Paralytic Shellfish Toxins in Surf Clams Mesodesma donacium during a Large Bloom of Alexandrium catenella Dinoflagellates Associated to an Intense Shellfish Mass Mortality
Abstract
1. Introduction
2. Results
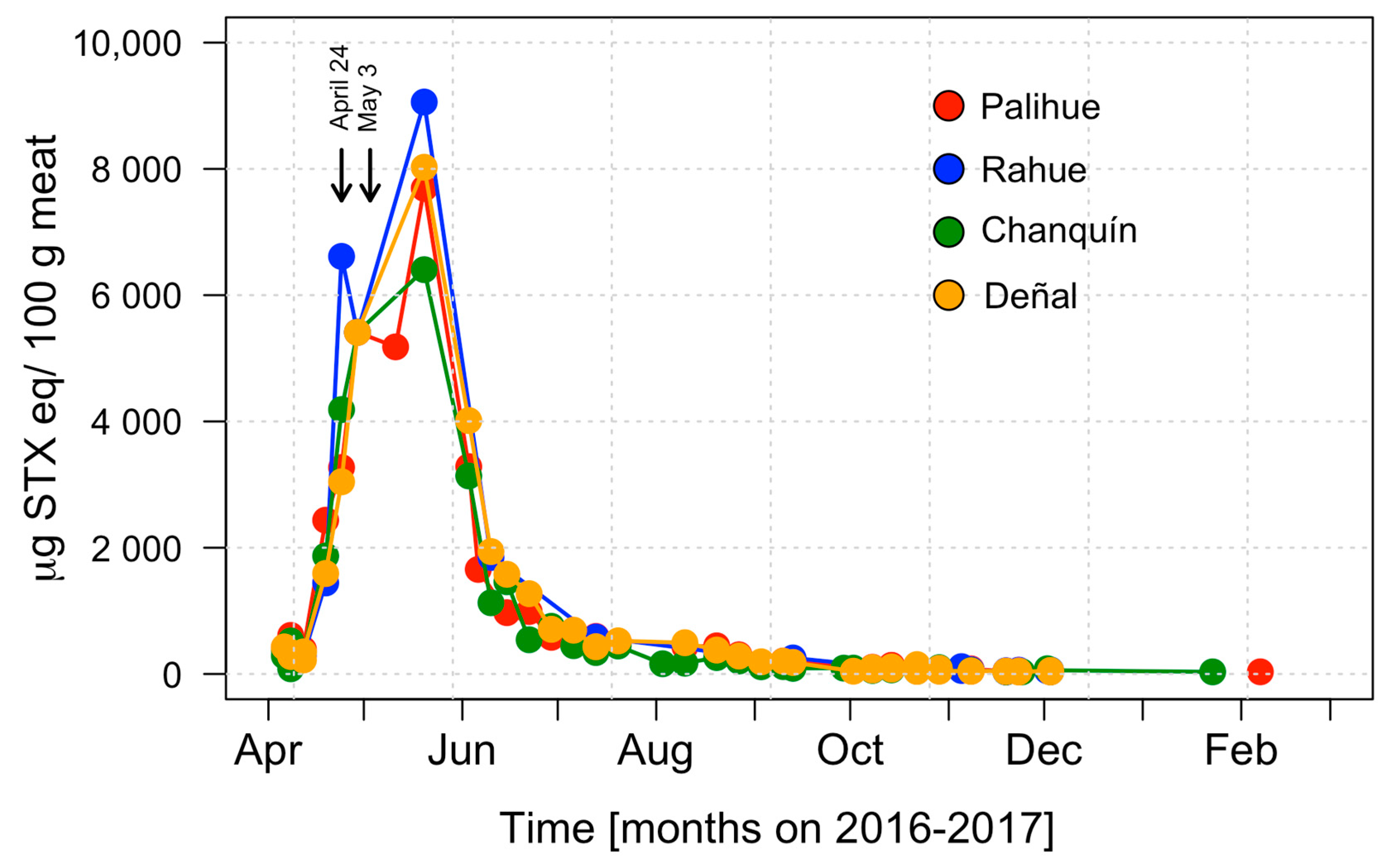
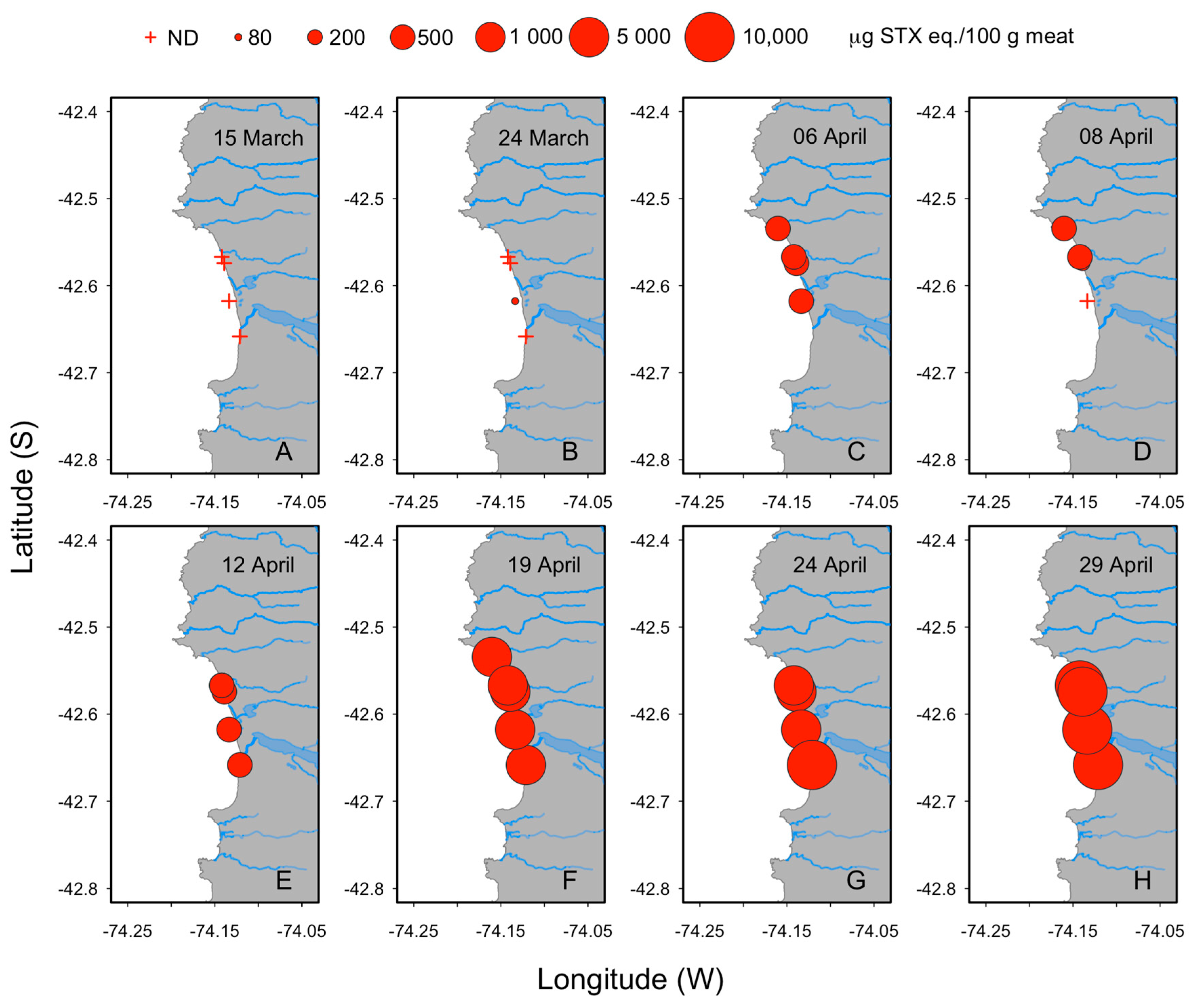
2.1. Toxicity of Surf Clams and Massive Beaching in Cucao Bay
