patil, C.K.; Campisi, J. P38mapk is a novel dna damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef]
- Pietsch, C.; Noser, J.; Wettstein, F.E.; Burkhardt, P. Unraveling the mechanisms involved in zearalenone-mediated toxicity in permanent fish cell cultures. Toxicon 2014, 88, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Shen, L.; Clayburgh, D.R.; Nalle, S.C.; Sullivan, E.A.; Meddings, J.B. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 2009, 136, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006, 119, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Kuang, S.Y. Optimal dietary protein level improved growth, disease resistance, intestinal immune and physical barrier function of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 55, 64–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Gao, J.; Liu, F.X.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011, 89, 3008. [Google Scholar] [CrossRef] [PubMed]
- Becci, P.J.; Voss, K.A.; Hess, F.G.; Gallo, M.A.; Parent, R.A.; Stevens, K.R.; Taylor, J.M. Long-term carcinogenicity and toxicity study of zearalenone in the rat. J. Appl. Toxicol. 2010, 2, 247–254. [Google Scholar] [CrossRef]
- Nuryono, N.; Noviandi, C.T.; Böhm, J.; Razzazi-Fazeli, E. A limited survey of zearalenone in Indonesian maize-based food and feed by ELISA and high performance liquid chromatography. Food Control 2005, 16, 65–71. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Jiang, W.D.; Liu, Y.; Wu, P.; Zhao, J.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary alpha-linolenic acid/linoleic acid ratios modulate intestinal immunity, tight junctions, anti-oxidant status and mrna levels of nf-kappa b p65, mlck and nrf2 in juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 51, 351–364. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Liang, J.J.; Liu, Y.J.; Tian, L.X.; Yang, H.J.; Liang, G.Y. Dietary available phosphorus requirement of juvenile grass carp (ctenopharyngodon idella). Aquac. Nutr. 2012, 18, 181–188. [Google Scholar] [CrossRef]
