The Role of Streptococcal and Staphylococcal Exotoxins and Proteases in Human Necrotizing Soft Tissue Infections
Abstract
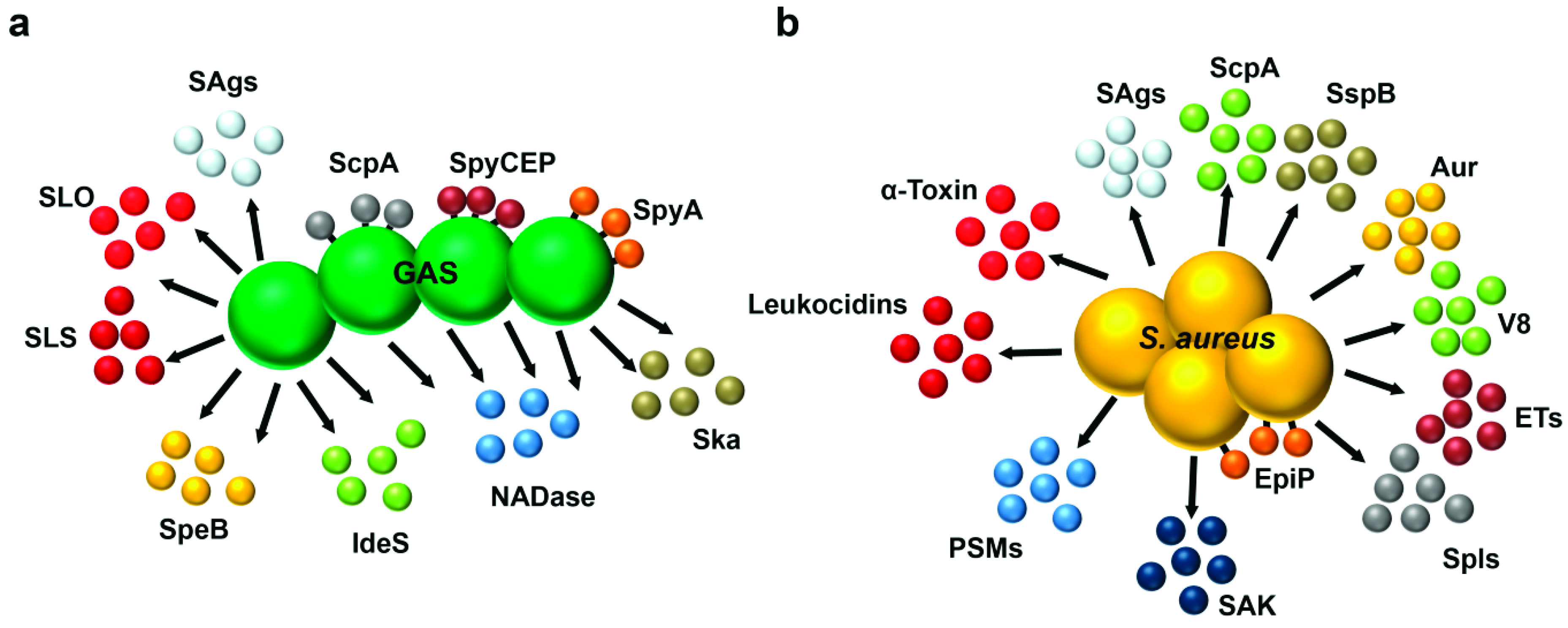
1. Introduction
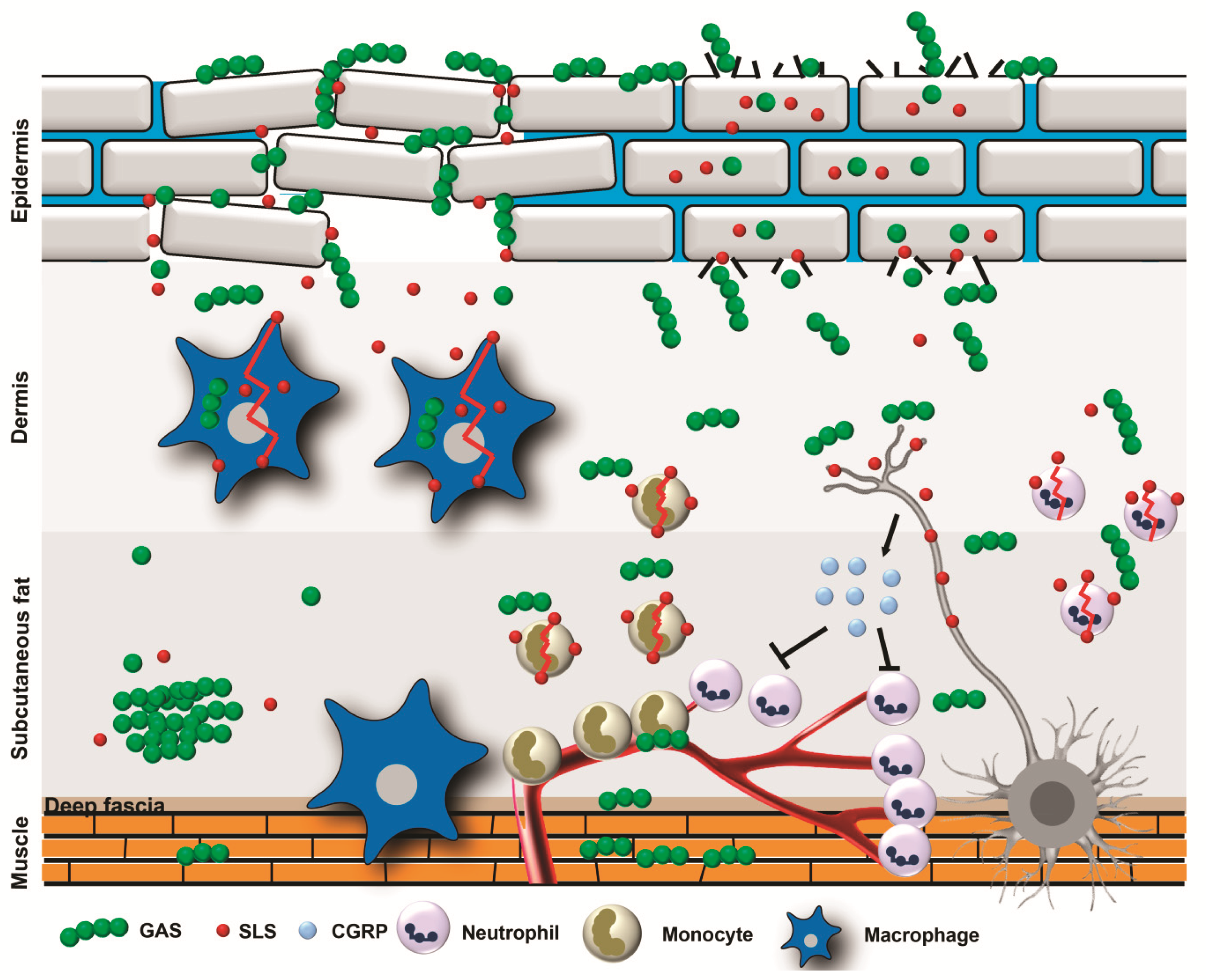
2. Pathophysiology of Type II NSTIs
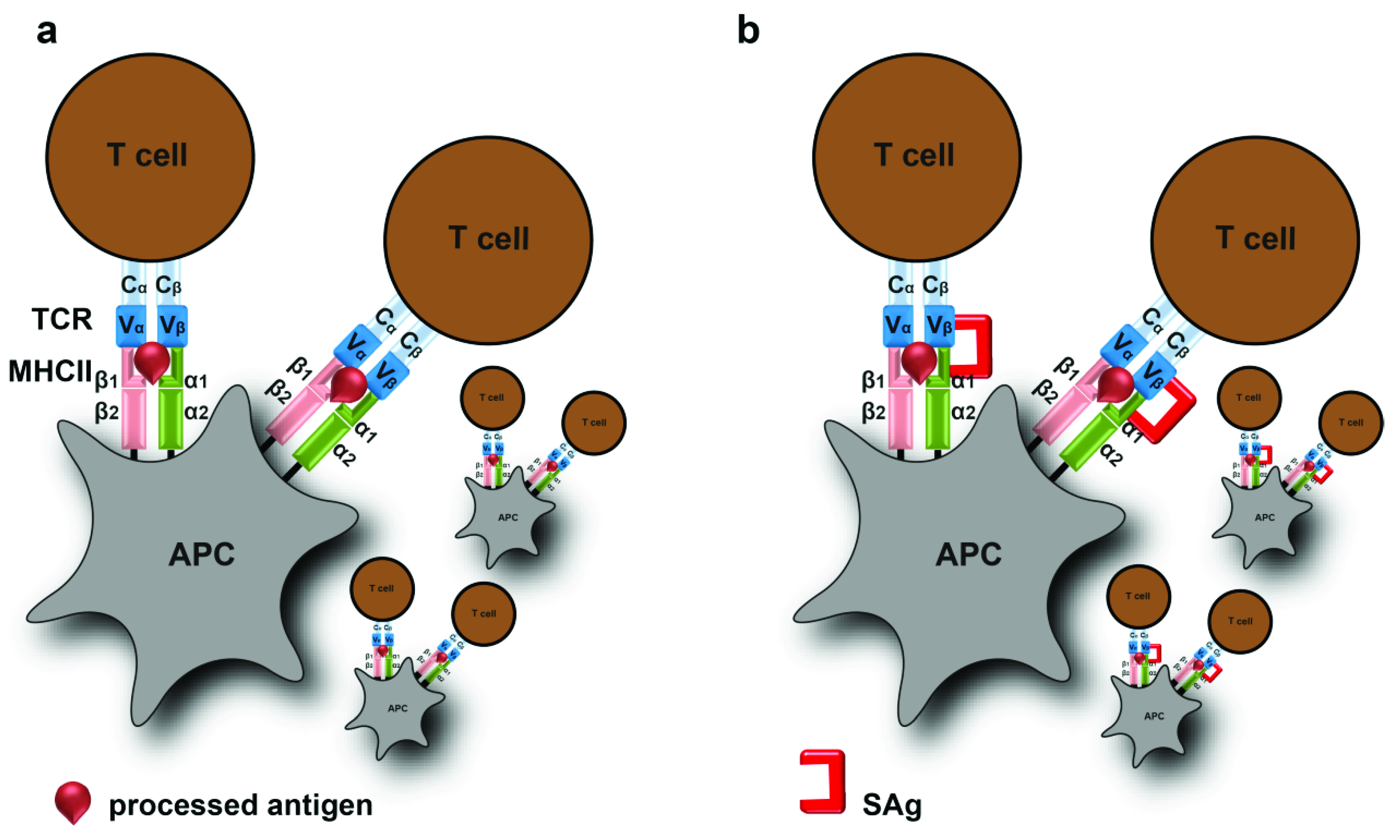
3. Superantigens and Toxic Shock Syndrome
4. Pore-Forming Toxins
4.1. GAS Pore-Forming Toxins
4.2. Staphylococcal Pore-Forming Toxins
5. Proteases and Other Immune-Modulatory Toxins
5.1. Streptococcal Proteases and Other Toxins
5.2. Staphylococcal Proteases and Other Toxins
5.3. Two Component Systems and Exotoxin Regulation
6. Treatment
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morgan, M.S. Diagnosis and management of necrotising fasciitis: A multiparametric approach. J. Hosp. Infect. 2010, 75, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Anaya, D.A.; McMahon, K.; Nathens, A.B.; Sullivan, S.R.; Foy, H.; Bulger, E. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch. Surg. 2005, 140, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Harbrecht, B.G.; Nash, N.A. Necrotizing soft tissue infections: A review. Surg. Infect. Larchmt. 2016, 17, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E. Necrotizing soft-Tissue infections. N. Engl. J. Med. 2017, 377, 2253–2265. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.; Lewis, F.; Hadley, K.; Blaisdell, F.W. Bacteriology of necrotizing fasciitis. Am. J. Surg. 1977, 134, 52–57. [Google Scholar] [CrossRef]
- Ustin, J.S.; Malangoni, M.A. Necrotizing soft-tissue infections. Crit. Care Med. 2011, 39, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Devaney, B.; Frawley, G.; Frawley, L.; Pilcher, D.V. Necrotising soft tissue infections: The effect of hyperbaric oxygen on mortality. Anaesth Intensive Care 2015, 43, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.E.; Sheil, F.; Ruston, J.C.; Butler, P.E. Necrotising soft tissue infection in a uk metropolitan population. Ann. R. Coll. Surg. Engl. 2015, 97, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Khamnuan, P.; Chongruksut, W.; Jearwattanakanok, K.; Patumanond, J.; Tantraworasin, A. Necrotizing fasciitis: Epidemiology and clinical predictors for amputation. Int. J. Gen. Med. 2015, 8, 195–202. [Google Scholar]
- Nordqvist, G.; Wallden, A.; Brorson, H.; Tham, J. Ten years of treating necrotizing fasciitis. Infect. Dis. 2015, 47, 319–325. [Google Scholar] [CrossRef]
- Miller, L.G.; Perdreau-Remington, F.; Rieg, G.; Mehdi, S.; Perlroth, J.; Bayer, A.S.; Tang, A.W.; Phung, T.O.; Spellberg, B. Necrotizing fasciitis caused by community-associated methicillin-Resistant staphylococcus aureus in los angeles. N. Engl. J. Med. 2005, 352, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Goh, T.; Goh, L.G.; Ang, C.H.; Wong, C.H. Early diagnosis of necrotizing fasciitis. Br. J. Surg. 2014, 101, e119–e125. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Otto, M.; Kreiswirth, B.N.; Chambers, H.F. Community-associated meticillin-Resistant staphylococcus aureus. Lancet 2010, 375, 1557–1568. [Google Scholar] [CrossRef]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Invasive methicillin-Resistant staphylococcus aureus infections in the united states. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group a streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Stevens, D.L.; Tanner, M.H.; Winship, J.; Swarts, R.; Ries, K.M.; Schlievert, P.M.; Kaplan, E. Severe group a streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 1989, 321, 1–7. [Google Scholar] [CrossRef]
- Nuwayhid, Z.B.; Aronoff, D.M.; Mulla, Z.D. Blunt trauma as a risk factor for group a streptococcal necrotizing fasciitis. Ann. Epidemiol. 2007, 17, 878–881. [Google Scholar] [CrossRef]
- Johansson, L.; Thulin, P.; Low, D.E.; Norrby-Teglund, A. Getting under the skin: The immunopathogenesis of streptococcus pyogenes deep tissue infections. Clin. Infect. Dis. 2010, 51, 58–65. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Kaul, R.; McGeer, A.; Low, D.E.; Green, K.; Schwartz, B. Population-based surveillance for group a streptococcal necrotizing fasciitis: Clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario group a streptococcal study. Am. J. Med. 1997, 103, 18–24. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Everett, E.D.; Dellinger, P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, E.L.; Montoya, J.G.; et al. Practice guidelines for the diagnosis and management of skin and soft-Tissue infections. Clin. Infect. Dis. 2005, 41, 1373–1406. [Google Scholar] [CrossRef] [PubMed]
- Simonart, T. Group a beta-Haemolytic streptococcal necrotising fasciitis: Early diagnosis and clinical features. Dermatology 2004, 208, 5–9. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.S.; Lesher, L.; Schlievert, P.M.; Rogers, T.; Villaume, L.G.; Danila, R.; Lynfield, R. Staphylococcal toxic shock syndrome 2000–2006: Epidemiology, clinical features, and molecular characteristics. PLoS ONE 2011, 6, e22997. [Google Scholar] [CrossRef] [PubMed]
- Wharton, M.; Chorba, T.L.; Vogt, R.L.; Morse, D.L.; Buehler, J.W. Case definitions for public health surveillance. MMWR Recomm. Rep. 1990, 39, 273–279. [Google Scholar]
- Hajjeh, R.A.; Reingold, A.; Weil, A.; Shutt, K.; Schuchat, A.; Perkins, B.A. Toxic shock syndrome in the united states: Surveillance update, 1979–1996. Emerg. Infect. Dis. 1999, 5, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.; Roux, X.; Huttner, B.; Pugin, J. Streptococcal toxic shock syndrome in the intensive care unit. Ann. Intensive Care 2018, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Low, D.E. Toxic shock syndrome: Major advances in pathogenesis, but not treatment. Crit. Care Clin. 2013, 29, 651–675. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.; Cohen, J. Superantigens: Microbial agents that corrupt immunity. Lancet Infect. Dis. 2002, 2, 156–162. [Google Scholar] [CrossRef]
- Commons, R.J.; Smeesters, P.R.; Proft, T.; Fraser, J.D.; Robins-Browne, R.; Curtis, N. Streptococcal superantigens: Categorization and clinical associations. Trends Mol. Med. 2014, 20, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Tuffs, S.W.; Haeryfar, S.M.M.; McCormick, J.K. Manipulation of innate and adaptive immunity by staphylococcal superantigens. Pathogens 2018, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Bohach, G.A.; Nair, S.P.; Hiramatsu, K.; Jouvin-Marche, E.; Mariuzza, R. International Nomenclature Committee for Staphylococcal, S. Standard nomenclature for the superantigens expressed by staphylococcus. J. Infect. Dis. 2004, 189, 2334–2336. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.M.; Schoettle, D.J.; Watson, D.W. Purification and physicochemical and biological characterization of a staphylococcal pyrogenic exotoxin. Infect. Immun. 1979, 23, 609–617. [Google Scholar] [PubMed]
- Bohach, G.A.; Fast, D.J.; Nelson, R.D.; Schlievert, P.M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit. Rev. Microbiol. 1990, 17, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Marrack, P.; Kappler, J. The staphylococcal enterotoxins and their relatives. Science 1990, 248, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Siemens, N.; Norrby-Teglund, A. Shocking superantigens promote establishment of bacterial infection. Proc. Natl. Acad. Sci. USA 2017, 114, 10000–10002. [Google Scholar] [CrossRef] [PubMed]
- Arad, G.; Levy, R.; Nasie, I.; Hillman, D.; Rotfogel, Z.; Barash, U.; Supper, E.; Shpilka, T.; Minis, A.; Kaempfer, R. Binding of superantigen toxins into the cd28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011, 9, e1001149. [Google Scholar] [CrossRef]
- Levy, R.; Rotfogel, Z.; Hillman, D.; Popugailo, A.; Arad, G.; Supper, E.; Osman, F.; Kaempfer, R. Superantigens hyperinduce inflammatory cytokines by enhancing the b7-2/cd28 costimulatory receptor interaction. Proc. Natl. Acad. Sci. USA 2016, 113, E6437–E6446. [Google Scholar] [CrossRef]
- Chatila, T.; Geha, R.S. Signal transduction by microbial superantigens via mhc class ii molecules. Immunol. Rev. 1993, 131, 43–59. [Google Scholar] [CrossRef]