2.2. Visual Observations of Surf Clams during Massive Beaching
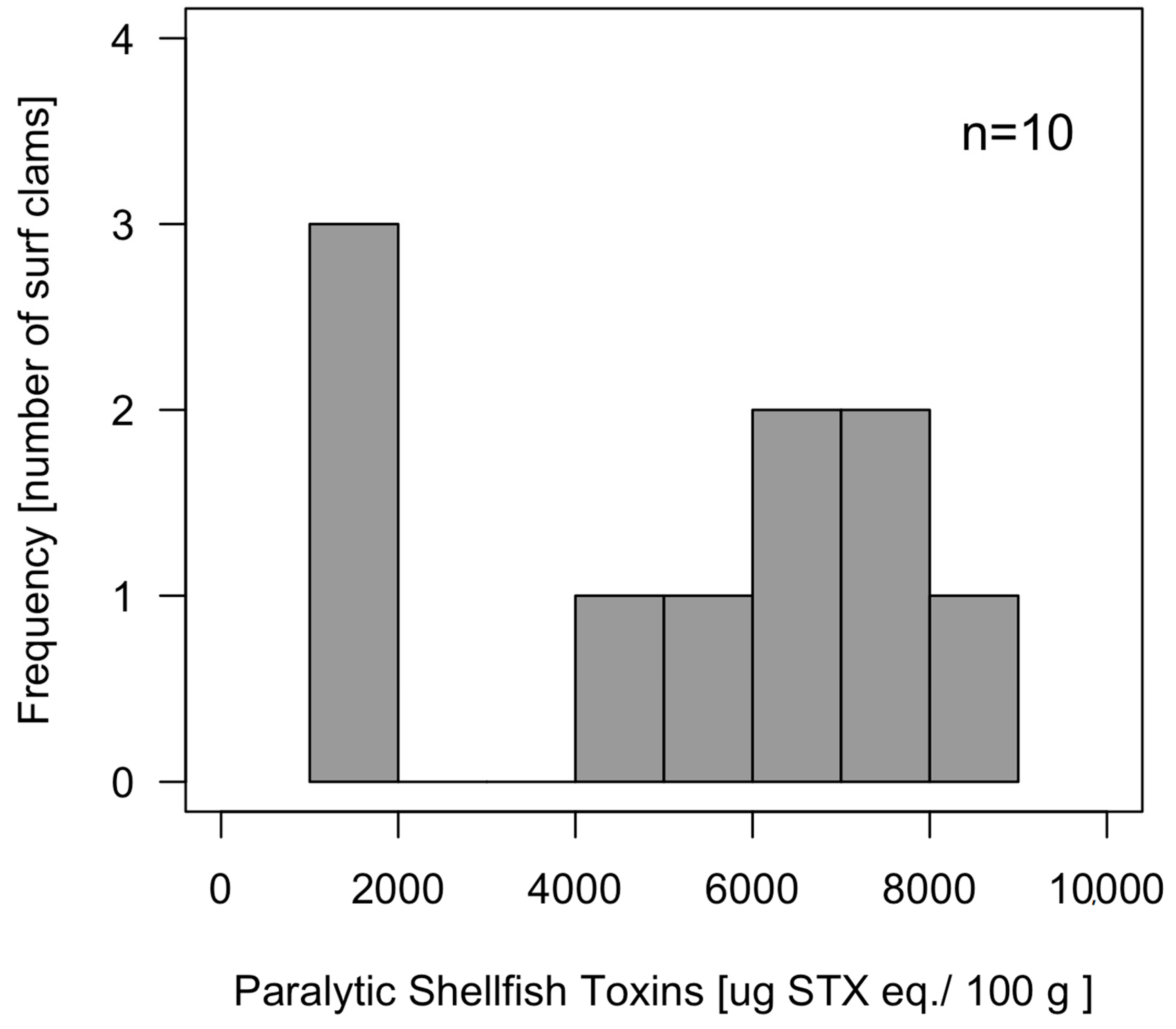
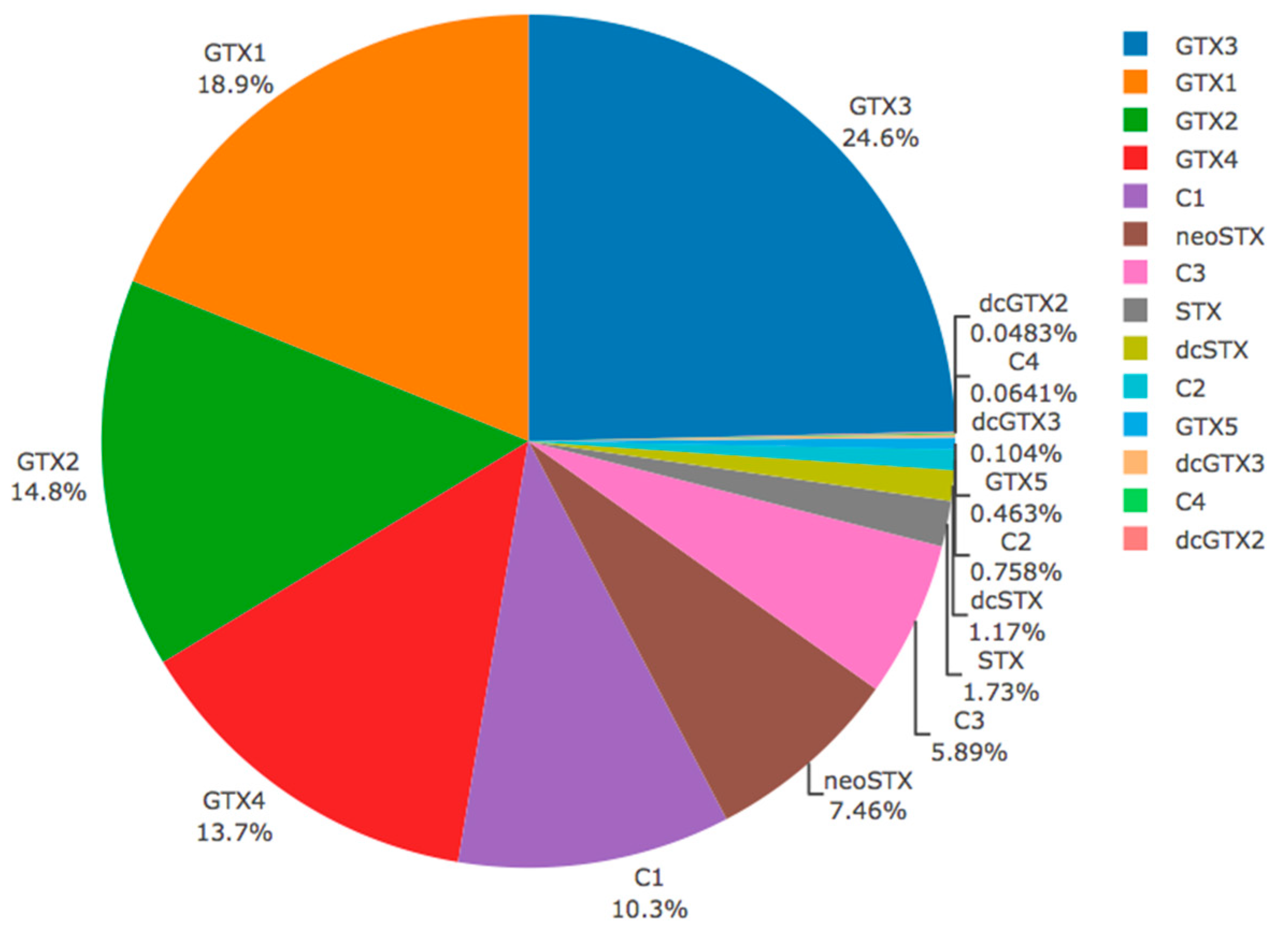
2.3. Toxicity and Toxin Profile of Whole Individuals
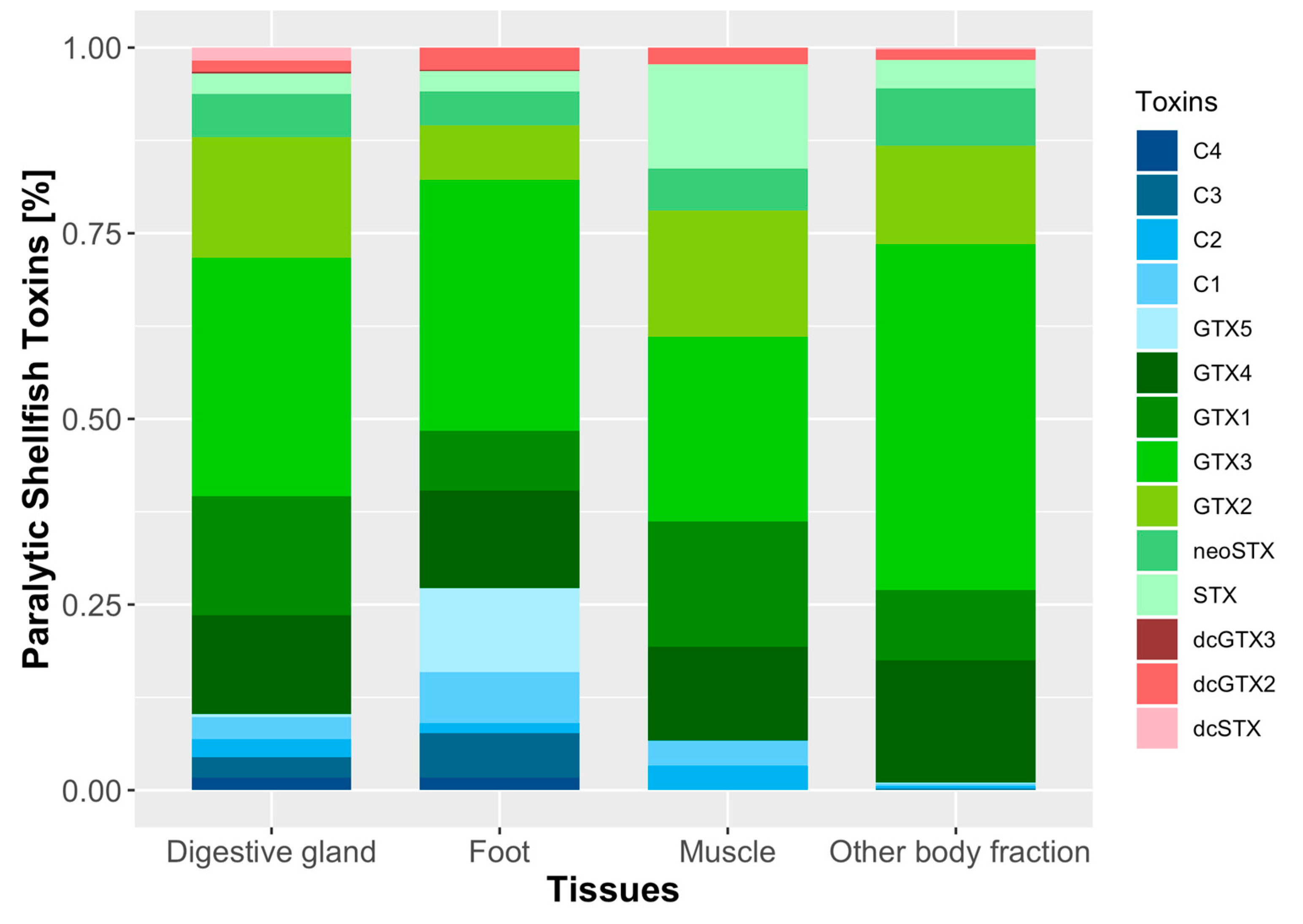
2.4. Toxicity and Toxin Profile of Surf Clam Tissues
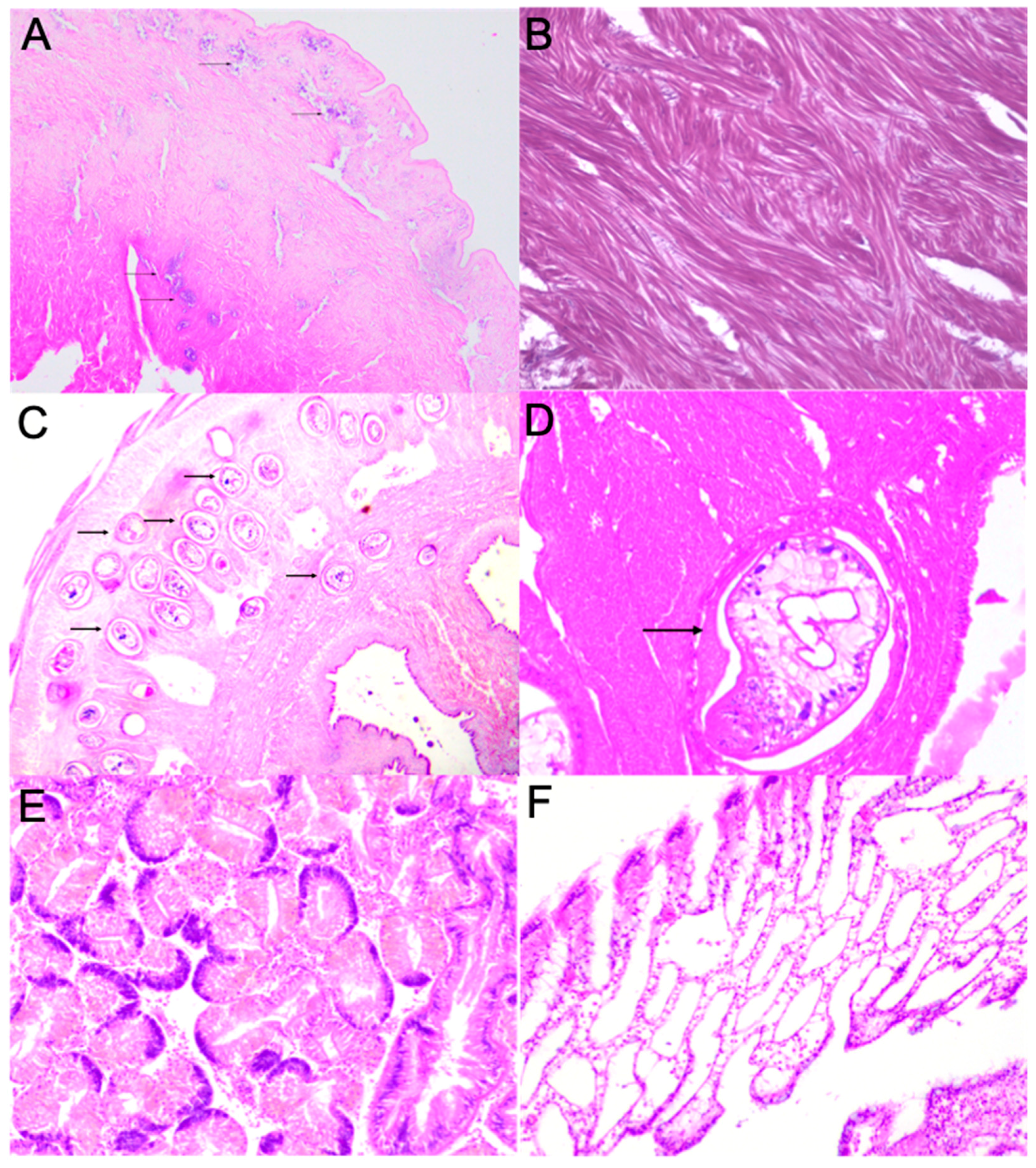
2.5. Surf Clam Histology
