Cholera Toxin Encapsulated within Several Vibrio cholerae O1 Serotype Inaba Outer Membrane Vesicles Lacks a Functional B-Subunit
Abstract
1. Introduction
2. Results
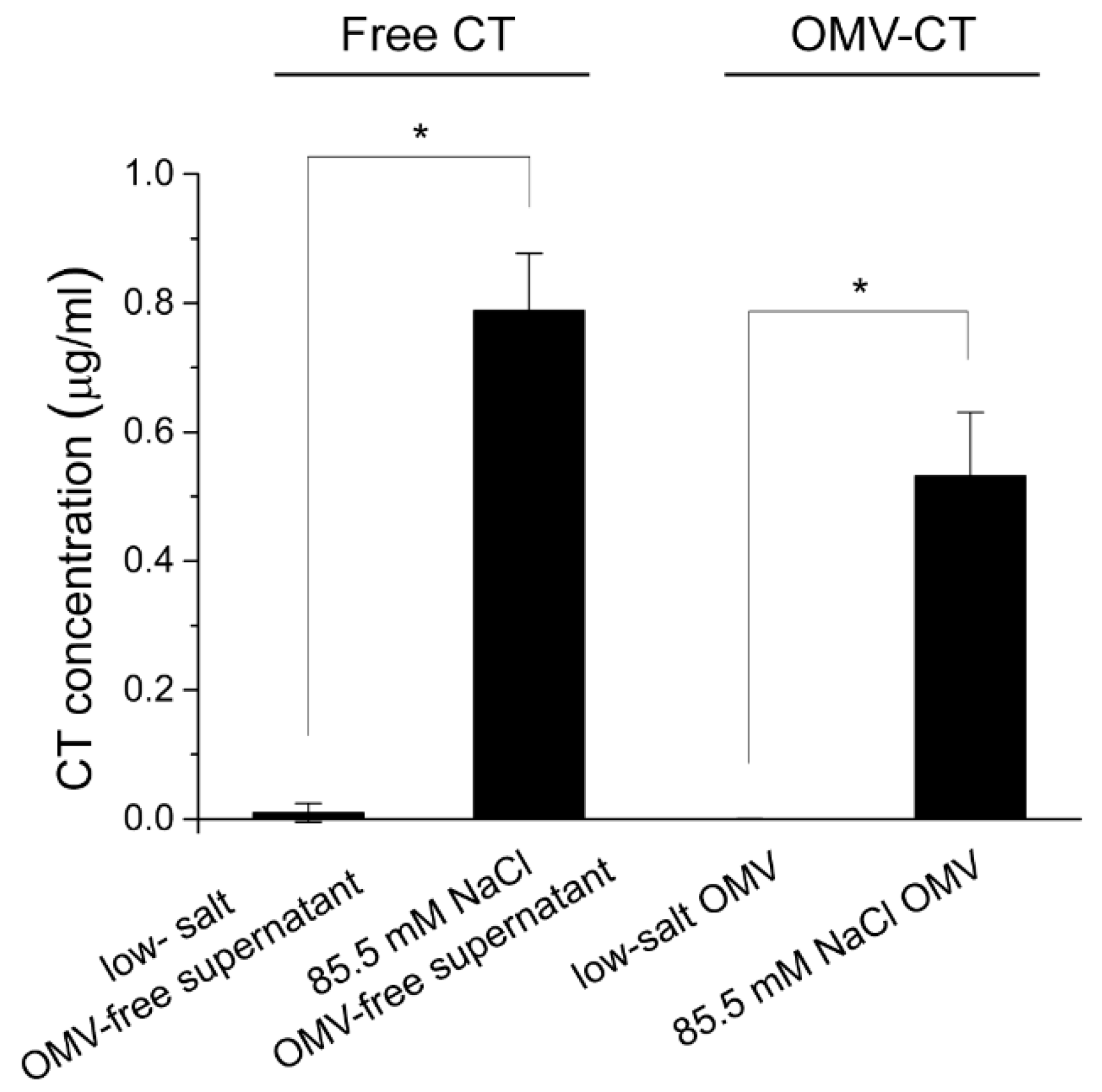
2.1. The Effect of Osmolarity of the Growth Medium on Association of CT with 569B OMVs
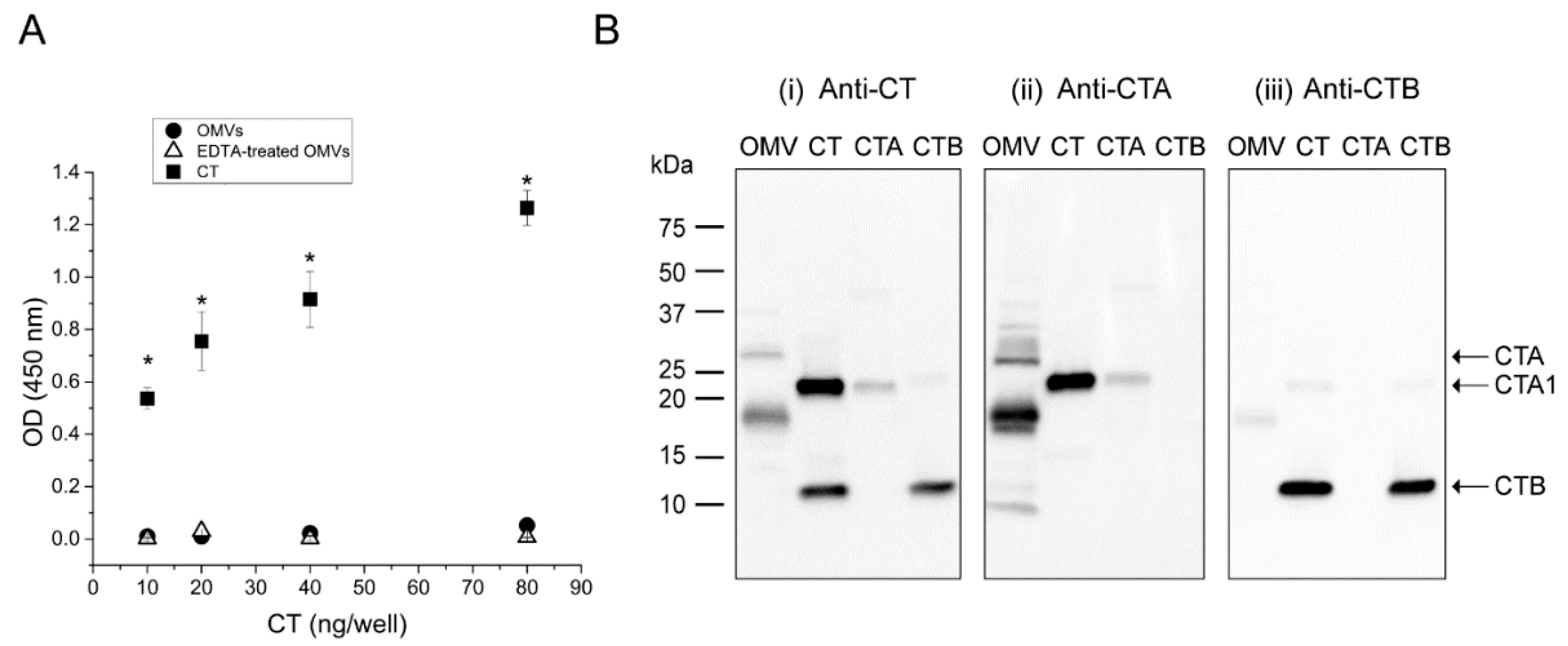
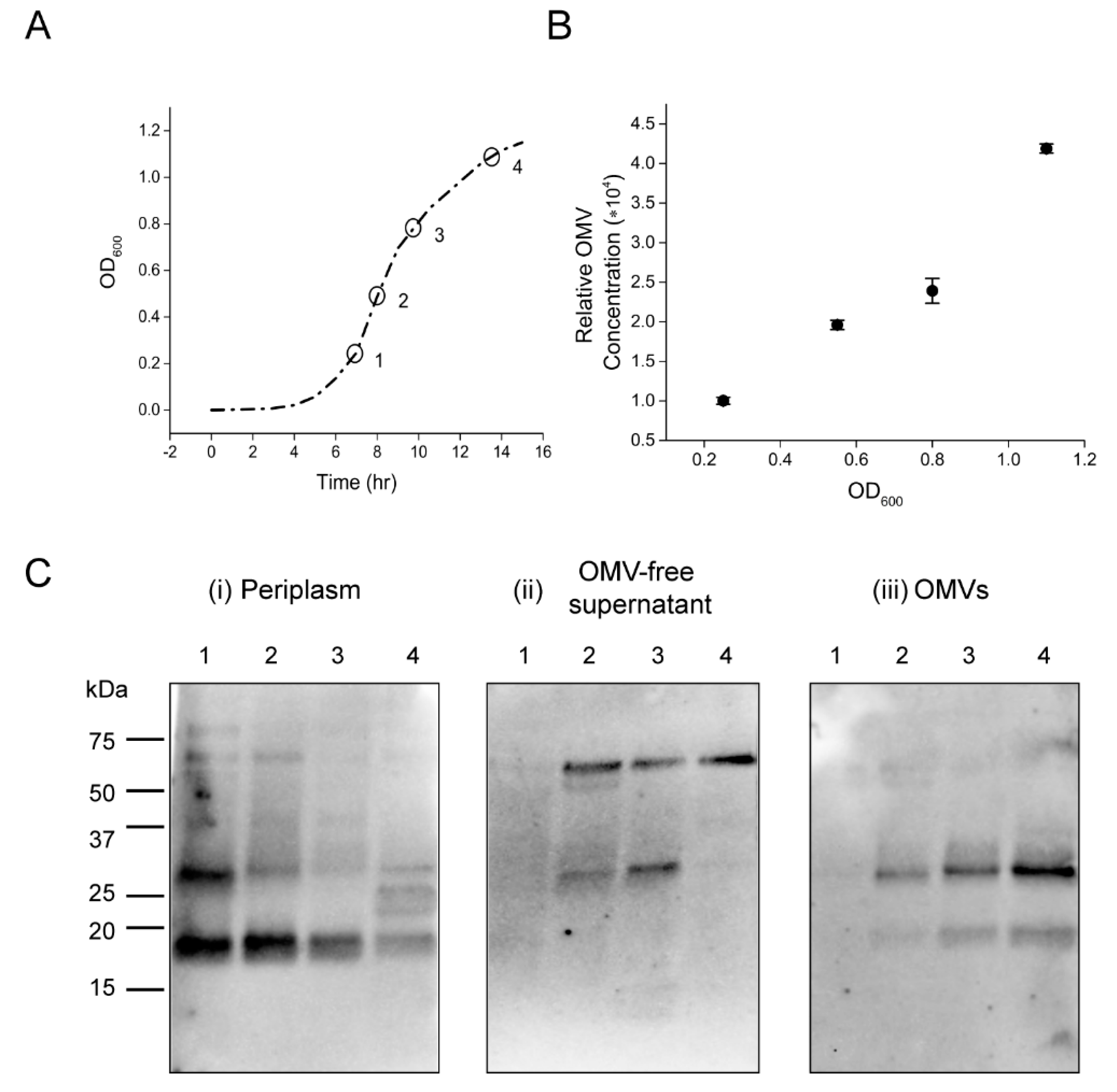
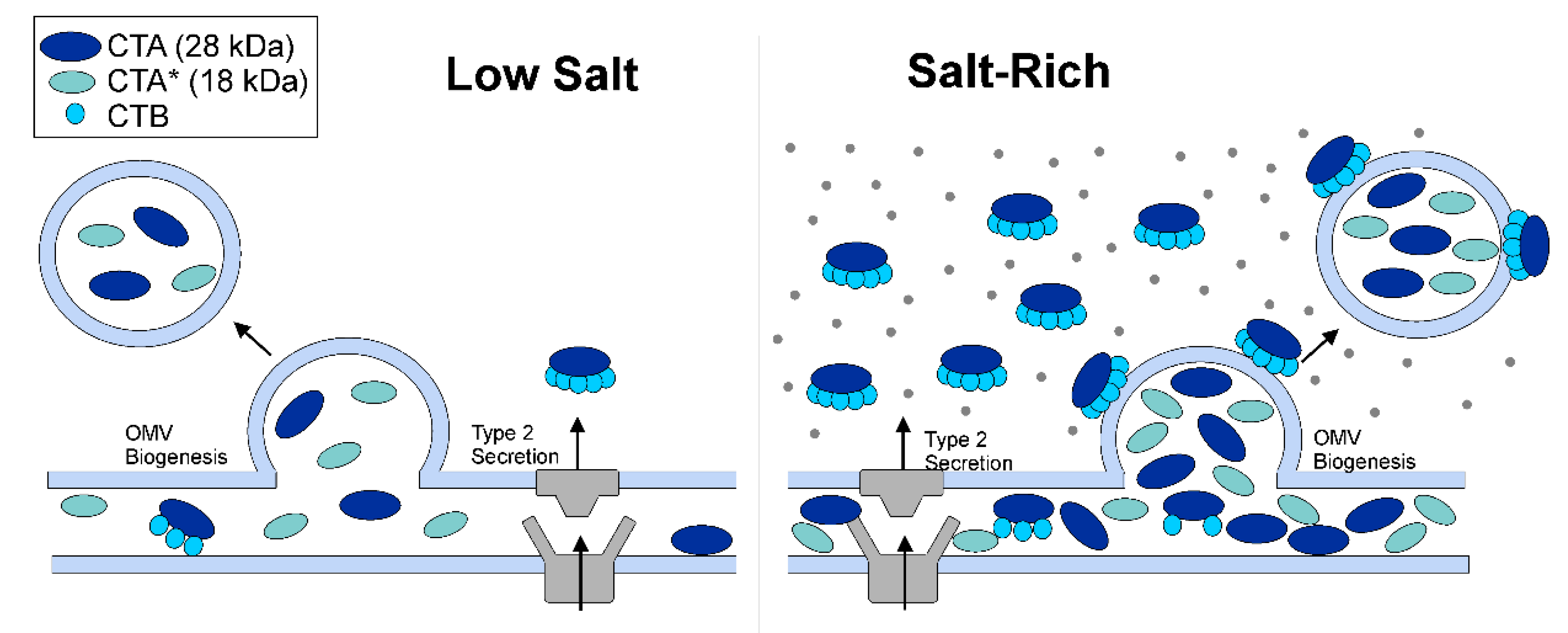
2.2. OMVs Isolated from Low-Salt V. cholerae Culture Do Not Carry Intact CT Holotoxin
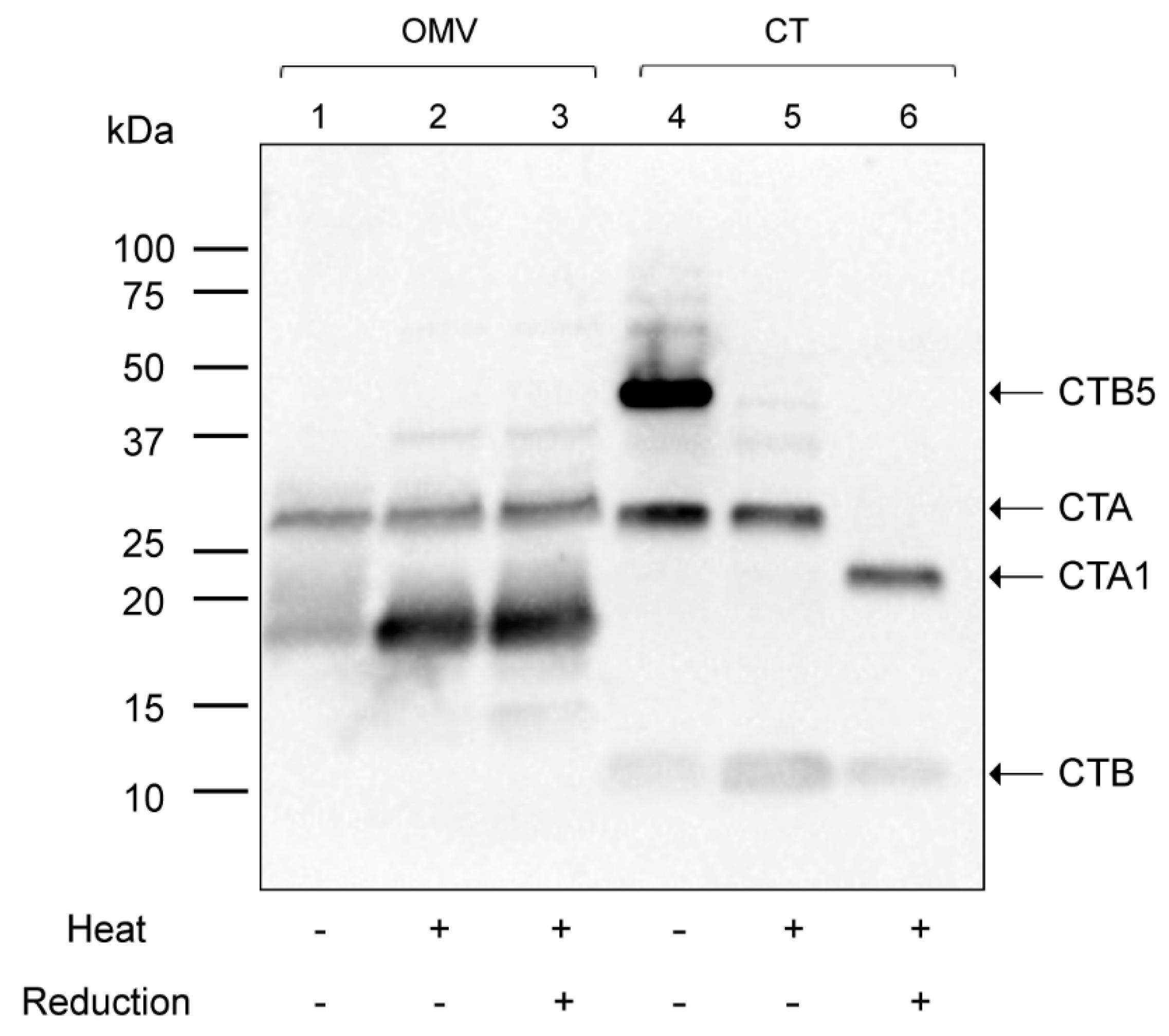
2.3. The CTB-Subunit Is Not Associated with 569B OMV-Encapsulated CT
2.4. OMVs Encapsulate CTA from the Periplasmic Pool
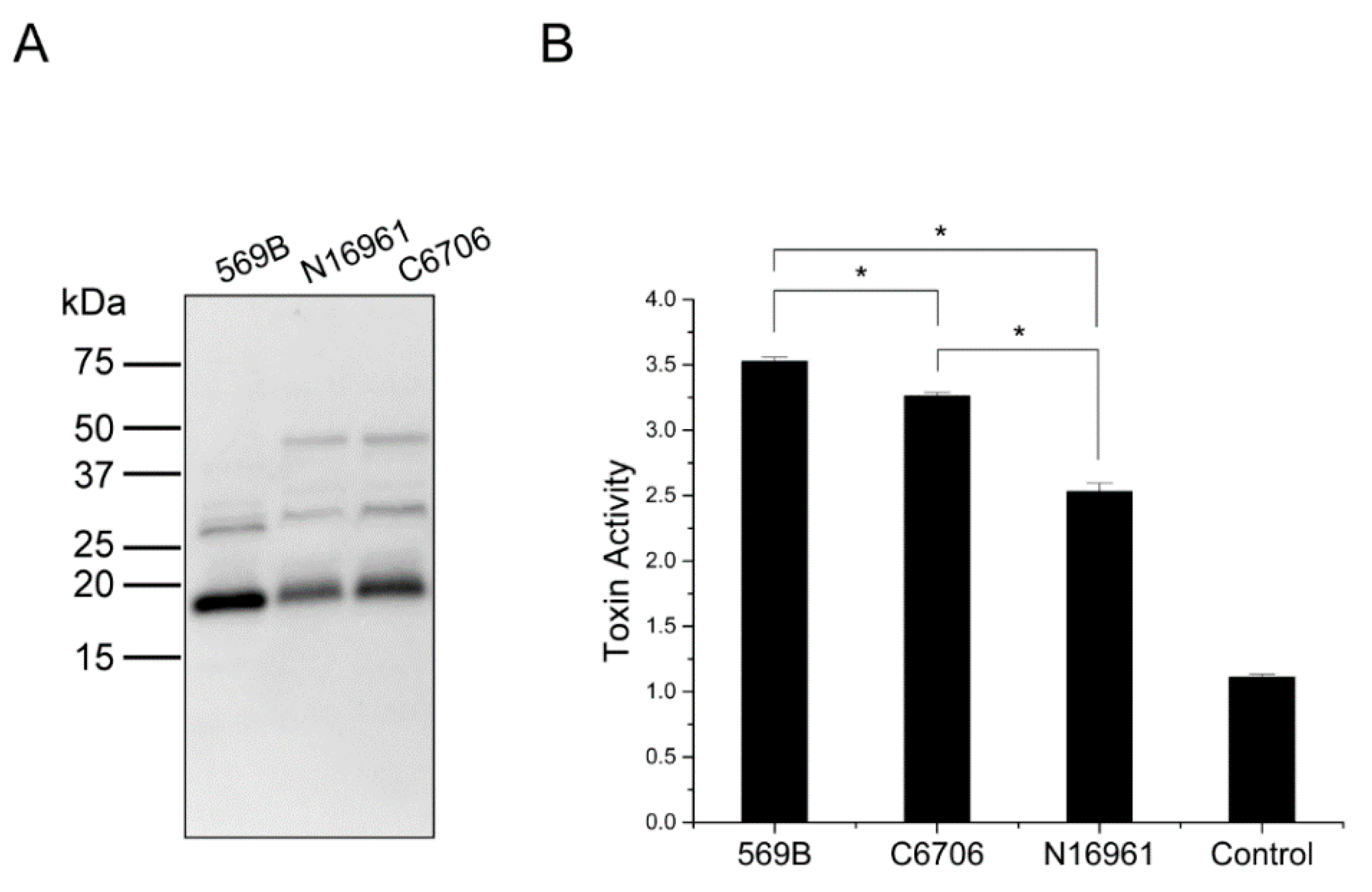
2.5. The Encapsulation of CTA in OMVs Is Conserved among OMVs from Several O1 Inaba Strains
2.6. The Encapsulation of CTA in OMVs Increases with an Increase in the Culture Osmolarity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Strains and Culturing Conditions
4.3. Vesicle Purification
4.4. GM1 ELISA
4.5. Western Blot Analysis
4.6. Isolation of Periplasm, OMVs, and OMV-Free Supernatant
4.7. Cytotoxicity Assay
4.8. Indirect ELISA
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, E.J.; Harris, J.B.; Morris Jr, J.G.; Calderwood, S.B.; Camilli, A. Cholera transmission: The host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 2009, 7, 693. [Google Scholar]
