A Gold Growth-Based Plasmonic ELISA for the Sensitive Detection of Fumonisin B1 in Maize
Abstract
1. Introduction
2. Results and Discussion
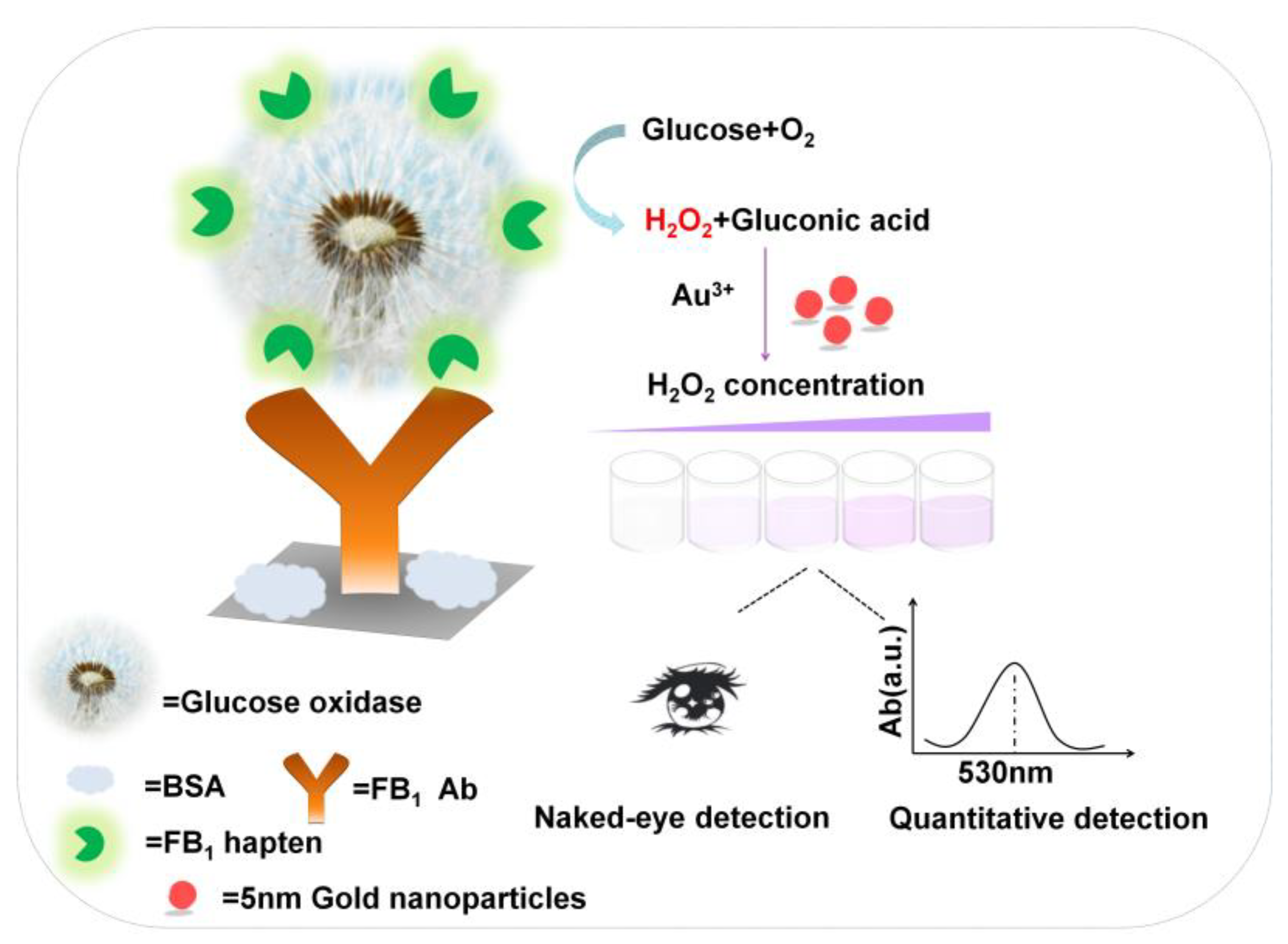
2.1. Principle of the Proposed pELISA Method
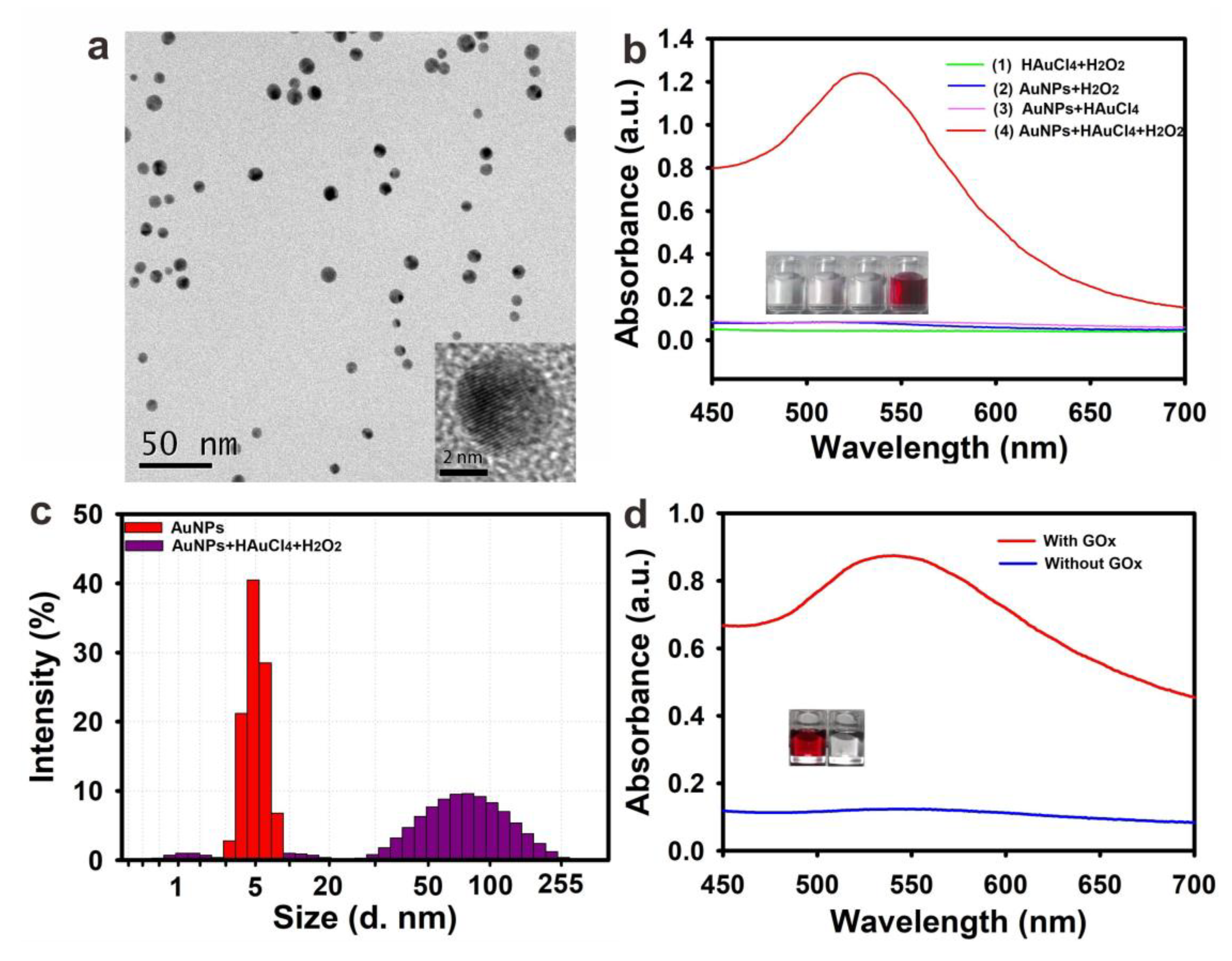
2.2. Feasibility of GOx Regulated AuNPs Growth
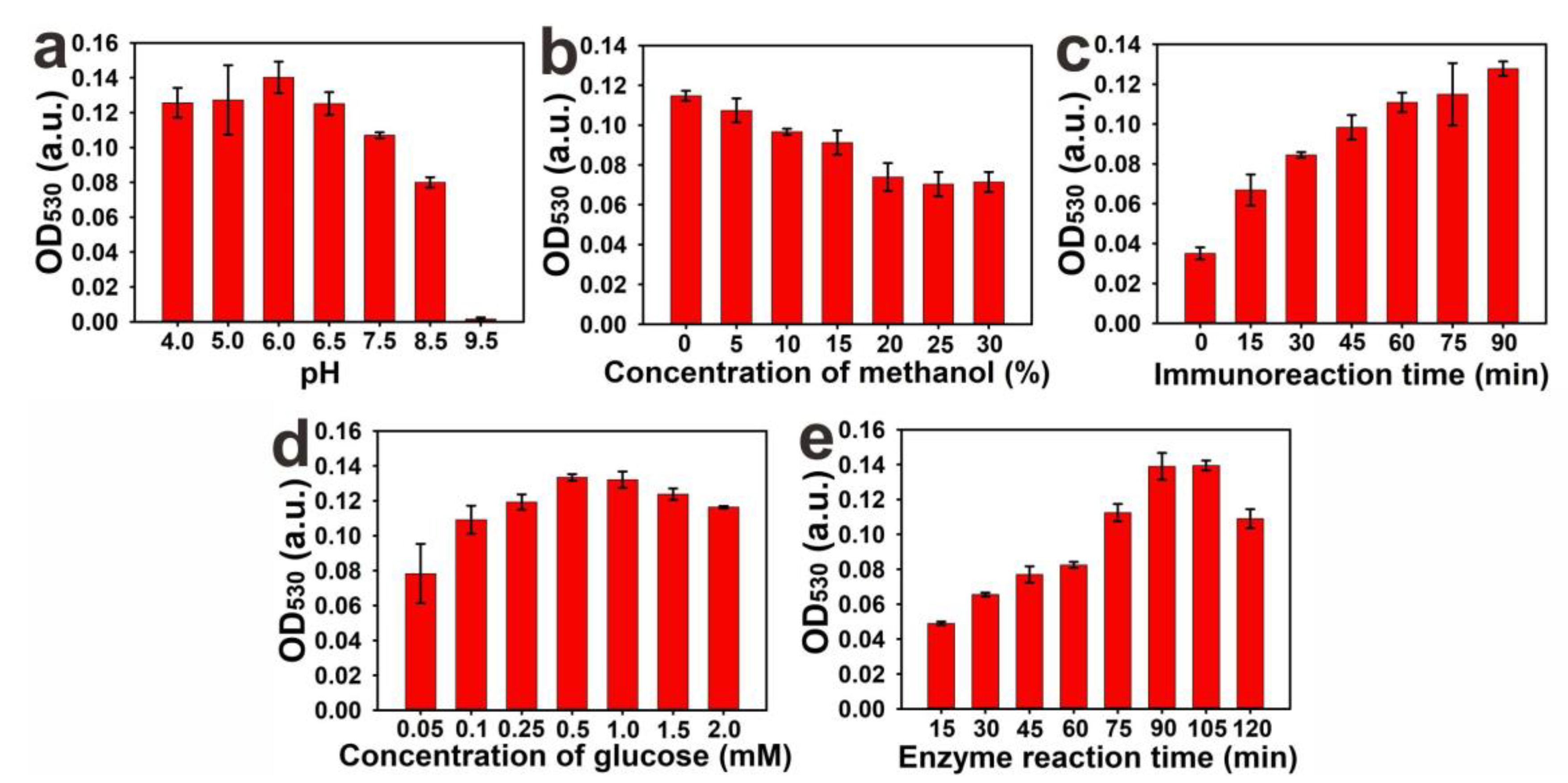
2.3. Optimization of the Parameters of pELISA
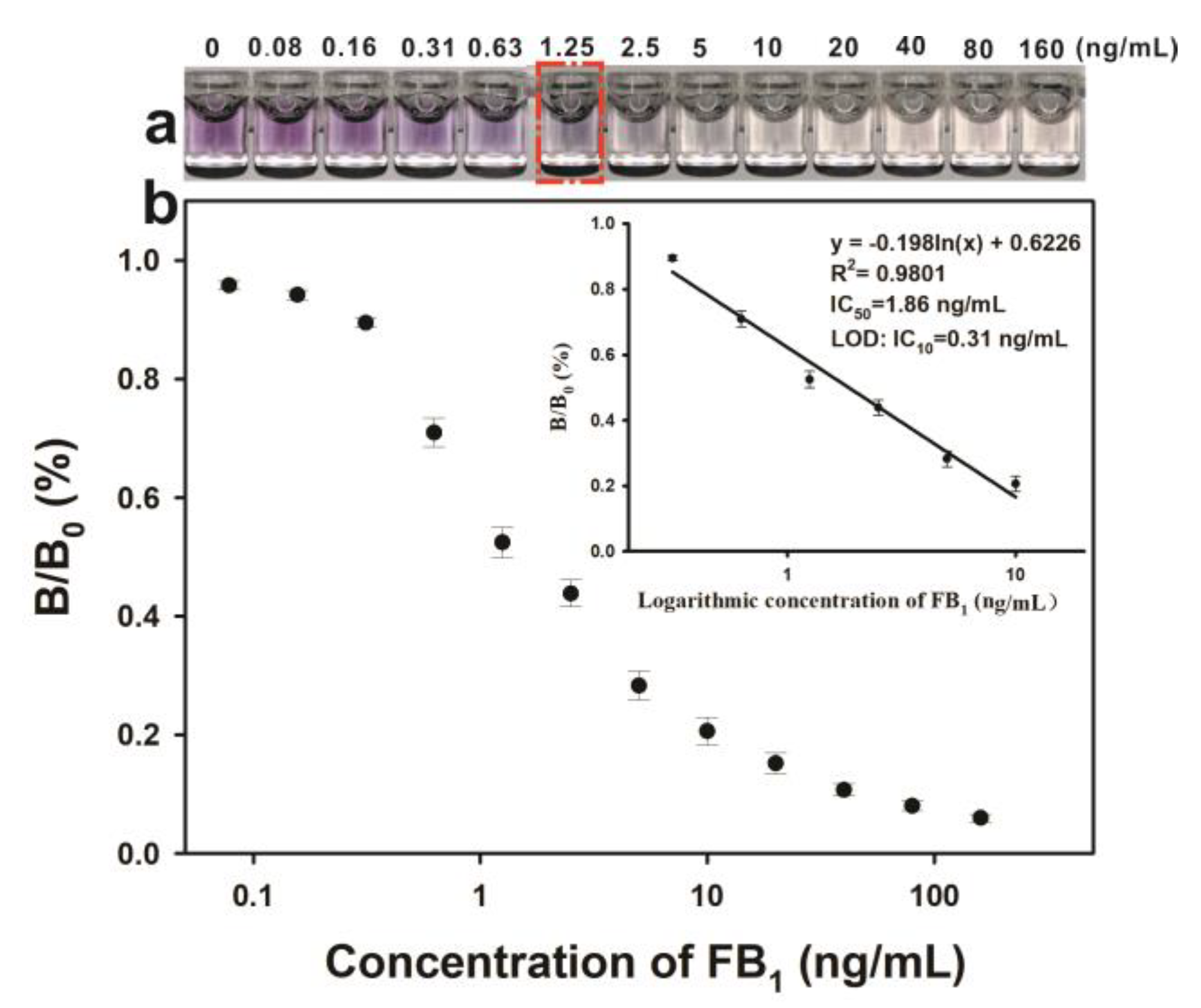
2.4. Analytical Performance of pELISA for the Sensitive Detection of FB1
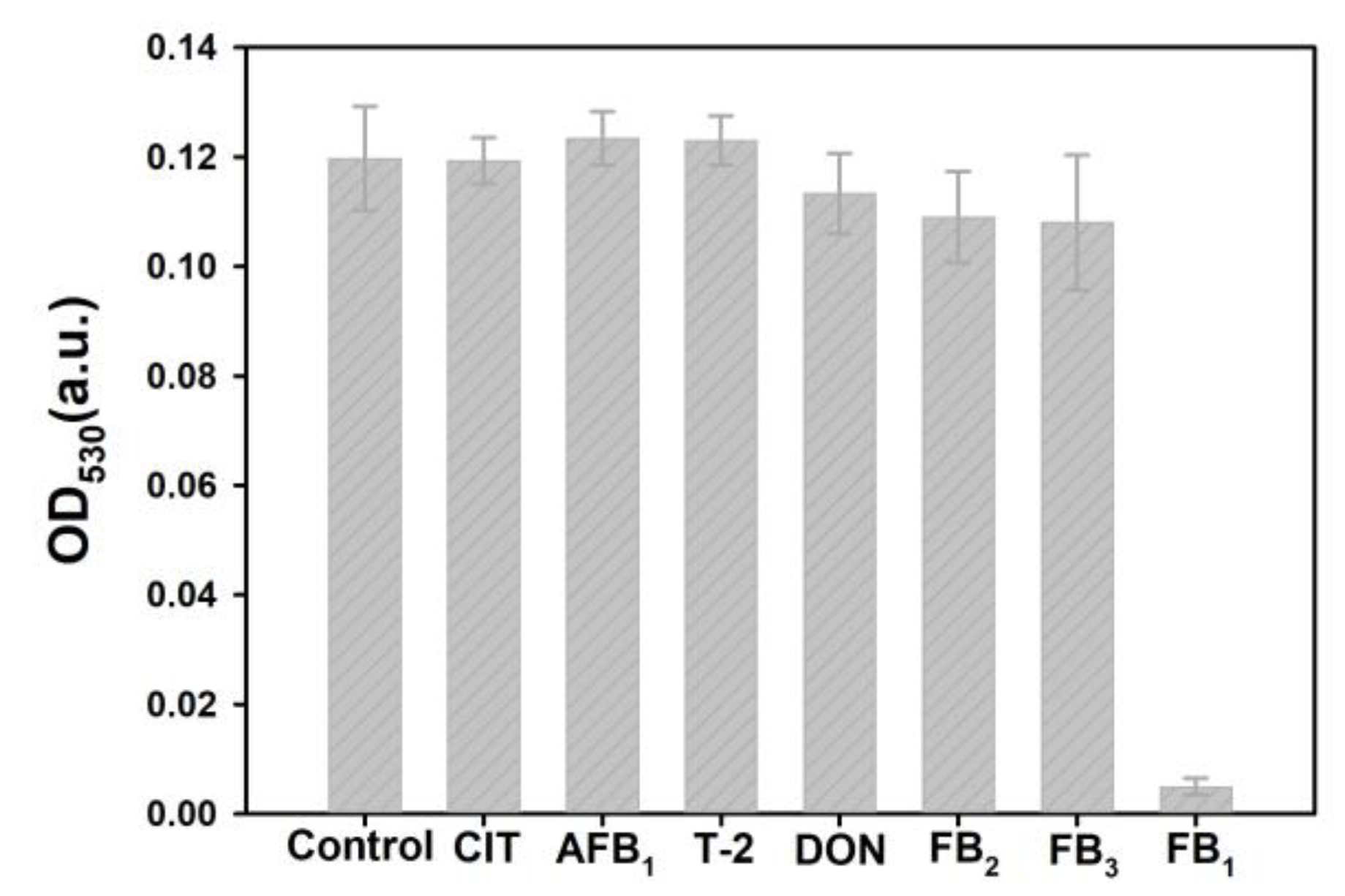
2.5. Selectivity of the Proposed Sensing System
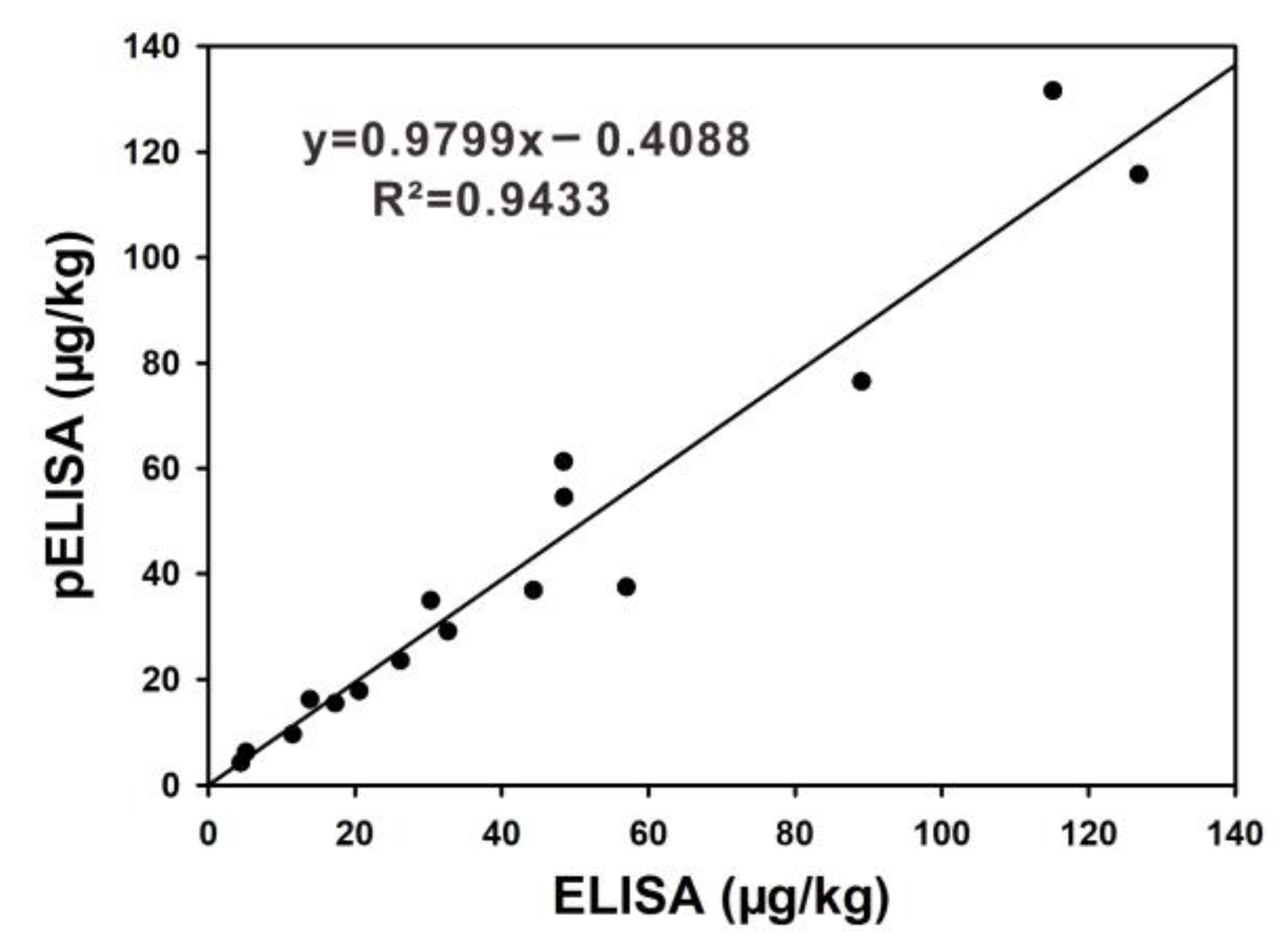
2.6. Validation of pELISA on Maize Samples
3. Conclusions
4. Materials and Methods
4.1. Regents
4.2. Apparatus
4.3. The Synthesis of Gold Seeds
4.4. Preparation of FB1-Labeled GOx
4.5. GOx Mediated Direct Competitive pELISA
4.6. Sample Preparation
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Omurtag, G.Z. Fumonisins, trichothecenes and zearalenone in cereals. Int. J. Mol. Sci. 2008, 9, 2062–2090. [Google Scholar]