3. Discussion
4. Materials and Methods
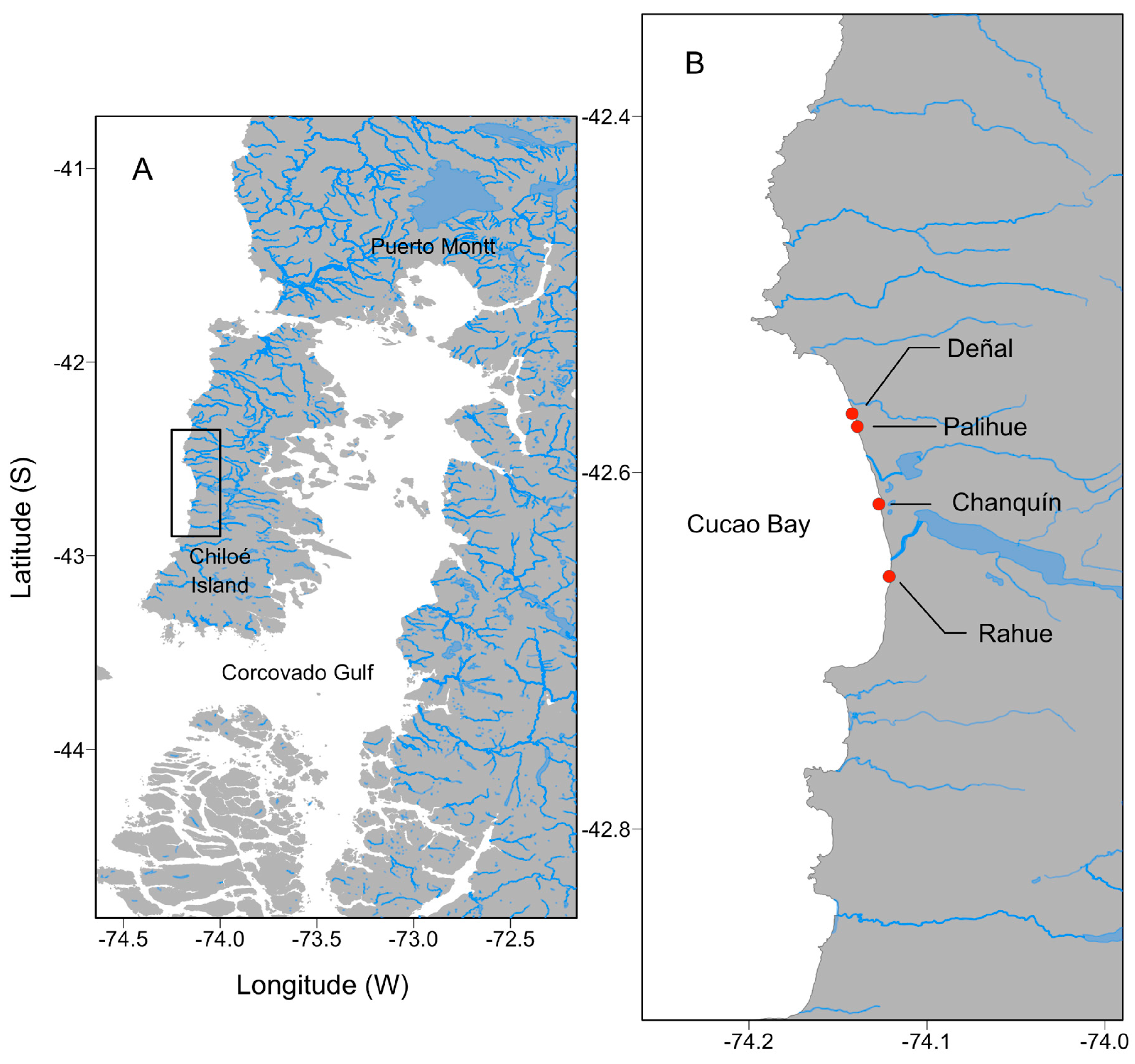
4.1. Study Area
4.2. Shellfish Sampling, Toxin Extraction, and Biological Method Analyses Used for Rutine Monitoring
4.3. Shellfish Sampling, Toxin Extraction, Chromatographic, and Histopathological Analyses during Beaching
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mons, M.N.; Van Egmond, H.P.; Speijers, G.J.A. Paralytic shellfish poisoning: A review. Rivm Rep. 1998, 388802005, 1–47. [Google Scholar]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef]
- Anderson, D.M. HABs in a changing world: A perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climactic and environmental change. In Harmful Algae 2012, Proceedings of the 15th International Conference on Harmful Algae, International Society for the Study of Harmful Algae (2014). ISBN 978-87-990827-4-2, 16p; Kim, H.G., Reguera, B., Hallegraeff, G., Lee, C.K., Han, M.S., Choi, J.K., Eds.; 2014. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4667985/ (accessed on 2 December 2015).