- Ji, H.; Li, J.; Liu, P. Regulation of growth performance and lipid metabolism by dietary n-3 highly unsaturated fatty acids in juvenile grass carp, Ctenopharyngodon idellus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2011, 159, 49–56. [Google Scholar] [CrossRef]
- Sveier, H.; Nordas, H.; Berge, G.E.; Lied, E. Dietary inclusion of crystalline D- and L-methionine: Effects on growth, feed and protein utilization, and digestibility in small and large Atlantic salmon (Salmon salar L.). Aquac. Nutr. 2015, 7, 169–181. [Google Scholar] [CrossRef]
- Xu, H.; Cao, L.; Zhang, Y.; Johnson, R.B.; Wei, Y.; Zheng, K.; Liang, M. Dietary arachidonic acid differentially regulates the gonadal steroidogenesis in the marine teleost, tongue sole (Cynoglossus semilaevis), depending on fish gender and maturation stage. Aquaculture 2017, 468, 378–385. [Google Scholar] [CrossRef]
- Chen, Y.P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A. Exogenous phospholipids supplementation improves growth and modulates immune response and physical barrier referring to NF-κB, TOR, MLCK and Nrf2 signaling factors in the intestine of juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2015, 47, 46–62. [Google Scholar] [CrossRef]
- Geraylou, Z.; Souffreau, C.; Rurangwa, E.; De, M.L.; Courtin, C.M.; Delcour, J.A.; Buyse, J.; Ollevier, F. Effects of dietary arabinoxylan-oligosaccharides (AXOS) and endogenous probiotics on the growth performance, non-specific immunity and gut microbiota of juvenile Siberian sturgeon (Acipenser baerii). Fish Shellfish Immunol. 2013, 35, 766–775. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis:15th Edition. Assoc. Anal. Chem. 1990, 9, VI. [Google Scholar] [CrossRef]
- Morán, P.; Marco-Rius, F.; Megías, M.; Covelo-Soto, L.; Pérez-Figueroa, A. Environmental induced methylation changes associated with seawater adaptation in brown trout. Aquaculture 2013, 392, 77–83. [Google Scholar] [CrossRef]