- Almagro-Moreno, S.; Pruss, K.; Taylor, R.K. Intestinal colonization dynamics of Vibrio cholerae. PLoS Pathog. 2015, 11, e1004787. [Google Scholar] [CrossRef]
- Faruque, S.M.; Albert, M.J.; Mekalanos, J.J. Epidemiology, Genetics, and Ecology of Toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 1998, 62, 1301–1314. [Google Scholar] [PubMed]
- Reidl, J.; Klose, K.E. Vibrio cholerae and cholera: Out of the water and into the host. Fems Microbiol. Rev. 2002, 26, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Mekalanos, J.; Collier, R.; Romig, W. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J. Biol. Chem. 1979, 254, 5855–5861. [Google Scholar] [PubMed]
- Sanchez, J.; Holmgren, J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell. Mol. Life Sci. 2008, 65, 1347–1360. [Google Scholar] [CrossRef]
- Bharati, K.; Ganguly, N.K. Cholera toxin: A paradigm of a multifunctional protein. Indian J. Med Res. 2011, 133, 179. [Google Scholar] [PubMed]
- Sikora, A.E. Proteins secreted via the type II secretion system: Smart strategies of Vibrio cholerae to maintain fitness in different ecological niches. PLoS Pathog. 2013, 9, e1003126. [Google Scholar] [CrossRef] [PubMed]