- Norrby-Teglund, A.; Thulin, P.; Gan, B.S.; Kotb, M.; McGeer, A.; Andersson, J.; Low, D.E. Evidence for superantigen involvement in severe group a streptococcal tissue infections. J. Infect. Dis. 2001, 184, 853–860. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Cahill, M.P.; Hostager, B.S.; Brosnahan, A.J.; Klingelhutz, A.J.; Gourronc, F.A.; Bishop, G.A.; Leung, D.Y.M. Staphylococcal superantigens stimulate epithelial cells through CD40 to produce chemokines. mBio 2019, 10, e00214-19. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Murayama, S.Y.; Sunaoshi, K.; Wajima, T.; Takahashi, M.; Masaki, J.; Kurokawa, I.; Ubukata, K. Nonhemolytic streptococcus pyogenes isolates that lack large regions of the sag operon mediating streptolysin s production. J. Clin. Microbiol. 2010, 48, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Higashi, D.L.; Biais, N.; Donahue, D.L.; Mayfield, J.A.; Tessier, C.R.; Rodriguez, K.; Ashfeld, B.L.; Luchetti, J.; Ploplis, V.A.; Castellino, F.J.; et al. Activation of band 3 mediates group a streptococcus streptolysin s-based beta-haemolysis. Nat. Microbiol. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Nizet, V.; Beall, B.; Bast, D.J.; Datta, V.; Kilburn, L.; Low, D.E.; De Azavedo, J.C. Genetic locus for streptolysin s production by group a streptococcus. Infect. Immun. 2000, 68, 4245–4254. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.M.; Cotter, P.D.; Hill, C.; Mitchell, D.A.; Ross, R.P. Streptolysin S-like virulence factors: The continuing saga. Nat. Rev. Microbiol. 2011, 9, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I. Is streptolysin s of group a streptococci a virulence factor? APMIS 1999, 107, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Bernheimer, A.W.; Schwartz, L.L. Lysosomal disruption by bacterial toxins. J. Bacteriol. 1964, 87, 1100–1104. [Google Scholar] [PubMed]
- Hryniewicz, W.; Pryjma, J. Effect of streptolysin s on human and mouse t and b lymphocytes. Infect. Immun. 1977, 16, 730–733. [Google Scholar]
- Keiser, H.; Weissmann, G.; Bernheimer, A.W. Studies on lysosomes. Iv. Solubilization of enzymes during mitochondrial swelling and disruption of lysosomes by streptolysin s and other hemolytic agents. J. Cell Biol. 1964, 22, 101–113. [Google Scholar] [CrossRef]
- Carr, A.; Sledjeski, D.D.; Podbielski, A.; Boyle, M.D.; Kreikemeyer, B. Similarities between complement-mediated and streptolysin S-Mediated hemolysis. J. Biol. Chem. 2001, 276, 41790–41796. [Google Scholar] [CrossRef]
- Betschel, S.D.; Borgia, S.M.; Barg, N.L.; Low, D.E.; De Azavedo, J.C. Reduced virulence of group a streptococcal TN916 mutants that do not produce streptolysin s. Infect. Immun. 1998, 66, 1671–1679. [Google Scholar] [PubMed]
- Sumitomo, T.; Nakata, M.; Higashino, M.; Jin, Y.; Terao, Y.; Fujinaga, Y.; Kawabata, S. Streptolysin s contributes to group a streptococcal translocation across an epithelial barrier. J. Biol. Chem. 2011, 286, 2750–2761. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, R.A.; Donahue, D.L.; Carothers, K.E.; Ross, J.N.; Ploplis, V.A.; Castellino, F.J.; Lee, S.W. Neutralization of streptolysin S-Dependent and independent inflammatory cytokine IL-1beta activity reduces pathology during early group a streptococcal skin infection. Front. Cell Infect. Microbiol. 2018, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Baddal, B.; Haarsma, R.; O’Seaghdha, M.; Yang, N.J.; Blake, K.J.; Portley, M.; Verri, W.A.; Dale, J.B.; Wessels, M.R.; et al. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 2018, 173, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Humar, D.; Datta, V.; Bast, D.J.; Beall, B.; De Azavedo, J.C.; Nizet, V. Streptolysin s and necrotising infections produced by group g streptococcus. Lancet 2002, 359, 124–129. [Google Scholar] [CrossRef]
- Miyoshi-Akiyama, T.; Takamatsu, D.; Koyanagi, M.; Zhao, J.; Imanishi, K.; Uchiyama, T. Cytocidal effect of streptococcus pyogenes on mouse neutrophils in vivo and the critical role of streptolysin s. J. Infect. Dis. 2005, 192, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bakleh, M.; Wold, L.E.; Mandrekar, J.N.; Harmsen, W.S.; Dimashkieh, H.H.; Baddour, L.M. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin. Infect. Dis. 2005, 40, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Linner, A.; Sunden-Cullberg, J.; Haggar, A.; Herwald, H.; Lore, K.; Treutiger, C.J.; Norrby-Teglund, A. Neutrophil-derived hyperresistinemia in severe acute streptococcal infections. J. Immunol. 2009, 183, 4047–4054. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Norrby-Teglund, A. Immunopathogenesis of streptococcal deep tissue infections. Curr. Top. Microbiol. Immunol. 2013, 368, 173–188. [Google Scholar] [PubMed]
- Datta, V.; Myskowski, S.M.; Kwinn, L.A.; Chiem, D.N.; Varki, N.; Kansal, R.G.; Kotb, M.; Nizet, V. Mutational analysis of the group a streptococcal operon encoding streptolysin s and its virulence role in invasive infection. Mol. Microbiol. 2005, 56, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Siemens, N.; Chakrakodi, B.; Shambat, S.M.; Morgan, M.; Bergsten, H.; Hyldegaard, O.; Skrede, S.; Arnell, P.; Madsen, M.B.; Johansson, L.; et al. Biofilm in group a streptococcal necrotizing soft tissue infections. JCI Insight 2016, 1, e87882. [Google Scholar] [CrossRef] [PubMed]
- Vajjala, A.; Biswas, D.; Tay, W.H.; Hanski, E.; Kline, K.A. Streptolysin-induced endoplasmic reticulum stress promotes group a streptococcal host-associated biofilm formation and necrotising fasciitis. Cell Microbiol. 2019, 21, e12956. [Google Scholar] [CrossRef] [PubMed]
- Barnett, T.C.; Cole, J.N.; Rivera-Hernandez, T.; Henningham, A.; Paton, J.C.; Nizet, V.; Walker, M.J. Streptococcal toxins: Role in pathogenesis and disease. Cell Microbiol. 2015, 17, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Keyel, P.A.; Roth, R.; Yokoyama, W.M.; Heuser, J.E.; Salter, R.D. Reduction of streptolysin o (slo) pore-forming activity enhances inflammasome activation. Toxins 2013, 5, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Timmer, A.M.; Timmer, J.C.; Pence, M.A.; Hsu, L.C.; Ghochani, M.; Frey, T.G.; Karin, M.; Salvesen, G.S.; Nizet, V. Streptolysin o promotes group a streptococcus immune evasion by accelerated macrophage apoptosis. J. Biol. Chem. 2009, 284, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Caparon, M.G. The nadase-negative variant of the streptococcus pyogenes toxin nad(+) glycohydrolase induces JNK1-Mediated programmed cellular necrosis. mBio 2016, 7, e02215-15. [Google Scholar] [CrossRef]
- Uchiyama, S.; Dohrmann, S.; Timmer, A.M.; Dixit, N.; Ghochani, M.; Bhandari, T.; Timmer, J.C.; Sprague, K.; Bubeck-Wardenburg, J.; Simon, S.I.; et al. Streptolysin o rapidly impairs neutrophil oxidative burst and antibacterial responses to group a streptococcus. Front. Immunol. 2015, 6, 581. [Google Scholar] [CrossRef]
- Sierig, G.; Cywes, C.; Wessels, M.R.; Ashbaugh, C.D. Cytotoxic effects of streptolysin O and streptolysin s enhance the virulence of poorly encapsulated group a streptococci. Infect. Immun. 2003, 71, 446–455. [Google Scholar] [CrossRef]
- Zhu, L.; Olsen, R.J.; Lee, J.D.; Porter, A.R.; DeLeo, F.R.; Musser, J.M. Contribution of secreted nadase and streptolysin o to the pathogenesis of epidemic serotype m1 streptococcus pyogenes infections. Am. J. Pathol. 2017, 187, 605–613. [Google Scholar] [CrossRef]
- Graham, M.R.; Smoot, L.M.; Migliaccio, C.A.; Virtaneva, K.; Sturdevant, D.E.; Porcella, S.F.; Federle, M.J.; Adams, G.J.; Scott, J.R.; Musser, J.M. Virulence control in group a streptococcus by a two-component gene regulatory system: Global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 2002, 99, 13855–13860. [Google Scholar] [CrossRef]
- Cole, J.N.; Barnett, T.C.; Nizet, V.; Walker, M.J. Molecular insight into invasive group a streptococcal disease. Nat. Rev. Microbiol. 2011, 9, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Hollands, A.; Sanderson-Smith, M.L.; Cole, J.N.; Kirk, J.K.; Henningham, A.; McArthur, J.D.; Dinkla, K.; Aziz, R.K.; Kansal, R.G.; et al. Dnase sda1 provides selection pressure for a switch to invasive group a streptococcal infection. Nat. Med. 2007, 13, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Sumby, P.; Whitney, A.R.; Graviss, E.A.; DeLeo, F.R.; Musser, J.M. Genome-Wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006, 2, e5. [Google Scholar] [CrossRef] [PubMed]
- Siemens, N.; Kittang, B.R.; Chakrakodi, B.; Oppegaard, O.; Johansson, L.; Bruun, T.; Mylvaganam, H.; Group, I.S.; Svensson, M.; Skrede, S.; et al. Increased cytotoxicity and streptolysin o activity in group g streptococcal strains causing invasive tissue infections. Sci. Rep. 2015, 5, 16945. [Google Scholar] [CrossRef]
- Johansson, L.; Thulin, P.; Sendi, P.; Hertzen, E.; Linder, A.; Akesson, P.; Low, D.E.; Agerberth, B.; Norrby-Teglund, A. Cathelicidin LL-37 in severe streptococcus pyogenes soft tissue infections in humans. Infect. Immun. 2008, 76, 3399–3404. [Google Scholar] [CrossRef] [PubMed]
- Love, J.F.; Tran-Winkler, H.J.; Wessels, M.R. Vitamin d and the human antimicrobial peptide ll-37 enhance group a streptococcus resistance to killing by human cells. mBio 2012, 3, e00394-12. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, J.; Rohde, M.; Siemens, N.; Kreikemeyer, B.; Bergman, P.; Johansson, L.; Norrby-Teglund, A. Ll-37 triggers formation of streptococcus pyogenes extracellular vesicle-like structures with immune stimulatory properties. J. Innate Immun. 2016, 8, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Grumann, D.; Nubel, U.; Broker, B.M. Staphylococcus aureus toxins-Their functions and genetics. Infect. Genet. Evol. 2014, 21, 583–592. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Kennedy, A.D.; Chen, L.; Bubeck Wardenburg, J.; Kobayashi, S.D.; Mathema, B.; Braughton, K.R.; Whitney, A.R.; Villaruz, A.E.; Martens, C.A.; et al. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2011, 108, 18091–18096. [Google Scholar] [CrossRef]