- Luo, J.B.; Feng, L.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q. The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol. 2014, 40, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Pagano, P.J.; Du, Y.; Cayatte, A.J.; Quinn, M.T.; Brecher, P.; Cohen, R.A. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ. Res. 1998, 82, 810. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Deng, J.; Mai, K.; Chen, L.; Mi, H.; Zhang, L. Effects of replacing soybean meal with rubber seed meal on growth, antioxidant capacity, non-specific immune response, and resistance to Aeromonas hydrophila in tilapia (Oreochromis niloticus × O. aureus). Fish Shellfish Immunol. 2015, 44, 436–444. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. [12] Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Baltacıoğlu, E.; Akalın, F.A.; Alver, A.; Değer, O.; Karabulut, E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. J. Clin. Periodontol. 2008, 53, 716–722. [Google Scholar] [CrossRef]
- Jiang, W.D.; Lin, F.; Yang, L.; Jiang, J.; Kai, H.; Li, S.H.; Zhou, X.Q. Lipid peroxidation, protein oxidant and antioxidant status of muscle, intestine and hepatopancreas for juvenile jian carp (Cyprinus carpio var. Jian) fed graded levels of myo-inositol. Food Chem. 2010, 120, 692–697. [Google Scholar] [CrossRef]
- Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Feng, L.; Zhou, X.Q. Copper exposure induces toxicity to the antioxidant system via the destruction of Nrf2/ARE signaling and caspase-3-regulated DNA damage in fish muscle: Amelioration by myo-inositol. Aquat. Toxicol. 2015, 159, 245–255. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jiang, W.D.; Wen, H.L.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A. Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with anti-oxidative damage referred to the Nrf2 and TOR signalling factors in young grass carp (Ctenopharyngodon idella): Avoid tryptophan deficiency or excess. Food Chem. 2016, 199, 210–219. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Li, S.H.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Effects of Dietary Copper on Growth, Digestive, and Brush Border Enzyme;Activities and Antioxidant Defense of Hepatopancreas and Intestine for;Young Grass Carp (Ctenopharyngodon idella). Biol. Trace Elem. Res. 2013, 155, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Feng, L.; Liu, Y.; Jiang, J.; Zhou, X.Q. Growth, digestive capacity and intestinal microflora of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary inositol. Aquac. Res. 2010, 40, 955–962. [Google Scholar] [CrossRef]
- Wang, K.-Z.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhang, Y.-A.; Zhou, X.-Q. Dietary gossypol reduced intestinal immunity and aggravated inflammation in on-growing grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2019, 86, 814–831. [Google Scholar] [CrossRef] [PubMed]
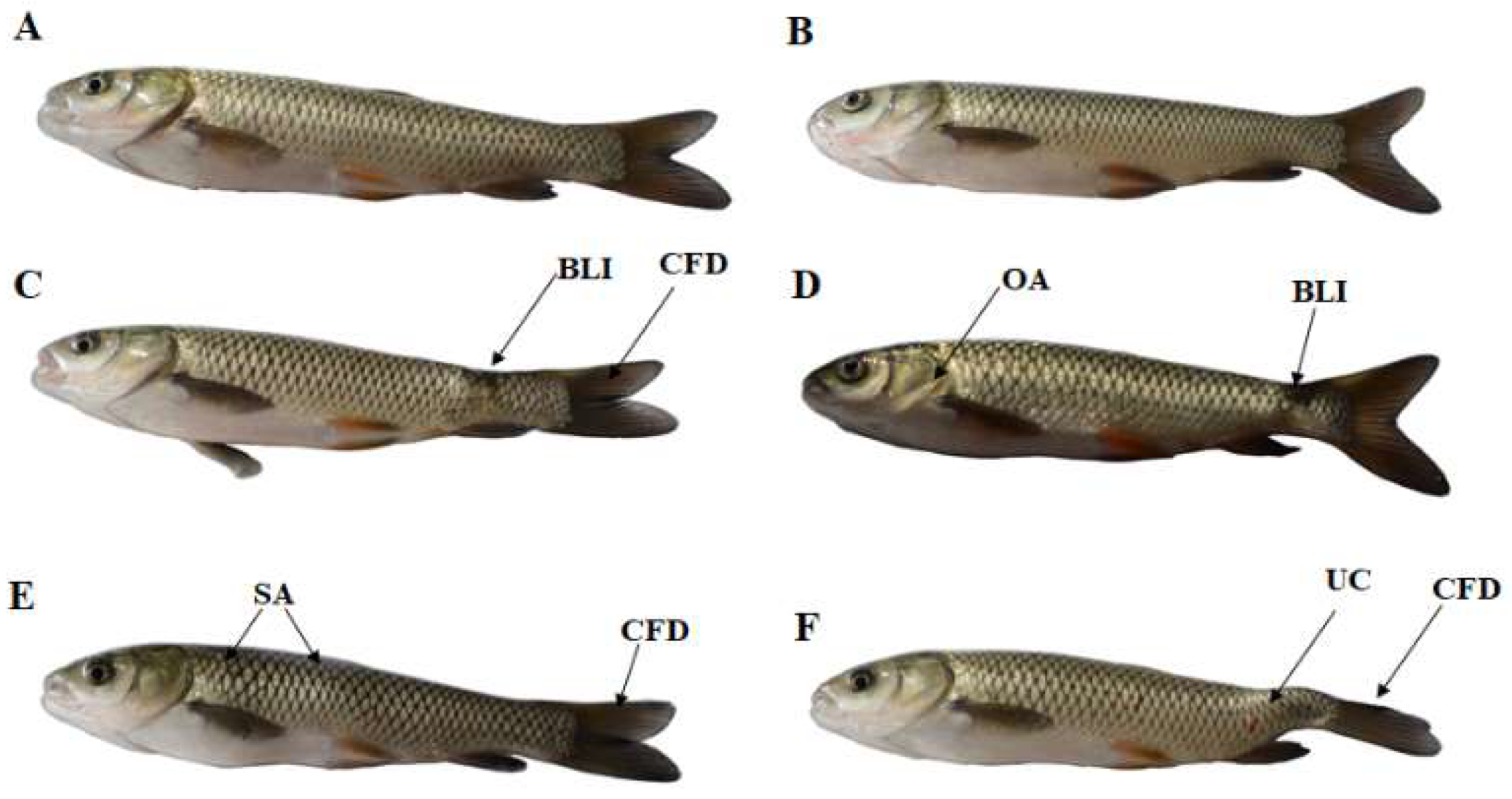
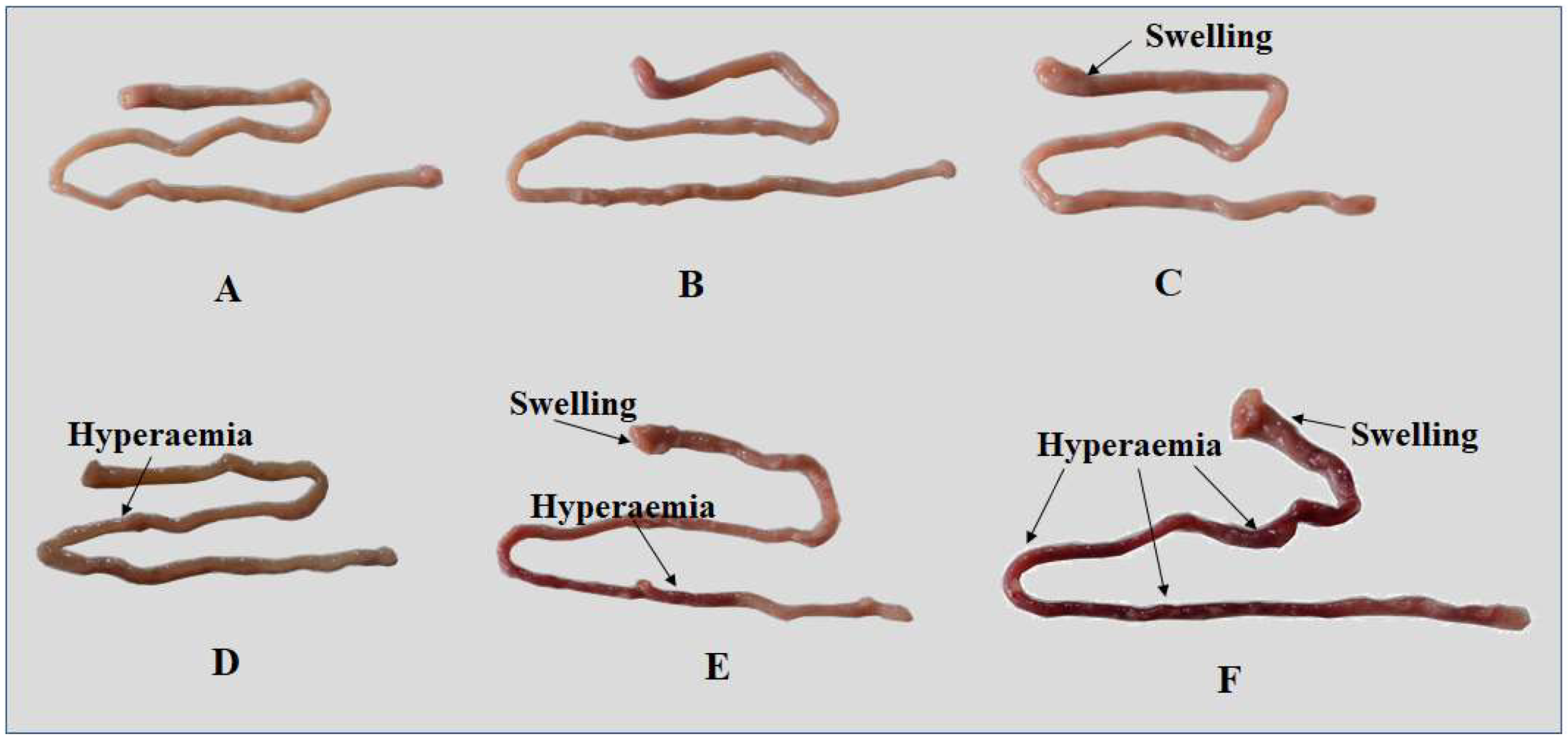

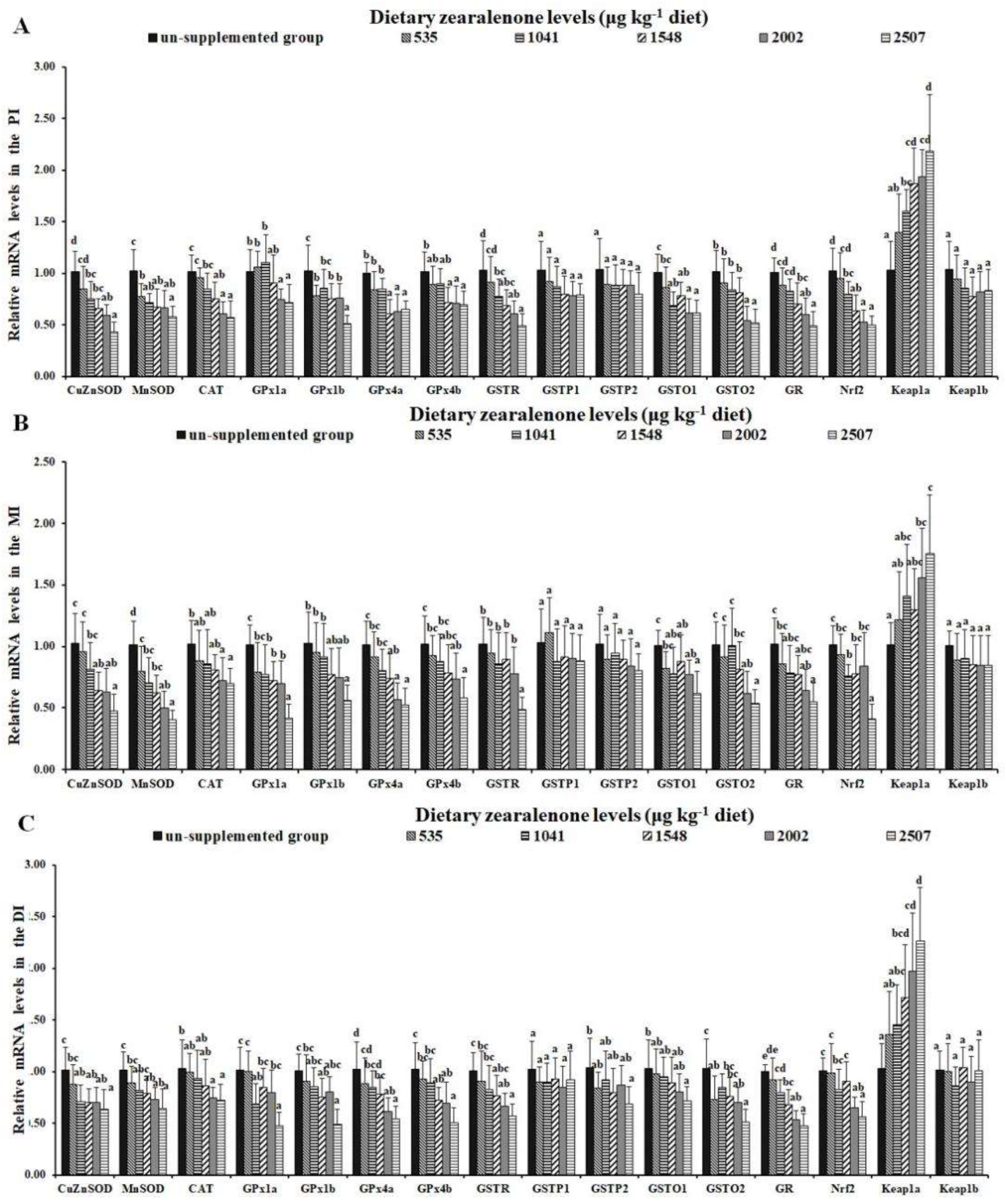
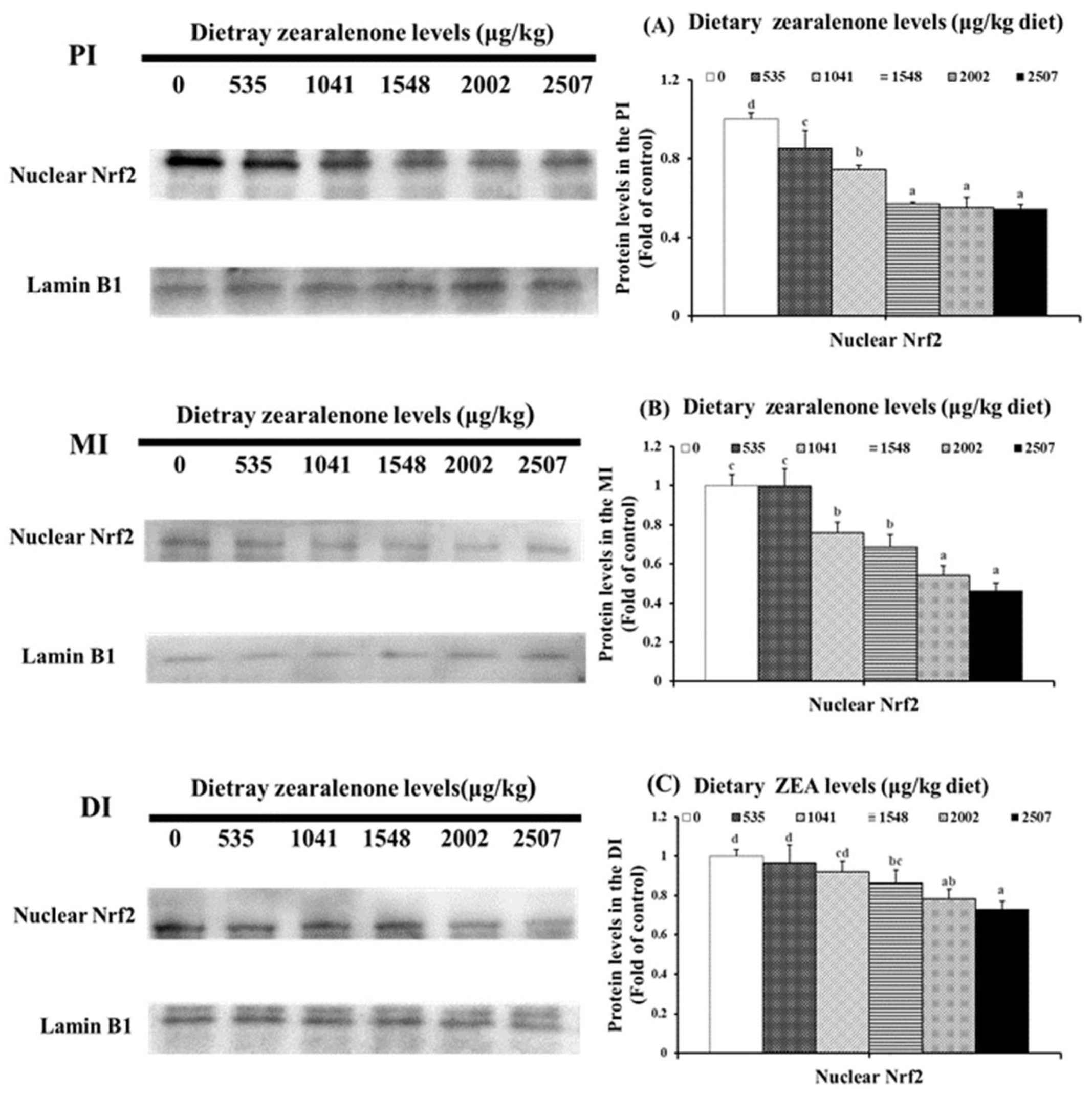

| Growth Indicators | Dietary ZEA Levels (μg/kg Diet) | |||||
|---|---|---|---|---|---|---|
| 0 | 535 | 1041 | 1548 | 2002 | 2507 | |
| IBW 1 | 15.35 ± 0.07 a | 15.31 ± 0.03 a | 15.29 ± 0.06 a | 15.36 ± 0.15 a | 15.29 ± 0.04 a | 15.45 ± 0.05 a |
| FBW 1 | 115.40 ± 1.11 e | 114.73 ± 0.68 e | 105.83 ± 1.81 d | 92.21 ± 0.97 c | 85.80 ± 1.12 b | 78.83 ± 0.39 a |
| PWG 1 | 651.99 ±9.73 e | 649.20 ± 5.79 e | 592.01 ± 5.27 d | 500.31 ± 1.86 c | 461.04 ± 8.37 b | 410.32 ± 1.00 a |
| SGR 1 | 2.882 ± 0.02 e | 2.877 ± 0.01 e | 2.763 ± 0.01 d | 2.560 ± 0.004 c | 2.464 ± 0.02 b | 2.328 ± 0.003 a |
| FI 1 | 118.47 ± 0.84 e | 118.34 ± 0.91 e | 112.11 ± 0.11 d | 102.73 ± 0.79 c | 95.68 ± 0.73 b | 90.02 ± 0.68 a |
| FE 1 | 0.845 ± 0.06 d | 0.840 ± 0.05 d | 0.808 ± 0.04 c | 0.748 ± 0.02 b | 0.737 ± 0.02 b | 0.704 ± 0.01 a |