- Wan, L.Y.M.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins b1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Anfossi, L.; Giovannoli, C.; Passini, C.; Goftman, V.; Goryacheva, I.; Baggiani, C. A fluorescent immunochromatographic strip test using quantum dots for fumonisins detection. Talanta 2016, 150, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Fasano, E.; Scognamiglio, G.; Nardone, A.; Triassi, M.; Cirillo, T. Exposure assessment to fumonisins B1, B2 and B3 through consumption of gluten-free foodstuffs intended for people affected by celiac disease. Food Chem. Toxicol. 2016, 97, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mi, T.; Oliveri Conti, G.; Yu, Q.; Wen, K.; Shen, J.; Ferrante, M.; Wang, Z. Development of a screening fluorescence polarization immunoassay for the simultaneous detection of fumonisins B1 and B2 in maize. J. Agric. Food Chem. 2015, 63, 4940–4946. [Google Scholar] [CrossRef] [PubMed]
- Kaltner, F.; Rampl, C.; Rychlik, M.; Zimmermann, T.; Rohe, A. Development and validation of a cost-effective hplc-fld method for routine analysis of fumonisins B1 and B2 in corn and corn products. Food Anal. Methods 2017, 10, 1349–1358. [Google Scholar] [CrossRef]
- Arranz, I.; Baeyens, W.; Weken, G.; Saeger, S.D.; Peteghem, C.V. Hplc determination of fumonisin mycotoxins. Crit. Rev. Food Sci. Nutr. 2004, 44, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sforza, S.; Dall’Asta, C.; Marchelli, R. Recent advances in mycotoxin determination in food and feed by hyphenated chromatographic techniques/mass spectrometry. Mass Spectrom. Rev. 2006, 25, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Fernández-Franzón, M.; Font, G.; Pena, A.; Silveira, I.; Lino, C.; Mañes, J. Analysis of fumonisins in corn-based food by liquid chromatography with fluorescence and mass spectrometry detectors. Food Chem. 2009, 112, 1031–1037. [Google Scholar] [CrossRef]
- Li, J.; Duan, H.; Xu, P.; Huang, X.; Xiong, Y. Effect of different-sized spherical gold nanoparticles grown layer by layer on the sensitivity of an immunochromatographic assay. RSC Adv. 2016, 6, 26178. [Google Scholar] [CrossRef]