- Tavares, A.; Nielsen, J.B.; Boye, K.; Rohde, S.; Paulo, A.C.; Westh, H.; Schonning, K.; de Lencastre, H.; Miragaia, M. Insights into alpha-Hemolysin (hla) evolution and expression among staphylococcus aureus clones with hospital and community origin. PLoS ONE 2014, 9, e98634. [Google Scholar] [CrossRef] [PubMed]
- Berube, B.J.; Bubeck Wardenburg, J. Staphylococcus aureus alpha-toxin: Nearly a century of intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Valeva, A.; Hellmann, N.; Walev, I.; Strand, D.; Plate, M.; Boukhallouk, F.; Brack, A.; Hanada, K.; Decker, H.; Bhakdi, S. Evidence that clustered phosphocholine head groups serve as sites for binding and assembly of an oligomeric protein pore. J. Biol. Chem. 2006, 281, 26014–26021. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tomita, T.; Yasuda, T. Membrane-damaging action of staphylococcal alpha-toxin on phospholipid-Cholesterol liposomes. Biochim. Biophys. Acta 1987, 898, 257–265. [Google Scholar] [CrossRef]
- Hildebrand, A.; Pohl, M.; Bhakdi, S. Staphylococcus-aureus alpha-Toxin-Dual mechanism of binding to target-Cells. J. Biol. Chem. 1991, 266, 17195–17200. [Google Scholar] [PubMed]
- Wilke, G.A.; Bubeck Wardenburg, J. Role of a disintegrin and metalloprotease 10 in staphylococcus aureus alpha-hemolysin-Mediated cellular injury. Proc. Natl. Acad. Sci. USA 2010, 107, 13473–13478. [Google Scholar] [CrossRef] [PubMed]
- Colciaghi, F.; Borroni, B.; Pastorino, L.; Marcello, E.; Zimmermann, M.; Cattabeni, F.; Padovani, A.; Di Luca, M. [alpha]-secretase adam10 as well as [alpha]apps is reduced in platelets and csf of alzheimer disease patients. Mol. Med. 2002, 8, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Maretzky, T.; Reiss, K.; Ludwig, A.; Buchholz, J.; Scholz, F.; Proksch, E.; de Strooper, B.; Hartmann, D.; Saftig, P. Adam10 mediates e-cadherin shedding and regulates epithelial cell-Cell adhesion, migration, and beta-Catenin translocation. Proc. Natl. Acad. Sci. USA 2005, 102, 9182–9187. [Google Scholar] [CrossRef]
- Maretzky, T.; Scholz, F.; Koten, B.; Proksch, E.; Saftig, P.; Reiss, K. Adam10-Mediated e-Cadherin release is regulated by proinflammatory cytokines and modulates keratinocyte cohesion in eczematous dermatitis. J. Investig. Dermatol. 2008, 128, 1737–1746. [Google Scholar] [CrossRef]
- Schulz, B.; Pruessmeyer, J.; Maretzky, T.; Ludwig, A.; Blobel, C.P.; Saftig, P.; Reiss, K. Adam10 regulates endothelial permeability and t-cell transmigration by proteolysis of vascular endothelial cadherin. Circ. Res. 2008, 102, 1192–1201. [Google Scholar] [CrossRef]
- Hattori, M.; Osterfield, M.; Flanagan, J.G. Regulated cleavage of a contact-mediated axon repellent. Science 2000, 289, 1360–1365. [Google Scholar] [CrossRef]
- Pan, D.; Rubin, G.M. Kuzbanian controls proteolytic processing of notch and mediates lateral inhibition during drosophila and vertebrate neurogenesis. Cell 1997, 90, 271–280. [Google Scholar] [CrossRef]
- Reiss, K.; Maretzky, T.; Ludwig, A.; Tousseyn, T.; de Strooper, B.; Hartmann, D.; Saftig, P. Adam10 cleavage of n-Cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005, 24, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Inoshima, I.; Inoshima, N.; Wilke, G.A.; Powers, M.E.; Frank, K.M.; Wang, Y.; Bubeck Wardenburg, J. A staphylococcus aureus pore-forming toxin subverts the activity of adam10 to cause lethal infection in mice. Nat. Med. 2011, 17, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.P.; Erickson, S.N.; Gough, P.J.; Garton, K.J.; Wille, P.T.; Raines, E.W.; Dunbar, A.J.; Dempsey, P.J. Adam10 mediates ectodomain shedding of the betacellulin precursor activated by p-aminophenylmercuric acetate and extracellular calcium influx. J. Biol. Chem. 2005, 280, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Woodin, A.M. Fractionation of a leucocidin from staphylococcus aureus. Bioch. J. 1959, 73, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Miles, G.; Movileanu, L.; Bayley, H. Subunit composition of a bicomponent toxin: Staphylococcal leukocidin forms an octameric transmembrane pore. Protein Sci. 2002, 11, 894–902. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Liu, X.; Day, C.J.; Chumbler, N.M.; James, D.B.; Alonzo, F.; Bode, N.J.; Lacy, D.B.; Jennings, M.P.; et al. Identification of a crucial residue required for staphylococcus aureus lukab cytotoxicity and receptor recognition. Infect. Immun. 2014, 82, 1268–1276. [Google Scholar] [CrossRef]
- Ventura, C.L.; Malachowa, N.; Hammer, C.H.; Nardone, G.A.; Robinson, M.A.; Kobayashi, S.D.; DeLeo, F.R. Identification of a novel staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS ONE 2010, 5, e11634. [Google Scholar] [CrossRef]
- Spaan, A.N.; Schiepers, A.; de Haas, C.J.; van Hooijdonk, D.D.; Badiou, C.; Contamin, H.; Vandenesch, F.; Lina, G.; Gerard, N.P.; Gerard, C.; et al. Differential interaction of the staphylococcal toxins panton-valentine leukocidin and gamma-hemolysin cb with human c5a receptors. J. Immunol. 2015, 195, 1034–1043. [Google Scholar] [CrossRef]
- Koop, G.; Vrieling, M.; Storisteanu, D.M.; Lok, L.S.; Monie, T.; van Wigcheren, G.; Raisen, C.; Ba, X.; Gleadall, N.; Hadjirin, N.; et al. Identification of lukpq, a novel, equid-Adapted leukocidin of staphylococcus aureus. Sci. Rep. 2017, 7, 40660. [Google Scholar] [CrossRef]
- Rainard, P.; Corrales, J.C.; Barrio, M.B.; Cochard, T.; Poutrel, B. Leucotoxic activities of staphylococcus aureus strains isolated from cows, ewes, and goats with mastitis: Importance of lukM/lukF’-PV leukotoxin. Clin. Diagn. Lab. Immunol. 2003, 10, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Gieldon, L.; Mysore, V.; Nippe, N.; Taxman, D.J.; Duncan, J.A.; Broglie, P.M.; Marketon, K.; Austermann, J.; Vogl, T.; et al. Staphylococcus aureus panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the nlrp3 inflammasome. J. Leukoc. Biol. 2012, 92, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and atp. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Melehani, J.H.; James, D.B.; DuMont, A.L.; Torres, V.J.; Duncan, J.A. Staphylococcus aureus leukocidin a/b (lukab) kills human monocytes via host nlrp3 and asc when extracellular, but not intracellular. PLoS Pathog. 2015, 11, e1004970. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Planillo, R.; Franchi, L.; Miller, L.S.; Nunez, G. A critical role for hemolysins and bacterial lipoproteins in staphylococcus aureus-Induced activation of the Nlrp3 inflammasome. J. Immunol. 2009, 183, 3942–3948. [Google Scholar] [CrossRef] [PubMed]
- Perret, M.; Badiou, C.; Lina, G.; Burbaud, S.; Benito, Y.; Bes, M.; Cottin, V.; Couzon, F.; Juruj, C.; Dauwalder, O.; et al. Cross-Talk between staphylococcus aureus leukocidins-Intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell Microbiol. 2012, 14, 1019–1036. [Google Scholar] [CrossRef]
- Graves, S.F.; Kobayashi, S.D.; Braughton, K.R.; Whitney, A.R.; Sturdevant, D.E.; Rasmussen, D.L.; Kirpotina, L.N.; Quinn, M.T.; DeLeo, F.R. Sublytic concentrations of staphylococcus aureus panton-Valentine leukocidin alter human pmn gene expression and enhance bactericidal capacity. J. Leukoc. Biol. 2012, 92, 361–374. [Google Scholar] [CrossRef]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.M.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.C.; van Kessel, K.P.M.; Vandenesch, F.; et al. The staphylococcal toxin panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 2013, 13, 584–594. [Google Scholar]
- Malachowa, N.; Kobayashi, S.D.; Freedman, B.; Dorward, D.W.; DeLeo, F.R. Staphylococcus aureus leukotoxin gh promotes formation of neutrophil extracellular traps. J. Immunol. 2013, 191, 6022–6029. [Google Scholar]
- Kaneko, J.; Kimura, T.; Kawakami, Y.; Tomita, T.; Kamio, Y. Panton-Valentine leukocidin genes in a phage-like particle isolated from mitomycin c-Treated staphylococcus aureus v8 (atcc 49775). Biosci. Biotechnol. Biochem. 1997, 61, 1960–1962. [Google Scholar] [CrossRef]
- Naimi, T.S.; LeDell, K.H.; Como-Sabetti, K.; Borchardt, S.M.; Boxrud, D.J.; Etienne, J.; Johnson, S.K.; Vandenesch, F.; Fridkin, S.; O’Boyle, C.; et al. Comparison of community-and health care-Associated methicillin-resistant staphylococcus aureus infection. JAMA 2003, 290, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Vandenesch, F.; Naimi, T.; Enright, M.C.; Lina, G.; Nimmo, G.R.; Heffernan, H.; Liassine, N.; Bes, M.; Greenland, T.; Reverdy, M.E.; et al. Community-Acquired methicillin-Resistant staphylococcus aureus carrying panton-valentine leukocidin genes: Worldwide emergence. Emerg. Infect. Dis. 2003, 9, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Piemont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of panton-valentine leukocidin-Producing staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Shallcross, L.J.; Fragaszy, E.; Johnson, A.M.; Hayward, A.C. The role of the panton-valentine leucocidin toxin in staphylococcal disease: A systematic review and meta-Analysis. Lancet Infect. Dis. 2013, 13, 43–54. [Google Scholar] [CrossRef]