| IL 2 | 31.85 ± 1.61 d | 30.72 ± 0.44 d | 28.49 ± 0.41 c | 25.47 ± 0.50 b | 24.27 ± 0.24 ab | 23.95 ± 0.10 a |
| ILI 2 | 140.89 ± 8.85 c | 139.75 ± 2.93 c | 131.41 ± 1.35 b | 121.18 ± 1.53 a | 120.08 ± 1.63 a | 118.55 ± 1.92 a |
| IW 2 | 2.39 ± 0.12 d | 2.24 ± 0.12 d | 1.97 ± 0.05 c | 1.64 ± 0.12 b | 1.47 ± 0.04 ab | 1.44 ± 0.11 a |
| ISI 2 | 1.99 ± 0.09 c | 2.00 ± 0.07 c | 1.83 ± 0.03 b | 1.71 ± 0.07 a | 1.69 ± 0.03 a | 2.49 ± 0.07 a |
| ZEA Content (ug/kg tissue) | ||||||
| PI 3 | n.d 4 | 29.64 ± 2.62 a | 41.26 ± 4.92 b | 56.82 ± 5.88 c | 69.19 ± 6.39 d | 87.97 ± 6.94 e |
| MI 3 | n.d 4 | 34.44 ± 3.15 a | 49.61 ± 4.89 b | 72.39 ± 5.80 c | 81.62 ± 7.91 c | 95.56 ± 12.78 d |
| DI 3 | n.d 4 | 37.02 ± 3.67 a | 58.09 ± 3.71 b | 76.34 ± 5.64 c | 87.48 ± 6.95 d | 102.46 ± 9.95 e |
| Item | Dietary ZEA Level (μg/kg Diet) | |||||
|---|---|---|---|---|---|---|
| 0 | 535 | 1041 | 1548 | 2002 | 2507 | |
| PI | ||||||
| ROS | 100.00 ± 10.97 a | 109.66 ± 8.81 ab | 113.90 ± 11.02 ab | 119.85 ± 12.10 bc | 131.45 ± 14.37 cd | 140.61 ± 12.95 d |
| MDA | 28.28 ± 1.72 a | 28.29 ± 2.74 a | 32.46 ± 3.16 b | 32.43 ± 2.53 b | 34.16 ± 3.45 b | 41.85 ± 3.43 c |
| PC | 5.82 ± 0.47 a | 5.88 ± 0.56 a | 6.90 ± 0.67 b | 7.56 ± 0.78 bc | 8.34 ± 0.87 cd | 8.59 ± 0.84 d |
| ASA | 184.37 ± 18.31 b | 186.21 ± 6.73 b | 181.99 ± 7.02 ab | 173.27 ± 12.90 ab | 171.67 ± 11.56 ab | 168.92 ± 6.46 a |
| AHR | 70.97 ± 6.67 b | 70.17 ± 4.72 b | 66.51 ± 3.01 ab | 65.73 ± 5.52 ab | 64.91 ± 4.56 ab | 62.05 ± 4.12 a |
| CuZnSOD | 11.17 ± 1.06 b | 10.42 ± 0.90 ab | 9.95 ± 0.80 a | 9.76 ± 0.28 a | 9.79 ± 0.84 a | 9.44 ± 0.32 a |
| MnSOD | 6.53 ± 0.45 b | 6.54 ± 0.39 b | 6.32 ± 0.61 ab | 6.67 ± 0.67 b | 5.83 ± 0.54 a | 5.67 ± 0.60 a |
| CAT | 3.37 ± 0.29 c | 3.31 ± 0.33 c | 3.07 ± 0.26 bc | 2.81 ± 0.21 b | 2.16 ± 0.16 a | 2.14 ± 0.22 a |
| GPx | 145.54 ± 12.87 c | 141.71 ± 13.58 bc | 145.81 ± 11.30 c | 132.24 ± 17.23 abc | 124.16 ± 10.42 ab | 126.55 ± 12.54 a |