- Usup, G.; Ahmad, A.; Matsuoka, K.; Lim, P.T.; Leaw, C.P. Biology, ecology and bloom dynamics of the toxic marine dinoflagellate Pyrodinium bahamense. Harmful Algae 2012, 14, 301–312. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y. Chemical and enzymatic transformation of paralytic shellfish toxins in marine organisms. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard-Le Denn, E., Gentien, P., Marcaillou-Le Baut, C., Eds.; Lavoisier Science Publishers: Paris, France, 1995; pp. 475–480. [Google Scholar]
- Oshima, Y.; Sugino, K.; Itakura, H.; Hirota, M.; Yasumoto, Y. Comparative studies on paralytic shellfish toxin profile of dinoflagellates and bivalves. In Toxic Marine Phytoplankton; Granelli, E., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Elsevier Science Publishing: New York, NY, USA, 1990; pp. 391–396. [Google Scholar]
- Negri, A.; Stirling, D.; Quilliam, M.; Blackburn, S.; Bolch, C.; Burton, I.; Eaglesham, G.; Thomas, K.; Walter, J.; Willis, R. Three novel hydroxybenzoate saxitoxin analogues isolated from the dinoflagellate Gymnodinium catenatum. Chem. Res. Toxicol. 2003, 16, 1029–1033. [Google Scholar] [CrossRef]
- Costa, P.R.; Robertson, A.; Quilliam, M.A. Toxin profile of Gymnodinium catenatum (Dinophyceae) from the Portuguese Coast, as determined by Liquid Chromatography Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 2046–2062. [Google Scholar] [CrossRef]
- Vale, P. New saxitoxin analogues in the marine environment: Developments in toxin chemistry, detection and biotransformation during the 2000s. Phytochem. Rev. 2010, 9, 525–535. [Google Scholar] [CrossRef]
- Dell’ Aversano, C.; Walter, J.A.; Burton, I.W.; Stirling, D.J.; Fattorusso, E.; Quilliam, M. Isolation and structure elucidation of new and unusual saxitoxin analogues from mussels. J. Nat. Prod. 2008, 71, 1518–1523. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Shumway, S.E. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquac. Soc. 1990, 21, 65–104. [Google Scholar] [CrossRef]
- Shumway, S.E.; Cucci, T.L. The effects of the toxic dinoflagellate Protogonyaulax tamarensis on the feeding and behaviour of bivalve molluscs. Aquat. Toxicol. 1987, 10, 9–97. [Google Scholar] [CrossRef]
- Bricelj, M.; Cembella, A.; Laby, D.; Shumway, S.; Cucci, T. Comparative physiological and behavioral responses to PSP toxins in two bivalve molluscs, the softshell Clam, Mya arenaria, and surfclam, Spisula solidissima. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovermental Oceanographic Comission of UNESCO: Paris, France, 1996; pp. 405–408. [Google Scholar]
- Gainey, L.F., Jr.; Shumway, S.E. A compendium of the responses of bivalve molluscs to toxic dinoflagellates. J. Shellfish Res. 1988, 7, 623–628. [Google Scholar]
- Lesser, M.P.; Shumway, S.E. Effects of toxic dinoflagellates on clearance rates and survival in juvenile bivalve molluscs. J. Shellfish Res. 1993, 12, 377–381. [Google Scholar]
- Hegaret, H.; Wikfors, G.H.; Shumway, S.E. Diverse feeding responses of five species of bivalve mollusc when exposed to three species of harmful algae. J. Shellfish Res. 2007, 26, 549–559. [Google Scholar] [CrossRef]
- Lassus, P.; Bardouil, M.; Beliaeff, B.; Masselin, P.; Naviner, M.; Truquet, P. Effect of a continuous supply of the toxic dinoflagellate Alexandrium minutum Halim on the feeding behavior of the Pacific oyster (Crassostrea gigas Thunberg). J. Shellfish Res. 1999, 18, 211–216. [Google Scholar]
- May, S.P.; Burkholder, J.A.M.; Shumway, S.E.; Hégaret, H.; Wikfors, G.H.; Frank, D. Effects of the toxic dinoflagellate Alexandrium monilatum on survival, grazing and behavioral response of three ecologically important bivalve molluscs. Harmful Algae 2010, 9, 281–293. [Google Scholar] [CrossRef]
- Tran, D.; Haberkorn, H.; Soudant, P.; Ciret, P.; Massabuau, J.-C. Behavioral responses of Crassostrea gigas exposed to the harmful algae Alexandrium minutum. Aquaculture 2010, 298, 338–345. [Google Scholar] [CrossRef]
- Fabioux, C.; Sulistiyani, Y.; Haberkorn, H.; Hégaret, H.; Amzil, Z.; Soudant, P. Exposure to toxic Alexandrium minutum activates the detoxifying and antioxidant systems in gills of the oyster Crassostrea gigas. Harmful Algae 2015, 48, 55–62. [Google Scholar] [CrossRef]