- Shukla, S.K.; Karow, M.E.; Brady, J.M.; Stemper, M.E.; Kislow, J.; Moore, N.; Wroblewski, K.; Chyou, P.H.; Warshauer, D.M.; Reed, K.D.; et al. Virulence genes and genotypic associations in nasal carriage, community-associated methicillin-Susceptible and methicillin-Resistant USA400 staphylococcus aureus isolates. J. Clin. Microbiol. 2010, 48, 3582–3592. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, F.; Torres, V.J. The bicomponent pore-forming leucocidins of staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2014, 78, 199–230. [Google Scholar] [CrossRef] [PubMed]
- Lipinska, U.; Hermans, K.; Meulemans, L.; Dumitrescu, O.; Badiou, C.; Duchateau, L.; Haesebrouck, F.; Etienne, J.; Lina, G. Panton-Valentine leukocidin does play a role in the early stage of staphylococcus aureus skin infections: A rabbit model. PLoS ONE 2011, 6, e22864. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; Whitney, A.R.; Braughton, K.R.; Gardner, D.J.; Long, D.; Bubeck Wardenburg, J.; Schneewind, O.; Otto, M.; Deleo, F.R. Comparative analysis of usa300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 2011, 204, 937–941. [Google Scholar] [CrossRef]
- Tromp, A.T.; Van Gent, M.; Abrial, P.; Martin, A.; Jansen, J.P.; De Haas, C.J.C.; Van Kessel, K.P.M.; Bardoel, B.W.; Kruse, E.; Bourdonnay, E.; et al. Human CD45 is an f-Component-Specific receptor for the staphylococcal toxin panton-valentine leukocidin. Nat. Microbiol. 2018, 3, 708–717. [Google Scholar] [CrossRef]
- Hermiston, M.L.; Xu, Z.; Weiss, A. Cd45: A critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003, 21, 107–137. [Google Scholar] [CrossRef]
- Dozois, A.; Thomsen, I.; Jimenez-Truque, N.; Soper, N.; Pearson, A.; Mohamed-Rambaran, P.; Dettorre, K.B.; Creech, C.B.; Wright, S.W. Prevalence and molecular characteristics of methicillin-Resistant staphylococcus aureus among skin and soft tissue infections in an emergency department in guyana. Emerg. Med. J. 2015, 32, 800–803. [Google Scholar] [CrossRef]
- Dumont, A.L.; Nygaard, T.K.; Watkins, R.L.; Smith, A.; Kozhaya, L.; Kreiswirth, B.N.; Shopsin, B.; Unutmaz, D.; Voyich, J.M.; Torres, V.J. Characterization of a new cytotoxin that contributes to staphylococcus aureus pathogenesis. Mol. Microbiol. 2011, 79, 814–825. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F.; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus lukab cytotoxin kills human neutrophils by targeting the cd11b subunit of the integrin mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [CrossRef]
- Alonzo, F.; Kozhaya, L.; Rawlings, S.A.; Reyes-Robles, T.; DuMont, A.L.; Myszka, D.G.; Landau, N.R.; Unutmaz, D.; Torres, V.J. Ccr5 is a receptor for staphylococcus aureus leukotoxin ed. Nature 2013, 493, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Robles, T.; Alonzo, F.; Kozhaya, L.; Lacy, D.B.; Unutmaz, D.; Torres, V.J. Staphylococcus aureus leukotoxin ed targets the chemokine receptors cxcr1 and cxcr2 to kill leukocytes and promote infection. Cell Host Microbe 2013, 14, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Reyes-Robles, T.; Badiou, C.; Cochet, S.; Boguslawski, K.M.; Yoong, P.; Day, C.J.; de Haas, C.J.; van Kessel, K.P.; Vandenesch, F.; et al. Staphylococcus aureus targets the duffy antigen receptor for chemokines (darc) to lyse erythrocytes. Cell Host Microbe 2015, 18, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Prevost, G.; Cribier, B.; Couppie, P.; Petiau, P.; Supersac, G.; Finck-Barbancon, V.; Monteil, H.; Piemont, Y. Panton-Valentine leucocidin and gamma-Hemolysin from staphylococcus aureus atcc 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 1995, 63, 4121–4129. [Google Scholar]
- Peacock, S.J.; Moore, C.E.; Justice, A.; Kantzanou, M.; Story, L.; Mackie, K.; O’Neill, G.; Day, N.P. Virulent combinations of adhesin and toxin genes in natural populations of staphylococcus aureus. Infect. Immun. 2002, 70, 4987–4996. [Google Scholar] [CrossRef]
- Fackrell, H.B.; Wiseman, G.M. Properties of the gamma haemolysin of staphylococcus aureus ‘smith 5r’. J. Gen. Microbiol. 1976, 92, 11–24. [Google Scholar] [CrossRef]
- Fackrell, H.B.; Wiseman, G.M. Production and purification of the gamma haemolysin of staphylococcus aureus ‘smith 5r’. J. Gen. Microbiol. 1976, 92, 1–10. [Google Scholar] [CrossRef]
- Spaan, A.N.; Vrieling, M.; Wallet, P.; Badiou, C.; Reyes-Robles, T.; Ohneck, E.A.; Benito, Y.; de Haas, C.J.; Day, C.J.; Jennings, M.P.; et al. The staphylococcal toxins gamma-Haemolysin ab and cb differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 2014, 5, 5438. [Google Scholar] [CrossRef] [PubMed]
- Mehlin, C.; Headley, C.M.; Klebanoff, S.J. An inflammatory polypeptide complex from staphylococcus epidermidis: Isolation and characterization. J. Exp. Med. 1999, 189, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.H.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinants for community-Associated mrsa. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Diep, B.A.; Villaruz, A.E.; Braughton, K.R.; Jiang, X.; DeLeo, F.R.; Chambers, H.F.; Lu, Y.; Otto, M. Evolution of virulence in epidemic community-Associated methicillin-resistant staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2009, 106, 5883–5888. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Otto, M. Phenol-Soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, D.; Gleske, A.K.; Rautenberg, M.; Wang, R.; Koberle, M.; Bohn, E.; Schoneberg, T.; Rabiet, M.J.; Boulay, F.; Klebanoff, S.J.; et al. Human formyl peptide receptor 2 senses highly pathogenic staphylococcus aureus. Cell Host Microbe 2010, 7, 463–473. [Google Scholar] [CrossRef]
- Cheung, G.Y.; Duong, A.C.; Otto, M. Direct and synergistic hemolysis caused by staphylococcus phenol-soluble modulins: Implications for diagnosis and pathogenesis. Microbes Infect. 2012, 14, 380–386. [Google Scholar] [CrossRef]
- Rasigade, J.P.; Trouillet-Assant, S.; Ferry, T.; Diep, B.A.; Sapin, A.; Lhoste, Y.; Ranfaing, J.; Badiou, C.; Benito, Y.; Bes, M.; et al. Psms of hypervirulent staphylococcus aureus act as intracellular toxins that kill infected osteoblasts. PLoS ONE 2013, 8, e63176. [Google Scholar] [CrossRef]
- Geiger, T.; Francois, P.; Liebeke, M.; Fraunholz, M.; Goerke, C.; Krismer, B.; Schrenzel, J.; Lalk, M.; Wolz, C. The stringent response of staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular psms expression. PLoS Pathog. 2012, 8, e1003016. [Google Scholar] [CrossRef]
- Surewaard, B.G.; de Haas, C.J.; Vervoort, F.; Rigby, K.M.; DeLeo, F.R.; Otto, M.; van Strijp, J.A.; Nijland, R. Staphylococcal alpha-Phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013, 15, 1427–1437. [Google Scholar] [CrossRef]
- Li, L.; Pian, Y.; Chen, S.; Hao, H.; Zheng, Y.; Zhu, L.; Xu, B.; Liu, K.; Li, M.; Jiang, H.; et al. Phenol-soluble modulin alpha4 mediates staphylococcus aureus-Associated vascular leakage by stimulating heparin-Binding protein release from neutrophils. Sci. Rep. 2016, 6, 29373. [Google Scholar] [CrossRef] [PubMed]
- Hodille, E.; Cuerq, C.; Badiou, C.; Bienvenu, F.; Steghens, J.P.; Cartier, R.; Bes, M.; Tristan, A.; Plesa, A.; Le, V.T.; et al. Delta hemolysin and phenol-soluble modulins, but not alpha hemolysin or panton-valentine leukocidin, induce mast cell activation. Front. Cell Infect. Microbiol. 2016, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Armbruster, N.S.; Gunter, M.; Henes, J.; Autenrieth, S.E. Staphylococcus aureus psm peptides modulate human monocyte-derived dendritic cells to prime regulatory t cells. Front. Immunol. 2018, 9, 2603. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Matsumoto, M.; Katayama, Y.; Oguma, R.; Wakabayashi, S.; Nygaard, T.; Saijo, S.; Inohara, N.; Otto, M.; Matsue, H.; et al. Staphylococcus aureus virulent psmalpha peptides induce keratinocyte alarmin release to orchestrate IL-17-Dependent skin inflammation. Cell Host Microbe 2017, 22, 667–677.e5. [Google Scholar] [CrossRef] [PubMed]
- Mairpady Shambat, S.; Chen, P.; Nguyen Hoang, A.T.; Bergsten, H.; Vandenesch, F.; Siemens, N.; Lina, G.; Monk, I.R.; Foster, T.J.; Arakere, G.; et al. Modelling staphylococcal pneumonia in a human 3d lung tissue model system delineates toxin-mediated pathology. Dis. Model. Mech. 2015, 8, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, A.J.; Mantz, M.J.; Squier, C.A.; Peterson, M.L.; Schlievert, P.M. Cytolysins augment superantigen penetration of stratified mucosa. J. Immunol. 2009, 182, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Gillman, A.N.; Breshears, L.M.; Kistler, C.K.; Finnegan, P.M.; Torres, V.J.; Schlievert, P.M.; Peterson, M.L. Epidermal growth factor receptor signaling enhances the proinflammatory effects of staphylococcus aureus gamma-toxin on the mucosa. Toxins 2017, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.D. A proteolytic enzyme produced by group a streptococci with special reference to its effect on the type-specific m antigen. J. Exp. Med. 1945, 81, 573–592. [Google Scholar] [CrossRef]