| GST | 61.88 ± 5.65 e | 62.94 ± 4.90 e | 54.02 ± 5.57 d | 46.43 ± 3.73 c | 38.41 ± 3.56 b | 32.98 ± 3.19 a |
| GR | 54.16 ± 4.48 e | 52.37 ± 4.63 e | 41.47 ± 3.96 d | 35.07 ± 2.98 c | 28.57 ± 3.57 b | 24.09 ± 2.13 a |
| GSH | 16.11 ± 1.27 b | 15.94 ± 1.17 b | 15.44 ± 1.65 ab | 14.58 ± 1.33 ab | 14.48 ± 1.42 ab | 14.05 ± 1.13 a |
| MI | ||||||
| ROS | 100.00 ± 9.38 a | 104.97 ± 3.32 ab | 116.17 ± 13.25 bc | 118.34 ± 7.52 c | 137.31 ± 11.22 d | 136.91 ± 9.74 d |
| MDA | 27.60 ± 2.43 a | 27.94 ± 2.85 a | 30.43 ± 3.26 ab | 33.32 ± 2.99 bc | 33.37 ± 1.83 bc | 34.67 ± 3.34 c |
| PC | 4.76 ± 0.42 a | 5.13 ± 0.49 a | 5.90 ± 0.54 b | 6.30 ± 0.57 b | 7.18 ± 0.34 c | 7.84 ± 0.92 c |
| ASA | 204.90 ± 19.85 c | 197.82 ± 13.47 bc | 182.98 ± 15.94 b | 161.31 ± 14.56 a | 159.77 ± 5.22 a | 154.46 ± 11.51 a |
| AHR | 69.87 ± 6.96 b | 68.91 ± 4.40 b | 65.31 ± 6.09 ab | 63.94 ± 3.41 ab | 60.08 ± 5.77 a | 58.77 ± 4.48 a |
| CuZnSOD | 9.26 ± 0.81 c | 9.05 ± 0.80 bc | 8.49 ± 0.26 ab | 8.34 ± 0.42 a | 8.24 ± 0.30 a | 8.21 ± 0.40 a |
| MnSOD | 6.55 ± 0.64 b | 6.48 ± 0.72 b | 6.02 ± 0.52 ab | 5.41 ± 0.47 a | 5.41 ± 0.35 a | 5.36 ± 0.24 a |
| CAT | 3.05 ± 0.30 b | 3.03 ± 0.29 b | 2.83 ± 0.24 ab | 2.75 ± 0.23 ab | 2.74 ± 0.24 ab | 2.55 ± 0.22 a |
| GPx | 130.65 ± 12.54 c | 127.53 ± 12.08 bc | 119.01 ± 10.78 abc | 114.97 ± 6.79 ab | 117.43 ± 4.18 ab | 110.51 ± 11.50 a |
| GST | 56.54 ± 5.20 c | 55.32 ± 5.54 c | 48.17 ± 4.24 b | 47.96 ± 3.33 b | 42.76 ± 4.37 a | 31.95 ± 2.69 a |
| GR | 35.53 ± 2.61 e | 29.00 ± 2.35 d | 28.53 ± 2.06 d | 25.34 ± 2.26 c | 21.43 ± 1.60 b | 17.77 ± 1.47 a |
| GSH | 15.39 ± 1.51 b | 15.26 ± 1.11 ab | 14.78 ± 0.41 ab | 14.26 ± 1.50 ab | 13.90 ± 1.20 ab | 13.74 ± 1.05 a |
| DI | ||||||
| ROS | 100.00 ± 8.85 a | 104.89 ± 8.97 ab | 115.86 ± 11.41 bc | 120.04 ± 10.65 c | 140.72 ± 13.19 d | 145.75 ± 9.05 d |
| MDA | 31.61 ± 2.85 a | 34.02 ± 2.83 ab | 34.04 ± 3.31 ab | 36.02 ± 2.40 b | 36.69 ± 2.96 b | 42.71 ± 4.11 c |