- Josephy, P.D.; Eling, T.; Mason, R.P. The horseradish peroxidase-catalyzed oxidation of 3,5,3’,5’-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J. Biol. Chem. 1982, 257, 3669–3675. [Google Scholar] [PubMed]
- Xiong, Y.; Pei, K.; Wu, Y.; Duan, H.; Lai, W.; Xiong, Y. Plasmonic ELISA based on enzyme-assisted etching of Au nanorods for the highly sensitive detection of aflatoxin B1 in corn samples. Sens. Actuators B Chem. 2018, 267, 320–327. [Google Scholar] [CrossRef]
- Liu, D.; Yang, J.; Wang, H.-F.; Wang, Z.; Huang, X.; Wang, Z.; Niu, G.; Hight Walker, A.; Chen, X. Glucose oxidase-catalyzed growth of gold nanoparticles enables quantitative detection of attomolar cancer biomarkers. Anal. Chem. 2014, 86, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.P.N.; Ahmed, S.; Abbas, A. Single-digit pathogen and attomolar detection with the naked eye using liposome-amplified plasmonic immunoassay. Nano Lett. 2015, 15, 6239–6246. [Google Scholar] [CrossRef] [PubMed]
- De La Rica, R.; Stevens, M.M. Plasmonic elisa for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, J.; Li, J.; Ju, H. A plasmonic colorimetric strategy for biosensing through enzyme guided growth of silver nanoparticles on gold nanostars. Biosens. Bioelectron. Chem. 2016, 78, 267–273. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, X.; Chen, X.; Zhang, W.; Ping, G.; Xiong, Y. Plasmonic elisa for naked-eye detection of ochratoxin a based on the tyramine-H2O2 amplification system. Sens. Actuators B. 2018, 259, 162–169. [Google Scholar] [CrossRef]
- Peng, C.; Duan, X.; Khamba, G.W.; Xie, Z. Highly sensitive “signal on” plasmonic elisa for small molecules by the naked eye. Anal. Methods 2014, 6, 9616–9621. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Wang, S.; Cheng, F.; Chen, L. Iodine-mediated etching of gold nanorods for plasmonic elisa based on colorimetric detection of alkaline phosphatase. ACS Appl. Mater. Interfaces 2015, 7, 27639. [Google Scholar] [CrossRef]
- Satija, J.; Punjabi, N.; Mishra, D.; Mukherji, S. Plasmonic-elisa: Expanding horizons. RSC Adv. 2016, 6, 85440–85456. [Google Scholar] [CrossRef]