- Yu, C.E.; Ferretti, J.J. Frequency of the erythrogenic toxin-B and toxin-C genes (speb and spec) among clinical isolates of group-a streptococci. Infect. Immun. 1991, 59, 211–215. [Google Scholar]
- Liu, T.Y.; Elliott, S.D. Streptococcal proteinase: The zymogen to enzyme transfromation. J. Biol. Chem. 1965, 240, 1138–1142. [Google Scholar]
- Nyberg, P.; Rasmussen, M.; Von Pawel-Rammingen, U.; Bjorck, L. Speb modulates fibronectin-Dependent internalization of streptococcus pyogenes by efficient proteolysis of cell-Wall-Anchored protein F1. Microbiology 2004, 150, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Raeder, R.; Woischnik, M.; Podbielski, A.; Boyle, M.D.P. A secreted streptococcal cysteine protease can cleave a surface-Expressed M1 protein and alter the immunoglobulin binding properties. Res. Microbiol. 1998, 149, 539–548. [Google Scholar] [CrossRef]
- Wexler, D.E.; Chenoweth, D.E.; Cleary, P.P. Mechanism of action of the group-a streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 1985, 82, 8144–8148. [Google Scholar] [CrossRef] [PubMed]
- Allhorn, M.; Olsen, A.; Collin, M. Endos from streptococcus pyogenes is hydrolyzed by the cysteine proteinase speb and requires glutamic acid 235 and tryptophans for igg glycan-Hydrolyzing activity. BMC Microbiol. 2008, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Pabst, M.J.; Jeng, A.; Kansal, R.; Low, D.E.; Nizet, V.; Kotb, M. Invasive m1t1 group a streptococcus undergoes a phase-Shift in vivo to prevent proteolytic degradation of multiple virulence factors by speb. Mol. Microbiol. 2004, 51, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.N.; McArthur, J.D.; McKay, F.C.; Sanderson-Smith, M.L.; Cork, A.J.; Ranson, M.; Rohde, M.; Itzek, A.; Sun, H.; Ginsburg, D.; et al. Trigger for group a streptococcal m1t1 invasive disease. FASEB J. 2006, 20, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Pinkney, M.; Kapur, V.; Smith, J.; Weller, U.; Palmer, M.; Glanville, M.; Messner, M.; Musser, J.M.; Bhakdi, S.; Kehoe, M.A. Different forms of streptolysin o produced by streptococcus pyogenes and by escherichia coli expressing recombinant toxin: Cleavage by streptococcal cysteine protease. Infect. Immun. 1995, 63, 2776–2779. [Google Scholar] [PubMed]
- Collin, M.; Olsen, A. Effect of speb and endos from streptococcus pyogenes on human immunoglobulins. Infect. Immun. 2001, 69, 7187–7189. [Google Scholar] [CrossRef]
- Collin, M.; Svensson, M.D.; Sjoholm, A.G.; Jensenius, J.C.; Sjobring, U.; Olsen, A. Endos and speb from streptococcus pyogenes inhibit immunoglobulin-Mediated opsonophagocytosis. Infect. Immun. 2002, 70, 6646–6651. [Google Scholar] [CrossRef]
- Kuo, C.F.; Lin, Y.S.; Chuang, W.J.; Wu, J.J.; Tsao, N. Degradation of complement 3 by streptococcal pyrogenic exotoxin b inhibits complement activation and neutrophil opsonophagocytosis. Infect. Immun. 2008, 76, 1163–1169. [Google Scholar] [CrossRef]
- Terao, Y.; Mori, Y.; Yamaguchi, M.; Shimizu, Y.; Ooe, K.; Hamada, S.; Kawabata, S. Group a streptococcal cysteine protease degrades c3 (c3b) and contributes to evasion of innate immunity. J. Biol. Chem. 2008, 283, 6253–6260. [Google Scholar] [CrossRef] [PubMed]
- Egesten, A.; Olin, A.I.; Linge, H.M.; Yadav, M.; Morgelin, M.; Karlsson, A.; Collin, M. Speb of streptococcus pyogenes differentially modulates antibacterial and receptor activating properties of human chemokines. PLoS ONE 2009, 4, e4769. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.; Majesky, M.W.; Li, L.L.; Black, R.A.; Musser, J.M. Cleavage of interleukin 1 beta (il-1 beta) precursor to produce active IL-1 beta by a conserved extracellular cysteine protease from streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 1993, 90, 7676–7680. [Google Scholar] [CrossRef] [PubMed]
- LaRock, C.N.; Todd, J.; LaRock, D.L.; Olson, J.; O’Donoghue, A.J.; Robertson, A.A.B.; Cooper, M.A.; Hoffman, H.M.; Nizet, V. IL-1beta is an innate immune sensor of microbial proteolysis. Sci. Immunol. 2016, 1, eaah3539. [Google Scholar] [CrossRef] [PubMed]
- Chella Krishnan, K.; Mukundan, S.; Alagarsamy, J.; Hur, J.; Nookala, S.; Siemens, N.; Svensson, M.; Hyldegaard, O.; Norrby-Teglund, A.; Kotb, M. Genetic architecture of group a streptococcal necrotizing soft tissue infections in the mouse. PLoS Pathog. 2016, 12, e1005732. [Google Scholar] [CrossRef] [PubMed]
- Matsuka, Y.V.; Pillai, S.; Gubba, S.; Musser, J.M.; Olmsted, S.B. Fibrinogen cleavage by the streptococcus pyogenes extracellular cysteine protease and generation of antibodies that inhibit enzyme proteolytic activity. Infect. Immun. 1999, 67, 4326–4333. [Google Scholar] [PubMed]
- Svensson, M.D.; Sjobring, U.; Luo, F.; Bessen, D.E. Roles of the plasminogen activator streptokinase and the plasminogen-associated m protein in an experimental model for streptococcal impetigo. Microbiology 2002, 148, 3933–3945. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.; Topouzis, S.; Majesky, M.W.; Li, L.L.; Hamrick, M.R.; Hamill, R.J.; Patti, J.M.; Musser, J.M. A conserved streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb. Pathog. 1993, 15, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Tamura, F.; Nakagawa, R.; Akuta, T.; Okamoto, S.; Hamada, S.; Maeda, H.; Kawabata, S.; Akaike, T. Proapoptotic effect of proteolytic activation of matrix metalloproteinases by streptococcus pyogenes thiol proteinase (streptococcus pyrogenic exotoxin b). Infect. Immun. 2004, 72, 4836–4847. [Google Scholar] [CrossRef] [PubMed]
- Darenberg, J.; Luca-Harari, B.; Jasir, A.; Sandgren, A.; Pettersson, H.; Schalen, C.; Norgren, M.; Romanus, V.; Norrby-Teglund, A.; Normark, B.H. Molecular and clinical characteristics of invasive group a streptococcal infection in sweden. Clin. Infect. Dis. 2007, 45, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Luca-Harari, B.; Darenberg, J.; Neal, S.; Siljander, T.; Strakova, L.; Tanna, A.; Creti, R.; Ekelund, K.; Koliou, M.; Tassios, P.T.; et al. Clinical and microbiological characteristics of severe streptococcus pyogenes disease in europe. J. Clin. Microbiol. 2009, 47, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Gubba, S.; Low, D.E.; Musser, J.M. Expression and characterization of group a streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human invasive disease episodes. Infect. Immun. 1998, 66, 765–770. [Google Scholar] [PubMed]
- Kansal, R.G.; McGeer, A.; Low, D.E.; Norrby-Teglund, A.; Kotb, M. Inverse relation between disease severity and expression of the streptococcal cysteine protease, speb, among clonal m1t1 isolates recovered from invasive group a streptococcal infection cases. Infect. Immun. 2000, 68, 6362–6369. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.E.; Norrby, A.; Bergholm, A.M.; Norgren, M. Aspects of pathogenesis of serious group—A streptococcal infections in sweden, 1988–1989. J. Infect. Dis. 1992, 166, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.; Wu, J.J.; Lin, K.Y.; Tsai, P.J.; Lee, S.C.; Jin, Y.T.; Lei, H.Y.; Lin, Y.S. Role of streptococcal pyrogenic exotoxin b in the mouse model of group a streptococcal infection. Infect. Immun. 1998, 66, 3931–3935. [Google Scholar]
- Lukomski, S.; Burns, E.H., Jr.; Wyde, P.R.; Podbielski, A.; Rurangirwa, J.; Moore-Poveda, D.K.; Musser, J.M. Genetic inactivation of an extracellular cysteine protease (speb) expressed by streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect. Immun. 1998, 66, 771–776. [Google Scholar]
- Lukomski, S.; Montgomery, C.A.; Rurangirwa, J.; Geske, R.S.; Barrish, J.P.; Adams, G.J.; Musser, J.M. Extracellular cysteine protease produced by streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 1999, 67, 1779–1788. [Google Scholar]
- Lukomski, S.; Sreevatsan, S.; Amberg, C.; Reichardt, W.; Woischnik, M.; Podbielski, A.; Musser, J.M. Inactivation of streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype m3 and m49 strains. J. Clin. Investig. 1997, 99, 2574–2580. [Google Scholar] [CrossRef]
- Ashbaugh, C.D.; Warren, H.B.; Carey, V.J.; Wessels, M.R. Molecular analysis of the role of the group a streptococcal cysteine protease, hyaluronic acid capsule, and m protein in a murine model of human invasive soft-tissue infection. J. Clin. Investig. 1998, 102, 550–560. [Google Scholar] [CrossRef]
- Ashbaugh, C.D.; Wessels, M.R. Absence of a cysteine protease effect on bacterial virulence in two murine models of human invasive group a streptococcal infection. Infect. Immun. 2001, 69, 6683–6688. [Google Scholar] [CrossRef]
- Von Pawel-Rammingen, U.; Johansson, B.P.; Bjorck, L. Ides, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin g. EMBO J. 2002, 21, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Von Pawel-Rammingen, U.; Johansson, B.P.; Tapper, H.; Bjorck, L. Streptococcus pyogenes and phagocytic killing. Nat. Med. 2002, 8, 1044–1045; author reply 1045–1046. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.P.; Cleary, P.P. Localization of the streptococcal C5a peptidase to the surface of group a streptococci. Infect. Immun. 1986, 53, 432–434. [Google Scholar] [PubMed]