| PC | 4.96 ± 0.43 a | 5.46 ± 0.42 ab | 6.38 ± 0.55 bc | 6.06 ± 0.59 c | 6.46 ± 0.69 c | 7.42 ± 0.68 d |
| ASA | 170.19 ± 14.86b | 166.70 ± 9.58 b | 162.05 ± 7.84 b | 147.57 ± 13.79 a | 143.45 ± 10.63 a | 134.84 ± 3.42 a |
| AHR | 51.85 ± 2.66 d | 50.86 ± 3.55 d | 47.95 ± 3.49 cd | 45.80 ± 2.34 bc | 42.18 ± 4.12 b | 37.07 ± 3.21 a |
| CuZnSOD | 8.18 ± 0.61 b | 8.18 ± 0.35 b | 8.14 ± 0.44 b | 7.36 ± 0.61 a | 7.25 ± 0.58 a | 7.04 ± 0.46 a |
| MnSOD | 5.98 ± 0.42 c | 6.00 ± 0.24 c | 5.56 ± 0.30 bc | 5.13 ± 0.48 ab | 5.07 ± 0.46 ab | 5.03 ± 0.44 a |
| CAT | 2.76 ± 0.22 c | 2.64 ± 0.26 c | 2.55 ± 0.22 c | 2.29 ± 0.20 b | 2.21 ± 0.19 b | 1.97 ± 0.13 a |
| GPx | 94.06 ± 9.11 d | 86.38 ± 8.29 cd | 78.56 ± 6.89 bc | 74.85 ± 7.34 b | 62.34 ± 5.68 a | 56.72 ± 5.59 a |
| GST | 48.14 ± 4.47 d | 46.82 ± 4.36 d | 41.32 ± 3.58 c | 38.57 ± 3.13 c | 31.30 ± 3.68 b | 25.41 ± 2.56 a |
| GR | 46.44 ± 4.61 d | 45.04 ± 2.66 d | 37.83 ± 3.76 c | 34.93 ± 3.43 bc | 32.43 ± 2.79 b | 27.59 ± 2.48 a |
| GSH | 13.43 ± 1.25 b | 13.34 ± 1.06 b | 12.75 ± 1.18 ab | 12.56 ± 1.18 ab | 12.35 ± 1.15 ab | 11.56 ± 0.81 a |
| Indicators | Broken-Line Analysis | R2 | The Doses of NOAEL for ZEA (μg/kg) |
|---|---|---|---|
| PWG | Y = −0.1243x + 712.2785; Ymax = 650.60 | 0.9847 | 496.22 |
| ROS | Y = 0.0216x + 92.408; Ymin = 102.44 | 0.9481 | 464.63 |
| Caspase-8 | Y = 0.0002x + 1.2174; Ymin = 1.16 | 0.9778 | 298.77 |
| Claudin-15b | Y = 0.0002x + 1.0318; Ymin = 1.12 | 0.9203 | 450.57 |
| Ingredients | Content (%) | Nutrients | Content (%) |
|---|---|---|---|
| Fish meal | 5.50 | Crude protein 4 | 32.24 |
| Casein | 21.67 | Crude lipid 4 | 5.03 |
| Gelatin | 6.00 | n-3 5 | 1.04 |
| α-starch | 23.00 | n-6 5 | 0.96 |
| Rice flour | 25.74 | Available phosphorus6 | 0.84 |
| Fish oil | 2.52 | ||
| Soybean oil | 1.81 | ||
| Cellulose | 5.00 | ||
| Ca(H2PO4)2 | 3.16 | ||
| Vitamin premix 1 | 1.00 | ||