- Cecchin, D.; De La Rica, R.; Bain, R.; Finnis, M.W.; Stevens, M.; Battaglia, G. Plasmonic elisa for the detection of gp120 at ultralow concentrations with the naked eye. Nanoscale 2014, 6, 9559–9562. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lorenzo, L.; De La Rica, R.; Álvarez-Puebla, R.A.; Liz-Marzán, L.M.; Stevens, M.M. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat. Mater. 2012, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Höller, R.P.; Dulle, M.; Thomä, S.; Mayer, M.; Steiner, A.M.; Förster, S.; Fery, A.; Kuttner, C.; Chanana, M. Protein-assisted assembly of modular 3d plasmonic raspberry-like core/satellite nanoclusters: Correlation of structure and optical properties. ACS Nano 2016, 10, 5740–5750. [Google Scholar] [CrossRef]
- Zhan, S.; Huang, X.; Chen, R.; Li, J.; Xiong, Y. Novel fluorescent elisa for the sensitive detection of zearalenone based on H2O2-sensitive quantum dots for signal transduction. Talanta 2016, 158, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Pei, K.; Wu, Y.; Xiong, Y. Colorimetric elisa based on glucose oxidase-regulated the color of acid–base indicator for sensitive detection of aflatoxin B1 in corn samples. Food Control 2017, 78, 317–323. [Google Scholar] [CrossRef]