- Cleary, P.P.; Prahbu, U.; Dale, J.B.; Wexler, D.E.; Handley, J. Streptococcal c5a peptidase is a highly specific endopeptidase. Infect. Immun. 1992, 60, 5219–5223. [Google Scholar] [PubMed]
- Ji, Y.D.; Carlson, B.; Kondagunta, A.; Cleary, P.P. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group a streptococcus. Infect. Immun. 1997, 65, 2080–2087. [Google Scholar] [PubMed]
- Lynskey, N.N.; Reglinski, M.; Calay, D.; Siggins, M.K.; Mason, J.C.; Botto, M.; Sriskandan, S. Multi-Functional mechanisms of immune evasion by the streptococcal complement inhibitor c5a peptidase. PLoS Pathog. 2017, 13, e1006493. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Grass, C.; Dan-Goor, M.; Maly, A.; Eran, Y.; Kwinn, L.A.; Nizet, V.; Ravins, M.; Jaffe, J.; Peyser, A.; Moses, A.E.; et al. Effect of a bacterial pheromone peptide on host chemokine degradation in group a streptococcal necrotising soft-Tissue infections. Lancet 2004, 363, 696–703. [Google Scholar] [CrossRef]
- Hidalgo-Grass, C.; Mishalian, I.; Dan-Goor, M.; Belotserkovsky, I.; Eran, Y.; Nizet, V.; Peled, A.; Hanski, E. A streptococcal protease that degrades cxc chemokines and impairs bacterial clearance from infected tissues. EMBO J. 2006, 25, 4628–4637. [Google Scholar] [CrossRef]
- Zinkernagel, A.S.; Timmer, A.M.; Pence, M.A.; Locke, J.B.; Buchanan, J.T.; Turner, C.E.; Mishalian, I.; Sriskandan, S.; Hanski, E.; Nizet, V. The il-8 protease spycep/scpc of group a streptococcus promotes resistance to neutrophil killing. Cell Host Microbe 2008, 4, 170–178. [Google Scholar] [CrossRef]
- Sumby, P.; Zhang, S.; Whitney, A.R.; Falugi, F.; Grandi, G.; Graviss, E.A.; Deleo, F.R.; Musser, J.M. A chemokine-degrading extracellular protease made by group a streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect. Immun. 2008, 76, 978–985. [Google Scholar] [CrossRef]
- Chiappini, N.; Seubert, A.; Telford, J.L.; Grandi, G.; Serruto, D.; Margarit, I.; Janulczyk, R. Streptococcus pyogenes spycep influences host-Pathogen interactions during infection in a murine air pouch model. PLoS ONE 2012, 7, e40411. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, F.; Ogawa, T.; Ogawa, M.; Madon, J.; Uchiyama, S.; Schuepbach, R.A.; Zinkernagel, A.S. The il-8 protease spycep is detrimental for group a streptococcus host-Cells interaction and biofilm formation. Front. Microbiol. 2014, 5, 339. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.C.; Ruiz, N.; Caparon, M. Cytolysin-mediated translocation (cmt): A functional equivalent of type iii secretion in gram-Positive bacteria. Cell 2001, 104, 143–152. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Caparon, M.G. The streptococcus pyogenes nad(+) glycohydrolase modulates epithelial cell parylation and hmgb1 release. Cell Microbiol. 2015, 17, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Velarde, J.J.; O’Seaghdha, M.; Baddal, B.; Bastiat-Sempe, B.; Wessels, M.R. Binding of nad(+)-glycohydrolase to streptolysin o stabilizes both toxins and promotes virulence of group a streptococcus. mBio 2017, 8, e01382-17. [Google Scholar] [CrossRef] [PubMed]
- Coye, L.H.; Collins, C.M. Identification of spya, a novel adp-Ribosyltransferase of streptococcus pyogenes. Mol. Microbiol. 2004, 54, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hoff, J.S.; DeWald, M.; Moseley, S.L.; Collins, C.M.; Voyich, J.M. Spya, a C3-like adp-Ribosyltransferase, contributes to virulence in a mouse subcutaneous model of streptococcus pyogenes infection. Infect. Immun. 2011, 79, 2404–2411. [Google Scholar] [CrossRef]
- Korotkova, N.; Hoff, J.S.; Becker, D.M.; Quinn, J.K.H.; Icenogle, L.M.; Moseley, S.L. Spya is a membrane-bound adp-ribosyltransferase of streptococcus pyogenes which modifies a streptococcal peptide, spyb. Mol. Microbiol. 2012, 83, 936–952. [Google Scholar] [CrossRef]
- Lin, A.E.; Beasley, F.C.; Keller, N.; Hollands, A.; Urbano, R.; Troemel, E.R.; Hoffman, H.M.; Nizet, V. A group a streptococcus adp-Ribosyltransferase toxin stimulates a protective interleukin 1beta-Dependent macrophage immune response. mBio 2015, 6, e00133. [Google Scholar] [CrossRef]
- Hynes, W.; Sloan, M. Secreted extracellular virulence factors. In Streptococcus Pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma, OK, USA, 2016. [Google Scholar]
- Kalia, A.; Bessen, D.E. Natural selection and evolution of streptococcal virulence genes involved in tissue-specific adaptations. J. Bacteriol. 2004, 186, 110–121. [Google Scholar] [CrossRef]
- Chandrahas, V.; Glinton, K.; Liang, Z.; Donahue, D.L.; Ploplis, V.A.; Castellino, F.J. Direct host plasminogen binding to bacterial surface m-protein in pattern d strains of streptococcus pyogenes is required for activation by its natural coinherited SK2b protein. J. Biol. Chem. 2015, 290, 18833–18842. [Google Scholar] [CrossRef] [PubMed]
- Siemens, N.; Patenge, N.; Otto, J.; Fiedler, T.; Kreikemeyer, B. Streptococcus pyogenes M49 plasminogen/plasmin binding facilitates keratinocyte invasion via integrin-integrin-linked kinase (ILK) pathways and protects from macrophage killing. J. Biol. Chem. 2011, 286, 21612–21622. [Google Scholar] [CrossRef] [PubMed]
- Boxrud, P.D.; Bock, P.E. Coupling of conformational and proteolytic activation in the kinetic mechanism of plasminogen activation by streptokinase. J. Biol. Chem. 2004, 279, 36642–36649. [Google Scholar] [CrossRef] [PubMed]
- Boxrud, P.D.; Verhamme, I.M.; Bock, P.E. Resolution of conformational activation in the kinetic mechanism of plasminogen activation by streptokinase. J. Biol. Chem. 2004, 279, 36633–36641. [Google Scholar] [CrossRef] [PubMed]
- Khil, J.; Im, M.; Heath, A.; Ringdahl, U.; Mundada, L.; Cary Engleberg, N.; Fay, W.P. Plasminogen enhances virulence of group a streptococci by streptokinase-dependent and streptokinase-independent mechanisms. J. Infect. Dis. 2003, 188, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ringdahl, U.; Homeister, J.W.; Fay, W.P.; Engleberg, N.C.; Yang, A.Y.; Rozek, L.S.; Wang, X.; Sjobring, U.; Ginsburg, D. Plasminogen is a critical host pathogenicity factor for group a streptococcal infection. Science 2004, 305, 1283–1286. [Google Scholar] [CrossRef]
- Shaw, L.; Golonka, E.; Potempa, J.; Foster, S.J. The role and regulation of the extracellular proteases of staphylococcus aureus. Microbiology 2004, 150, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, G.R. Role of metalloprotease in activation of the precursor of staphylococcal protease. J. Bacteriol. 1978, 136, 607–613. [Google Scholar] [PubMed]
- Ohbayashi, T.; Irie, A.; Murakami, Y.; Nowak, M.; Potempa, J.; Nishimura, Y.; Shinohara, M.; Imamura, T. Degradation of fibrinogen and collagen by staphopains, cysteine proteases released from staphylococcus aureus. Microbiology 2011, 157, 786–792. [Google Scholar] [CrossRef]
- Potempa, J.; Dubin, A.; Korzus, G.; Travis, J. Degradation of elastin by a cysteine proteinase from staphylococcus aureus. J. Biol. Chem. 1988, 263, 2664–2667. [Google Scholar]
- Laarman, A.J.; Mijnheer, G.; Mootz, J.M.; van Rooijen, W.J.; Ruyken, M.; Malone, C.L.; Heezius, E.C.; Ward, R.; Milligan, G.; van Strijp, J.A.; et al. Staphylococcus aureus staphopain a inhibits CXCR2-Dependent neutrophil activation and chemotaxis. EMBO J. 2012, 31, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Smagur, J.; Guzik, K.; Magiera, L.; Bzowska, M.; Gruca, M.; Thogersen, I.B.; Enghild, J.J.; Potempa, J. A new pathway of staphylococcal pathogenesis: Apoptosis-like death induced by staphopain B in human neutrophils and monocytes. J. Innate. Immun. 2009, 1, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Smagur, J.; Guzik, K.; Bzowska, M.; Kuzak, M.; Zarebski, M.; Kantyka, T.; Walski, M.; Gajkowska, B.; Potempa, J. Staphylococcal cysteine protease staphopain b (sspb) induces rapid engulfment of human neutrophils and monocytes by macrophages. Biol. Chem. 2009, 390, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Prokesova, L.; Potuznikova, B.; Potempa, J.; Zikan, J.; Radl, J.; Hachova, L.; Baran, K.; Porwit-Bobr, Z.; John, C. Cleavage of human immunoglobulins by serine proteinase from staphylococcus aureus. Immunol. Lett. 1992, 31, 259–265. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Takai, T.; Nakamura, T.; Mitsuishi, K.; Gunawan, H.; Suto, H.; Ogawa, T.; Wang, X.L.; Ikeda, S.; Okumura, K.; et al. Staphylococcus aureus extracellular protease causes epidermal barrier dysfunction. J. Investig. Dermatol. 2010, 130, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.L.; Prachi, P.; Minasov, G.; Shuvalova, L.; Ruan, J.; Dubrovska, I.; Winsor, J.; Giraldi, M.; Biagini, M.; Liberatori, S.; et al. Structure and protective efficacy of the staphylococcus aureus autocleaving protease epip. FASEB J. 2014, 28, 1780–1793. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Nobile, G.; Rindi, S.; Speziale, P. Staphylococcus aureus manipulates innate immunity through own and host-Expressed proteases. Front. Cell Infect. Microbiol. 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Schmidt, J.J.; Johnson-Winegar, A.D.; Spero, L.; Iandolo, J.J. Sequence determination and comparison of the exfoliative toxin a and toxin b genes from staphylococcus aureus. J. Bacteriol. 1987, 169, 3904–3909. [Google Scholar] [CrossRef]