- Pugsley, A.P. The complete general secretory pathway in Gram-negative bacteria. Microbiol. Rev. 1993, 57, 50–108. [Google Scholar] [PubMed]
- Douzi, B.; Filloux, A.; Voulhoux, R. On the path to uncover the bacterial type II secretion system. Philos. Trans. R. Soc. B 2012, 367, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, Y.; Wolf, A.A.; Rodighiero, C.; Wheeler, H.; Tsai, B.; Allen, L.; Jobling, M.G.; Rapoport, T.; Holmes, R.K.; Lencer, W.I. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulm. Mol. Biol. Cell 2003, 14, 4783–4793. [Google Scholar] [CrossRef]
- Feng, Y.; Jadhav, A.P.; Rodighiero, C.; Fujinaga, Y.; Kirchhausen, T.; Lencer, W.I. Retrograde transport of cholera toxin from the plasma membrane to the endoplasmic reticulum requires the trans-Golgi network but not the Golgi apparatus in Exo2-treated cells. Embo Rep. 2004, 5, 596–601. [Google Scholar] [CrossRef]
- Tsai, B.; Rodighiero, C.; Lencer, W.I.; Rapoport, T.A. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 2001, 104, 937–948. [Google Scholar] [CrossRef]
- Taylor, M.; Banerjee, T.; Ray, S.; Tatulian, S.A.; Teter, K. Protein-disulfide isomerase displaces the cholera toxin A1 subunit from the holotoxin without unfolding the A1 subunit. J. Biol. Chem. 2011, 286, 22090–22100. [Google Scholar] [CrossRef] [PubMed]