| Mineral premix 2 | 2.00 | ||
| Choline chloride (50%) | 1.00 | ||
| ZEA premix 3 | 1.00 | ||
| Ethoxyquin (30%) | 0.05 | ||
| DL-Methionine (99%) | 0.45 | ||
| L-Tryptophan (99.2%) | 0.05 | ||
| Threonine (98.5%) | 0.05 |
| Growth Indicators | Formula |
|---|---|
| PWG | 100 × [FBW (g/fish) − IBW (g/fish)]/IBW (g/fish) |
| SGR | 100 × [In (mean final weight − In (mean initial weight)]/days |
| FE | [FBW (g/fish) − IBW (g/fish)]/FI (g/fish) |
| ILI | 100 × intestine length (cm)/total body length (cm) |
| ISI | 100 × wet intestine weight (g)/wet body weight (g) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-L.; Zhou, X.-Q.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Wang, S.-W.; Kuang, S.-Y.; Tang, L.; Feng, L. Effects of Dietary Zearalenone on Oxidative Stress, Cell Apoptosis, and Tight Junction in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins 2019, 11, 333. https://doi.org/10.3390/toxins11060333
Wang Y-L, Zhou X-Q, Jiang W-D, Wu P, Liu Y, Jiang J, Wang S-W, Kuang S-Y, Tang L, Feng L. Effects of Dietary Zearalenone on Oxidative Stress, Cell Apoptosis, and Tight Junction in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins. 2019; 11(6):333. https://doi.org/10.3390/toxins11060333
Chicago/Turabian StyleWang, Ya-Li, Xiao-Qiu Zhou, Wei-Dan Jiang, Pei Wu, Yang Liu, Jun Jiang, Shang-Wen Wang, Sheng-Yao Kuang, Ling Tang, and Lin Feng. 2019. "Effects of Dietary Zearalenone on Oxidative Stress, Cell Apoptosis, and Tight Junction in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella)" Toxins 11, no. 6: 333. https://doi.org/10.3390/toxins11060333
APA StyleWang, Y.-L., Zhou, X.-Q., Jiang, W.-D., Wu, P., Liu, Y., Jiang, J., Wang, S.-W., Kuang, S.-Y., Tang, L., & Feng, L. (2019). Effects of Dietary Zearalenone on Oxidative Stress, Cell Apoptosis, and Tight Junction in the Intestine of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins, 11(6), 333. https://doi.org/10.3390/toxins11060333