- Amagai, M.; Yamaguchi, T.; Hanakawa, Y.; Nishifuji, K.; Sugai, M.; Stanley, J.R. Staphylococcal exfoliative toxin b specifically cleaves desmoglein 1. J. Investig. Dermatol. 2002, 118, 845–850. [Google Scholar] [CrossRef]
- Reed, S.B.; Wesson, C.A.; Liou, L.E.; Trumble, W.R.; Schlievert, P.M.; Bohach, G.A.; Bayles, K.W. Molecular characterization of a novel staphylococcus aureus serine protease operon. Infect. Immun. 2001, 69, 1521–1527. [Google Scholar] [CrossRef]
- Popowicz, G.M.; Dubin, G.; Stec-Niemczyk, J.; Czarny, A.; Dubin, A.; Potempa, J.; Holak, T.A. Functional and structural characterization of spl proteases from staphylococcus aureus. J. Mol. Biol. 2006, 358, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Stentzel, S.; Teufelberger, A.; Nordengrun, M.; Kolata, J.; Schmidt, F.; van Crombruggen, K.; Michalik, S.; Kumpfmuller, J.; Tischer, S.; Schweder, T.; et al. Staphylococcal serine protease-like proteins are pacemakers of allergic airway reactions to staphylococcus aureus. J. Allergy Clin. Immunol. 2017, 139, 492–500.e8. [Google Scholar] [CrossRef] [PubMed]
- Banbula, A.; Potempa, J.; Travis, J.; Fernandez-Catalan, C.; Mann, K.; Huber, R.; Bode, W.; Medrano, F. Amino-Acid sequence and three-dimensional structure of the staphylococcus aureus metalloproteinase at 1.72 a resolution. Structure 1998, 6, 1185–1193. [Google Scholar] [CrossRef]
- Potempa, J.; Watorek, W.; Travis, J. The inactivation of human plasma alpha 1-Proteinase inhibitor by proteinases from staphylococcus aureus. J. Biol. Chem. 1986, 261, 14330–14334. [Google Scholar]
- Burlak, C.; Hammer, C.H.; Robinson, M.A.; Whitney, A.R.; McGavin, M.J.; Kreiswirth, B.N.; Deleo, F.R. Global analysis of community-Associated methicillin-Resistant staphylococcus aureus exoproteins reveals molecules produced in vitro and during infection. Cell Microbiol. 2007, 9, 1172–1190. [Google Scholar] [CrossRef]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wojcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J.; et al. Degradation of human antimicrobial peptide LL-37 by staphylococcus aureus-Derived proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [CrossRef] [PubMed]
- Laarman, A.J.; Ruyken, M.; Malone, C.L.; van Strijp, J.A.; Horswill, A.R.; Rooijakkers, S.H. Staphylococcus aureus metalloprotease aureolysin cleaves complement c3 to mediate immune evasion. J. Immunol. 2011, 186, 6445–6453. [Google Scholar] [CrossRef]
- Peetermans, M.; Vanassche, T.; Liesenborghs, L.; Lijnen, R.H.; Verhamme, P. Bacterial pathogens activate plasminogen to breach tissue barriers and escape from innate immunity. Crit. Rev. Microbiol. 2016, 42, 866–882. [Google Scholar] [CrossRef]
- Bokarewa, M.I.; Jin, T.; Tarkowski, A. Staphylococcus aureus: Staphylokinase. Int. J. Biochem. Cell Biol. 2006, 38, 504–509. [Google Scholar] [CrossRef]
- Braff, M.H.; Jones, A.L.; Skerrett, S.J.; Rubens, C.E. Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase-dependent fibrinolysis. J. Infect. Dis. 2007, 195, 1365–1372. [Google Scholar] [CrossRef]
- Jin, T.; Bokarewa, M.; Foster, T.; Mitchell, J.; Higgins, J.; Tarkowski, A. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J. Immunol. 2004, 172, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Nobile, G.; Gianotti, V.; Zapotoczna, M.; Foster, T.J.; Geoghegan, J.A.; Speziale, P. Molecular interactions of human plasminogen with fibronectin-binding protein b (fnbpb), a fibrinogen/fibronectin-Binding protein from staphylococcus aureus. J. Biol. Chem. 2016, 291, 18148–18162. [Google Scholar] [CrossRef] [PubMed]
- Rooijakkers, S.H.; van Wamel, W.J.; Ruyken, M.; van Kessel, K.P.; van Strijp, J.A. Anti-Opsonic properties of staphylokinase. Microbes Infect. 2005, 7, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Churchward, G. The two faces of janus: Virulence gene regulation by covR/S in group a streptococci. Mol. Microbiol. 2007, 64, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Jenul, C.; Horswill, A.R. Regulation of staphylococcus aureus virulence. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Bronner, S.; Stoessel, P.; Gravet, A.; Monteil, H.; Prevost, G. Variable expressions of staphylococcus aureus bicomponent leucotoxins semiquantified by competitive reverse transcription-PCR. Appl. Environ. Microb. 2000, 66, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; Whitney, A.R.; Kobayashi, S.D.; Sturdevant, D.E.; Kennedy, A.D.; Braughton, K.R.; Shabb, D.W.; Diep, B.A.; Chambers, H.F.; Otto, M.; et al. Global changes in staphylococcus aureus gene expression in human blood. PLoS ONE 2011, 6, e18617. [Google Scholar] [CrossRef] [PubMed]
- Loughman, J.A.; Fritz, S.A.; Storch, G.A.; Hunstad, D.A. Virulence gene expression in human community-Acquired staphylococcus aureus infection. J. Infect. Dis. 2009, 199, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Bronesky, D.; Wu, Z.; Marzi, S.; Walter, P.; Geissmann, T.; Moreau, K.; Vandenesch, F.; Caldelari, I.; Romby, P. Staphylococcus aureus rnaiii and its regulon link quorum sensing, stress responses, metabolic adaptation, and regulation of virulence gene expression. Annu. Rev. Microbiol. 2016, 70, 299–316. [Google Scholar] [CrossRef]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci—An overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef]
- Novick, R.P.; Ross, H.F.; Projan, S.J.; Kornblum, J.; Kreiswirth, B.; Moghazeh, S. Synthesis of staphylococcal virulence factors is controlled by a regulatory rna molecule. EMBO J. 1993, 12, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Sakoulas, G.; McIntyre, L.M.; Meka, V.G.; Arbeit, R.D.; Cabell, C.H.; Stryjewski, M.E.; Eliopoulos, G.M.; Reller, L.B.; Corey, G.R.; et al. Persistent bacteremia due to methicillin-Resistant staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-Induced platelet microbicidal protein. J. Infect. Dis. 2004, 190, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Shopsin, B.; Drlica-Wagner, A.; Mathema, B.; Adhikari, R.P.; Kreiswirth, B.N.; Novick, R.P. Prevalence of agr dysfunction among colonizing staphylococcus aureus strains. J. Infect. Dis. 2008, 198, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Mairpady Shambat, S.; Siemens, N.; Monk, I.R.; Mohan, D.B.; Mukundan, S.; Krishnan, K.C.; Prabhakara, S.; Snall, J.; Kearns, A.; Vandenesch, F.; et al. A point mutation in agrc determines cytotoxic or colonizing properties associated with phenotypic variants of st22 mrsa strains. Sci. Rep. 2016, 6, 31360. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.; Wang, R.; Khan, B.A.; Sturdevant, D.E.; Otto, M. Role of the accessory gene regulator agr in community-Associated methicillin-Resistant staphylococcus aureus pathogenesis. Infect. Immun. 2011, 79, 1927–1935. [Google Scholar] [CrossRef]
- Liu, Q.; Yeo, W.S.; Bae, T. The saers two-Component system of staphylococcus aureus. Genes 2016, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, A.T.; Calzolari, A.; Cataldi, A.A.; Bogni, C.; Nagel, R. The sae locus of staphylococcus aureus encodes a two-component regulatory system. FEMS Microbiol. Lett 1999, 177, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Voyich, J.M.; Vuong, C.; DeWald, M.; Nygaard, T.K.; Kocianova, S.; Griffith, S.; Jones, J.; Iverson, C.; Sturdevant, D.E.; Braughton, K.R.; et al. The saeR/S gene regulatory system is essential for innate immune evasion by staphylococcus aureus. J. Infect. Dis. 2009, 199, 1698–1706. [Google Scholar] [CrossRef]
- Geiger, T.; Goerke, C.; Mainiero, M.; Kraus, D.; Wolz, C. The virulence regulator sae of staphylococcus aureus: Promoter activities and response to phagocytosis-related signals. J. Bacteriol. 2008, 190, 3419–3428. [Google Scholar] [CrossRef]
- Novick, R.P.; Jiang, D. The staphylococcal saers system coordinates environmental signals with agr quorum sensing. Microbiology 2003, 149, 2709–2717. [Google Scholar] [CrossRef]
- Baroja, M.L.; Herfst, C.A.; Kasper, K.J.; Xu, S.X.; Gillett, D.A.; Li, J.; Reid, G.; McCormick, J.K. The saers two-Component system is a direct and dominant transcriptional activator of toxic shock syndrome toxin 1 in staphylococcus aureus. J. Bacteriol. 2016, 198, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Zurek, O.W.; Nygaard, T.K.; Watkins, R.L.; Pallister, K.B.; Torres, V.J.; Horswill, A.R.; Voyich, J.M. The role of innate immunity in promoting saeR/S-Mediated virulence in staphylococcus aureus. J. Innate Immun. 2014, 6, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Engleberg, N.C.; Heath, A.; Miller, A.; Rivera, C.; DiRita, V.J. Spontaneous mutations in the csrrs two-Component regulatory system of streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 2001, 183, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.B.; Hjortrup, P.B.; Hansen, M.B.; Lange, T.; Norrby-Teglund, A.; Hyldegaard, O.; Perner, A. Immunoglobulin g for patients with necrotising soft tissue infection (instinct): A randomised, blinded, placebo-controlled trial. Intensive Care Med. 2017, 43, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Polzik, P.; Johansson, P.I.; Hyldegaard, O. How biomarkers reflect the prognosis and treatment of necrotising soft tissue infections and the effects of hyperbaric oxygen therapy: The protocol of the prospective cohort protreat study conducted at a tertiary hospital in copenhagen, denmark. BMJ Open 2017, 7, e017805. [Google Scholar] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of america. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [PubMed]