- Wernick, N.L.; Chinnapen, D.J.-F.; Cho, J.A.; Lencer, W.I. Cholera toxin: An intracellular journey into the cytosol by way of the endoplasmic reticulum. Toxins 2010, 2, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Lencer, W.I.; Hirst, T.R.; Holmes, R.K. Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta (Bba)-Mol. Cell Res. 1999, 1450, 177–190. [Google Scholar] [CrossRef]
- Chatterjee, D.; Chaudhuri, K. Vibrio cholerae O395 Outer Membrane Vesicles Modulate Intestinal Epithelial Cells in a NOD1 Protein-dependent Manner and Induce Dendritic Cell-mediated Th2/Th17 Cell Responses. J. Biol. Chem. 2013, 288, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Rasti, E.S.; Schappert, M.L.; Brown, A.C. Association of Vibrio cholerae 569B outer membrane vesicles with host cells occurs in a GM1-independent manner. Cell. Microbiol. 2018, 20, e12828. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef]
- Miller, V.L.; Mekalanos, J.J. A novel suicide vector and its use in construction of insertion mutations: Osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1988, 170, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Gardel, C.L.; Mekalanos, J.J. Regulation of cholera toxin by temperature, pH, and osmolarity. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 235, pp. 517–526. [Google Scholar]