- Neves, R.A.F.F.; Figueiredo, G.M.; Valentin, J.L.; da Silva Scardua, P.M.; Hégaret, H. Immunological and physiological responses of the periwinkle Littorina littorea during and after exposure to the toxic dinoflagellate Alexandrium minutum. Aquat. Toxicol. 2015, 160, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Lassudrie, M.; Wikfors, G.H.; Sunila, I.; Alix, J.H.; Dixon, M.S.; Combot, D.; Soudant, P.; Fabioux, C.; Hégaret, H. Physiological and pathological changes in the eastern oyster Crassostrea virginica infested with the trematode Bucephalus sp. and exposed to the toxic dinoflagellate Alexandrium fundyense. J. Invertebr. Pathol. 2015, 126, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Borcier, E.; Morvezen, R.; Boudry, P.; Miner, P.; Charrier, G.; Laroche, J.; Hegaret, H. Effects of bioactive extracellular compounds and paralytic shellfish toxins produced by Alexandrium minutum on growth and behaviour of juvenile great scallops Pecten maximus. Aquat. Toxicol. 2017, 184, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Horstman, D.A. Reported red-water outbreaks and their effects on fauna of the west and south coasts of South Africa, 1959–1980. Fish. Bull. South. Afr. 1981, 15, 71–88. [Google Scholar]
- Popkiss, M.E.E.; Horstman, D.A.; Harpur, D. Paralytic shellfish poisoning: A report of 17 cases in Cape Town. South Afr. Med. J. 1979, 55, 1017–1023. [Google Scholar]
- Wardle, W.J.; Ray, S.M.; Aldrich, A.S. Mortality of marine organisms associated with offshore summer blooms of the toxic dinoflagellate Gonyaulax monilata Howell at Galveston, Texas. In Proceedings of the First International Conference on Toxic Dinoflagellate Blooms; LoCicero, V.R., Ed.; Massachusetts Science and Technology Foundation: Wakefield, MA, USA, 1975; pp. 257–263. [Google Scholar]
- Guzmán, L.; Campodonico, I.; Antunovic, M. Estudios sobre un florecimiento toxico causado por Gonyaulax catenella en Magallanes. IV. Distribución y niveles de veneno paralítico de los mariscos (noviembre de 1972–noviembre de 1973). Inst. Patagon. 1975, 6, 209–223. [Google Scholar]
- Lembeye, G.; Marcos, N.; Sfeir, A.; Molinet, C.; Jara, F. Seguimiento de la toxicidad en recursos pesqueros de importancia comercial en la X y XI región; Informe Final Proyecto FIP 97/49; Universidad Austral de Chile: Puerto Montt, Chile, 1998; Volume 89. [Google Scholar]
- García, C.; Mardones, P.; Sfeir, A.; Lagos, N. Simultaneous presence of Paralytic and Diarrheic Shellfish Poisoning toxins in Mytilus chilensis samples collected in the Chiloe Island, Austral Chilean Fjords. Biol. Res. 2004, 37, 721–731. [Google Scholar] [CrossRef]
- Muñoz, P.; Avaria, S.; Sievers, H.; Prado, R. Presencia de dinoflagelados toxicos del genero Dinophysis en el seno Aysén, Chile. Rev. Biol. Mar. 1992, 27, 187–212. [Google Scholar]
- Aguilera-Belmonte, A.; Inostroza, I.; Franco, J.M.; Riobo, P.; Gómez, P.I. The growth, toxicity and genetic characterization of seven strains of Alexandrium catenella (Whedon and Kofoid) Balech 1985 (Dinophyceae) isolated during the 2009 summer outbreak in southern Chile. Harmful Algae 2011, 12, 105–112. [Google Scholar] [CrossRef]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Díaz, M.; Labra, G.; Figueroa, R. Coupling planktonic and benthic shifts during a bloom of Alexandrium catenella in southern Chile: Implications for bloom dynamics and recurrence. Harmful Algae 2014, 40, 9–22. [Google Scholar] [CrossRef]
- Guzmán, L.; Pacheco, H.; Pizarro, G.; Alárcon, C. Alexandrium catenella y veneno paralizante de los mariscos en Chile. In Floraciones Algales Nocivas en el Cono Sur Americano; Sar, E.A., Ferrario, M.E., Reguera, B., Eds.; Instituto Español de Oceanografía: Madrid, Spain, 2002; Volume 11, pp. 235–255. [Google Scholar]
- Mardones, J.; Clément, A.; Rojas, X.; Aparicio, C. Alexandrium catenella during 2009 in Chilean waters, and recent expansion to coastal ocean. Harmful Algae News 2010, 41, 8–9. [Google Scholar]
- Molinet, C.; Lafón, A.; Lembeye, G.; Moreno, C.A. Patrones de distribución espacial y temporal de floraciones de Alexandrium catenella (Whedon & Kofoid) Balech 1985, en aguas interiores de la Patagonia noroccidental de Chile. Rev. Chil. Hist. Nat. 2003, 76, 681–698. [Google Scholar]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Díaz, M.; Labra, G.; Figueroa, R.I. Species diversity and abundance of dinoflagellate resting cysts seven months after a bloom of Alexandrium catenella in two contrasting coastal systems of the Chilean Inland Sea. Eur. J. Phycol. 2018, 3, 410–421. [Google Scholar] [CrossRef]
- Guzmán, L.; Espinoza-González, O.; Pinilla, E.; Martinez, R.; Carbonell, P.; Calderón, M.J.; Lopez, L.; Hernández, C. The Alexandrium catenella and PSP outbreak in the Chilean coast, the first in the open coast of the South East Pacific Ocean. In The 17th International Conference of Harmful Algae; Odebrecht, C., Cintra, F., Proença, L., Mafra, L., Schramm, M., Garlet, N., Alves, T., Eds.; Florianopolis, Brazil, 2016; Volume 74. [Google Scholar]
- Hernández, C.; Díaz, P.A.; Molinet, C.; Seguel, M. Exceptional climate anomalies and northwards expansion of Paralytic Shellfish Poisoning outbreaks in Southern Chile. Harmful Algae News 2016, 54, 1–2. [Google Scholar]