- Freischlag, J.A.; Ajalat, G.; Busuttil, R.W. Treatment of necrotizing soft tissue infections. The need for a new approach. Am. J. Surg. 1985, 149, 751–755. [Google Scholar] [CrossRef]
- McHenry, C.R.; Piotrowski, J.J.; Petrinic, D.; Malangoni, M.A. Determinants of mortality for necrotizing soft-tissue infections. Ann. Surg. 1995, 221, 558–563. [Google Scholar] [CrossRef]
- Bucca, K.; Spencer, R.; Orford, N.; Cattigan, C.; Athan, E.; McDonald, A. Early diagnosis and treatment of necrotizing fasciitis can improve survival: An observational intensive care unit cohort study. ANZ J. Surg. 2013, 83, 365–370. [Google Scholar] [CrossRef]
- Hadeed, G.J.; Smith, J.; O’Keeffe, T.; Kulvatunyou, N.; Wynne, J.L.; Joseph, B.; Friese, R.S.; Wachtel, T.L.; Rhee, P.M.; El-Menyar, A.; et al. Early surgical intervention and its impact on patients presenting with necrotizing soft tissue infections: A single academic center experience. J. Emerg. Trauma Shock 2016, 9, 22–27. [Google Scholar]
- Stevens, D.L.; Gibbons, A.E.; Bergstrom, R.; Winn, V. The eagle effect revisited: Efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 1988, 158, 23–28. [Google Scholar] [PubMed]
- Sriskandan, S.; McKee, A.; Hall, L.; Cohen, J. Comparative effects of clindamycin and ampicillin on superantigenic activity of streptococcus pyogenes. J. Antimicrob. Chemother. 1997, 40, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Linner, A.; Darenberg, J.; Sjolin, J.; Henriques-Normark, B.; Norrby-Teglund, A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: A comparative observational study. Clin. Infect. Dis. 2014, 59, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, F.; Zurcher, C.; Tarnutzer, A.; Schilcher, K.; Neff, A.; Keller, N.; Marques Maggio, E.; Poyart, C.; Schuepbach, R.A.; Zinkernagel, A.S. Clindamycin affects group a streptococcus virulence factors and improves clinical outcome. J. Infect. Dis. 2017, 215, 269–277. [Google Scholar] [PubMed]
- Coyle, E.A.; Cha, R.; Rybak, M.J. Influences of linezolid, penicillin, and clindamycin, alone and in combination, on streptococcal pyrogenic exotoxin a release. Antimicrob. Agents Chemother. 2003, 47, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- DeMuri, G.P.; Sterkel, A.K.; Kubica, P.A.; Duster, M.N.; Reed, K.D.; Wald, E.R. Macrolide and clindamycin resistance in group a streptococci isolated from children with pharyngitis. Pediatr. Infect. Dis. J. 2017, 36, 342–344. [Google Scholar] [CrossRef]
- Eckmann, C.; Dryden, M. Treatment of complicated skin and soft-Tissue infections caused by resistant bacteria: Value of linezolid, tigecycline, daptomycin and vancomycin. Eur. J. Med. Res. 2010, 15, 554–563. [Google Scholar]
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65, iii35–iii44. [Google Scholar] [CrossRef]
- Bounthavong, M.; Hsu, D.I. Efficacy and safety of linezolid in methicillin-Resistant staphylococcus aureus (mrsa) complicated skin and soft tissue infection (cssti): A meta-Analysis. Curr. Med. Res. Opin. 2010, 26, 407–421. [Google Scholar] [CrossRef]
- Itani, K.M.; Dryden, M.S.; Bhattacharyya, H.; Kunkel, M.J.; Baruch, A.M.; Weigelt, J.A. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-Resistant staphylococcus aureus. Am. J. Surg. 2010, 199, 804–816. [Google Scholar] [CrossRef]
- Stevens, D.L.; Herr, D.; Lampiris, H.; Hunt, J.L.; Batts, D.H.; Hafkin, B. Linezolid versus vancomycin for the treatment of methicillin-resistant staphylococcus aureus infections. Clin. Infect. Dis. 2002, 34, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Bayer, A.S.; Arias, C.A. Mechanism of action and resistance to daptomycin in staphylococcus aureus and enterococci. Cold Spring Harb. Perspect. Med. 2016, 6, a026997. [Google Scholar] [CrossRef] [PubMed]
- Mairpady Shambat, S.; Haggar, A.; Vandenesch, F.; Lina, G.; van Wamel, W.J.; Arakere, G.; Svensson, M.; Norrby-Teglund, A. Levels of alpha-Toxin correlate with distinct phenotypic response profiles of blood mononuclear cells and with agr background of community-Associated staphylococcus aureus isolates. PLoS ONE 2014, 9, e106107. [Google Scholar] [CrossRef] [PubMed]
- Norrby-Teglund, A.; Ihendyane, N.; Kansal, R.; Basma, H.; Kotb, M.; Andersson, J.; Hammarstrom, L. Relative neutralizing activity in polyspecific igm, iga, and igg preparations against group a streptococcal superantigens. Clin. Infect. Dis. 2000, 31, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Norrby-Teglund, A.; Kaul, R.; Low, D.E.; McGeer, A.; Newton, D.W.; Andersson, J.; Andersson, U.; Kotb, M. Plasma from patients with severe invasive group a streptococcal infections treated with normal polyspecific igg inhibits streptococcal superantigen-induced t cell proliferation and cytokine production. J. Immunol. 1996, 156, 3057–3064. [Google Scholar] [PubMed]
- Norrby-Teglund, A.; Low, D.E.; McGeer, A.; Kotb, M. Superantigenic activity produced by group a streptococcal isolates is neutralized by plasma from IVIG-Treated streptococcal toxic shock syndrome patients. Adv. Exp. Med. Biol. 1997, 418, 563–566. [Google Scholar] [PubMed]
- Tarnutzer, A.; Andreoni, F.; Keller, N.; Zurcher, C.; Norrby-Teglund, A.; Schupbach, R.A.; Zinkernagel, A.S. Human polyspecific immunoglobulin attenuates group a streptococcal virulence factor activity and reduces disease severity in a murine necrotizing fasciitis model. Clin. Microbiol. Infect. 2018, 25, 512.e7–512.e13. [Google Scholar] [CrossRef] [PubMed]
- Norrby-Teglund, A.; Muller, M.P.; McGeer, A.; Gan, B.S.; Guru, V.; Bohnen, J.; Thulin, P.; Low, D.E. Successful management of severe group a streptococcal soft tissue infections using an aggressive medical regimen including intravenous polyspecific immunoglobulin together with a conservative surgical approach. Scand. J. Infect. Dis. 2005, 37, 166–172. [Google Scholar] [CrossRef]
- Parks, T.; Wilson, C.; Curtis, N.; Norrby-Teglund, A.; Sriskandan, S. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: A systematic review and meta-analysis. Clin. Infect. Dis. 2018, 67, 1434–1436. [Google Scholar] [CrossRef]
- Shaw, J.J.; Psoinos, C.; Emhoff, T.A.; Shah, S.A.; Santry, H.P. Not just full of hot air: Hyperbaric oxygen therapy increases survival in cases of necrotizing soft tissue infections. Surg. Infect. 2014, 15, 328–335. [Google Scholar] [CrossRef]
- Wang, C.; Schwaitzberg, S.; Berliner, E.; Zarin, D.A.; Lau, J. Hyperbaric oxygen for treating wounds: A systematic review of the literature. Arch. Surg. 2003, 138, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Simonsen, U.; Garred, P.; Hyldegaard, O. Biomarkers of necrotising soft tissue infections: Aspects of the innate immune response and effects of hyperbaric oxygenation-the protocol of the prospective cohort bionec study. BMJ Open 2015, 5, e006995. [Google Scholar] [CrossRef] [PubMed]
| Leukocidin | Other Names | Receptors | Human Cell Targets |
|---|---|---|---|
| PVL | PVL-LukSV | C5aR1 C5aR2 | Neutrophils Monocytes Macrophages |
| LukAB | LukGH | CD11b | Neutrophils Monocytes Macrophages Dendritic cells |
| LukED | CCR5 CXCR1 CXCR2 DARC | T cells Neutrophils Monocytes Dendritic cells Erythrocytes | |
| HlgAB | γ-hemolysin γ-toxin | CXCR1 CXCR2 CXCR4 CCR2 DARC | Neutrophils Monocytes Macrophages Erythrocytes |
| HlgCB | Leukocidin γ-hemolysin γ-toxin | C5aR1 C5aR2 | Neutrophils Monocytes Macrophages |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumba, P.; Mairpady Shambat, S.; Siemens, N. The Role of Streptococcal and Staphylococcal Exotoxins and Proteases in Human Necrotizing Soft Tissue Infections. Toxins 2019, 11, 332. https://doi.org/10.3390/toxins11060332
Shumba P, Mairpady Shambat S, Siemens N. The Role of Streptococcal and Staphylococcal Exotoxins and Proteases in Human Necrotizing Soft Tissue Infections. Toxins. 2019; 11(6):332. https://doi.org/10.3390/toxins11060332
Chicago/Turabian StyleShumba, Patience, Srikanth Mairpady Shambat, and Nikolai Siemens. 2019. "The Role of Streptococcal and Staphylococcal Exotoxins and Proteases in Human Necrotizing Soft Tissue Infections" Toxins 11, no. 6: 332. https://doi.org/10.3390/toxins11060332
APA StyleShumba, P., Mairpady Shambat, S., & Siemens, N. (2019). The Role of Streptococcal and Staphylococcal Exotoxins and Proteases in Human Necrotizing Soft Tissue Infections. Toxins, 11(6), 332. https://doi.org/10.3390/toxins11060332