- Cong, Y.; Bowdon, H.R.; Elson, C.O. Identification of an immunodominant T cell epitope on cholera toxin. Eur. J. Immunol. 1996, 26, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Wallerström, G.; Fredriksson, M.; Ångström, J.; Holmgren, J. Detoxification of Cholera Toxin without Removal of Its Immunoadjuvanticity by the Addition of (STa-related) Peptides to the Catalytic Subunit. A Potential New Strategy to Generate Immunostimulants for Vaccination. J. Biol. Chem. 2002, 277, 33369–33377. [Google Scholar] [CrossRef]
- Iwanaga, M.; Yamamoto, K.; Higa, N.; Ichinose, Y.; Nakasone, N.; Tanabe, M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 1986, 30, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Hirst, T.R.; Holmgren, J. Conformation of protein secreted across bacterial outer membranes: A study of enterotoxin translocation from Vibrio cholerae. Proc. Natl. Acad. Sci. USA 1987, 84, 7418–7422. [Google Scholar] [CrossRef]
- Hardy, S.; Holmgren, J.; Johansson, S.; Sanchez, J.; Hirst, T.R. Coordinated assembly of multisubunit proteins: Oligomerization of bacterial enterotoxins in vivo and in vitro. Proc. Natl. Acad. Sci. USA 1988, 85, 7109–7113. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, K.V.; Sandkvist, M.; Hol, W.G. The type II secretion system: Biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol. 2012, 10, 336. [Google Scholar] [CrossRef]