- Duan, H.; Chen, X.; Xu, W.; Fu, J.; Xiong, Y.; Wang, A. Quantum-dot submicrobead-based immunochromatographic assay for quantitative and sensitive detection of zearalenone. Talanta 2015, 132, 126–131. [Google Scholar] [CrossRef]
- Anderson, G.P.; Kowtha, V.A.; Taitt, C.R. Detection of fumonisin b1 and ochratoxin a in grain products using microsphere-based fluid array immunoassays. Toxins 2010, 2, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Quan, Y.; Lee, N.; Kennedy, I.R. Rapid determination of fumonisin b1 in food samples by enzyme-linked immunosorbent assay and colloidal gold immunoassay. J. Sci. Food Agric. 2006, 54, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
| Spiked FB1 (mg/kg) | Average a | Recovery (%) | Standard Deviation | CV (%) |
|---|---|---|---|---|
| 2.40 | 2.28 | 95.04 | 2.41 | 2.54 |
| 1.20 | 1.06 | 88.33 | 10.16 | 11.85 |
| 0.30 | 0.35 | 116.67 | 14.89 | 13.20 |
| 0.15 | 0.17 | 113.33 | 8.15 | 6.89 |
| 0.08 | 0.08 | 104.00 | 2.81 | 3.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, S.; Zheng, L.; Zhou, Y.; Wu, K.; Duan, H.; Huang, X.; Xiong, Y. A Gold Growth-Based Plasmonic ELISA for the Sensitive Detection of Fumonisin B1 in Maize. Toxins 2019, 11, 323. https://doi.org/10.3390/toxins11060323
Zhan S, Zheng L, Zhou Y, Wu K, Duan H, Huang X, Xiong Y. A Gold Growth-Based Plasmonic ELISA for the Sensitive Detection of Fumonisin B1 in Maize. Toxins. 2019; 11(6):323. https://doi.org/10.3390/toxins11060323
Chicago/Turabian StyleZhan, Shengnan, Lingyan Zheng, Yaofeng Zhou, Kesheng Wu, Hong Duan, Xiaolin Huang, and Yonghua Xiong. 2019. "A Gold Growth-Based Plasmonic ELISA for the Sensitive Detection of Fumonisin B1 in Maize" Toxins 11, no. 6: 323. https://doi.org/10.3390/toxins11060323
APA StyleZhan, S., Zheng, L., Zhou, Y., Wu, K., Duan, H., Huang, X., & Xiong, Y. (2019). A Gold Growth-Based Plasmonic ELISA for the Sensitive Detection of Fumonisin B1 in Maize. Toxins, 11(6), 323. https://doi.org/10.3390/toxins11060323