- Hirst, T.R.; Sanchez, J.; Kaper, J.B.; Hardy, S.; Holmgren, J. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc. Natl. Acad. Sci. USA 1984, 81, 7752–7756. [Google Scholar] [CrossRef]
- Sánchez, J.; Holmgren, J. Cholera toxin—a foe & a friend. Indian J. Med Res. 2011, 133, 153. [Google Scholar]
- Reichow, S.L.; Korotkov, K.V.; Hol, W.G.; Gonen, T. Structure of the cholera toxin secretion channel in its closed state. Nat. Struct. Mol. Biol. 2010, 17, 1226. [Google Scholar] [CrossRef]
- Reichow, S.L.; Korotkov, K.V.; Gonen, M.; Sun, J.; Delarosa, J.R.; Hol, W.G.; Gonen, T. The binding of cholera toxin to the periplasmic vestibule of the type II secretion channel. Channels 2011, 5, 215–218. [Google Scholar] [CrossRef]
- Connell, T.D.; Metzger, D.J.; Wang, M.; Jobling, M.G.; Holmes, R.K. Initial studies of the structural signal for extracellular transport of cholera toxin and other proteins recognized by Vibrio cholerae. Infect. Immun. 1995, 63, 4091–4098. [Google Scholar]
- Booth, B.; Boesman-Finkelstein, M.; Finkelstein, R. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect. Immun. 1984, 45, 558–560. [Google Scholar]
- Sikora, A.E.; Zielke, R.A.; Lawrence, D.A.; Andrews, P.C.; Sandkvist, M. Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J. Biol. Chem. 2011, 286, 16555–16566. [Google Scholar] [CrossRef]
- Lencer, W.I.; Constable, C.; Moe, S.; Rufo, P.A.; Wolf, A.; Jobling, M.G.; Ruston, S.P.; Madara, J.L.; Holmes, R.K.; Hirst, T.R. Proteolytic Activation of Cholera Toxin and Escherichia coli Labile Toxin by Entry into Host Epithelial Cells. Signal transduction by a protease-resistant toxin variant. J. Biol. Chem. 1997, 272, 1556–15568. [Google Scholar] [CrossRef]
- Soussi, M.; Santamaria, M.; Ocana, A.; Lluch, C. Effects of salinity on protein and lipopolysaccharide pattern in a salt-tolerant strain of Mesorhizobium ciceri. J. Appl. Microbiol. 2001, 90, 476–481. [Google Scholar] [CrossRef]
- Aguilar, A.; Merino, S.; Rubires, X.; Tomas, J.M. Influence of osmolarity on lipopolysaccharides and virulence of Aeromonas hydrophila serotype O: 34 strains grown at 37 degrees C. Infect. Immun. 1997, 65, 1245–1250. [Google Scholar] [PubMed]
- Horstman, A.L.; Kuehn, M.J. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 2000, 275, 12489–12496. [Google Scholar] [CrossRef]
- Horstman, A.L.; Kuehn, M.J. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J. Biol. Chem. 2002, 277, 32538–32545. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, H.; Witholt, B. Heat-labile enterotoxin in Escherichia coli. Kinetics of association of subunits into periplasmic holotoxin. J. Biol. Chem. 1985, 260, 16037–16044. [Google Scholar] [PubMed]
- Quan, S.; Hiniker, A.; Collet, J.-F.; Bardwell, J.C. Isolation of bacteria envelope proteins. In Bacterial Cell Surfaces; Springer: Berlin/Heidelberg, Germany, 2013; pp. 359–366. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasti, E.S.; Brown, A.C. Cholera Toxin Encapsulated within Several Vibrio cholerae O1 Serotype Inaba Outer Membrane Vesicles Lacks a Functional B-Subunit. Toxins 2019, 11, 207. https://doi.org/10.3390/toxins11040207
Rasti ES, Brown AC. Cholera Toxin Encapsulated within Several Vibrio cholerae O1 Serotype Inaba Outer Membrane Vesicles Lacks a Functional B-Subunit. Toxins. 2019; 11(4):207. https://doi.org/10.3390/toxins11040207
Chicago/Turabian StyleRasti, Elnaz S., and Angela C. Brown. 2019. "Cholera Toxin Encapsulated within Several Vibrio cholerae O1 Serotype Inaba Outer Membrane Vesicles Lacks a Functional B-Subunit" Toxins 11, no. 4: 207. https://doi.org/10.3390/toxins11040207
APA StyleRasti, E. S., & Brown, A. C. (2019). Cholera Toxin Encapsulated within Several Vibrio cholerae O1 Serotype Inaba Outer Membrane Vesicles Lacks a Functional B-Subunit. Toxins, 11(4), 207. https://doi.org/10.3390/toxins11040207