- Alamo, V.V.; Valdivieso, V.M. Lista sistemática de moluscos marinos del Perú; Segunda edición, revisada y actualizada; Instituto del Mar del Perú: Callao, Perú, 1997; p. 183. [Google Scholar]
- Tarifeño, E. Studies on the biology of surf clam Mesodesma donacium (Lamarck, 1818) (Bivalvia: Mesodesmatidae) from Chilean sandy beaches. Ph.D. Dissertation, University of California, Los Angeles, CA, USA, 1980. [Google Scholar]
- Guzmán, N.; Saá, S.; Ortlieb, L. Catálogo descriptivo de los moluscos litorales (Gastropoda y Pelecypoda) de la zona de Antofagasta, 23 S (Chile). Est. Ocea. 1998, 17, 17–86. [Google Scholar]
- Jaramillo, E.; Pino, M.; Filun, L.; Gonzalez, M. Longshore distribution of Mesodesma donacium (Bivalvia: Mesodes-matidae) on a sandy beach of the south of Chile. Veliger 1994, 37, 192–200. [Google Scholar]
- Ortega, L.; Castilla, J.C.; Espino, M.; Yamashiro, C.; Defeo, O. Effects of fishing, market price, and climate on two South American clam species. Mar. Ecol. Prog. Ser. 2012, 469, 71–82. [Google Scholar] [CrossRef]
- Riascos, J.M.; Carstensen, D.; Laudien, J.; Arntz, W.E.; Oliva, M.E.; Guntner, A.; Heilmayer, O. Thriving and declining: Climate variability shaping life-history and population persistence of Mesodesma donacium in the Humboldt Upwelling System. Mar. Ecol. Prog. Ser. 2009, 385, 151–163. [Google Scholar] [CrossRef]
- García, C.; Pérez, F.; Contreras, C.; Figueroa, D.; Barriga, A.; López-Rivera, A.; Araneda, O.F.; Contreras, H.R. Saxitoxins and okadaic acid group: Accumulation and distribution in invertebrate marine vectors from Southern Chile. Food Addit. Contam. Part A 2015, 32, 984–1002. [Google Scholar] [CrossRef]
- Molinet, C.; Niklitschek, E.; Seguel, M.; Díaz, P. Trends of natural accumulation and detoxification of paralytic shellfish poison in two bivalves from the Northwest Patagonian inland sea. Revista de Biologia Marina y Oceanografia 2010, 45, 195–204. [Google Scholar] [CrossRef]
- Compagnon, D.; Lembeye, G.; Marcos, N.; Ruíz-Tagle, N.; Lagos, N. Accumulation of Paralytic Shellfish Poisoning toxins in the bivalve Aulacomya ater and two carnivorous gastropods Concholepas concholepas and Argobuccinum ranelliformes during an Alexandrium catenella bloom in southern Chile. J. Shellfish Res. 1998, 17, 67–73. [Google Scholar]
- Díaz, P.A.; Álvarez, A.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019. [Google Scholar] [CrossRef]
- White, A.W.; Shumway, S.E.; Nassif, J.; Whittaker, D.K. Variation in Levels of Paralytic Shellfish Toxins among Individual Shellfish. In Toxin Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1993; pp. 441–446. [Google Scholar]
- Cembella, A.D.; Shumway, S.E.; Lewis, N.I.I. Anatomical distribution and spatio-temporal variation in paralytic shellfish toxin composition in two bivalve species from the Gulf of Maine. J. Shellfish Res. 1993, 12, 389–403. [Google Scholar]
- Curtis, K.M.; Trainer, V.L.; Shumway, S.E. Paralytic shellfish toxins in geoduck clams (Panope abrupta): Variability, anatomical distribution, and comparison of two toxin detection methods. J. Shellfish Res. 2000, 19, 313–319. [Google Scholar]
- Pousse, E.; Flye-Sainte-Marie, J.; Alunno-Bruscia, M.; Hegaret, H.; Jean, F. Sources of paralytic shellfish toxin accumulation variability in the Pacific oyster Crassostrea gigas. Toxicon 2018, 144, 14–22. [Google Scholar] [CrossRef]
- Mardsen, I.D.; Shumway, S.E. The effect of a toxic dinoflagellate (Alexandrium tamarense) on the oxygen uptake of juvenile filter-feeding bivalve molluscs. Comp. Biochem. Physiol. 1993, 106, 769–773. [Google Scholar]
- Bardouil, M.; Bohec, M.; Cormerais, M.; Bougrier, S.; Lassus, P. Experimental study of the effects of a toxic microalgal diet on feeding of the oyster Crassostrea gigas Thunberg. J. Shellfish Res. 1993, 12, 417–422. [Google Scholar]
- Bricelj, V.M.; Shumway, S. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Li, S.C.; Wang, W.X.; Hsieh, D.P.H. Effects of toxic dinoflagellate Alexandrium tamarense on the energy budgets and growth of two marine bivalves. Mar. Environ. Res. 2002, 53, 145–160. [Google Scholar] [CrossRef]
- Navarro, J.M.; González, K.; Cisternas, B.A.; López, J.A.; Chaparro, O.R.; Segura, C.; Cordova, M.; Suarez Isla, B.; Fernández-Reiriz, M.J.; Labarta, U. Contrasting physiological responses of two populations of the razor clam Tagelus dombeii with different histories of exposure to paralytic shellfish poisoning (PSP). PLoS ONE 2014, 9, 9. [Google Scholar] [CrossRef]
- Bougrier, S.; Lassus, P.; Bardouil, M.; Masselin, P.; Truquet, P. Paralytic shellfish poison accumulation yields and feeding time activity in the Pacific oyster (Crassostrea gigas) and king scallop (Pecten maximus). Aquat. Living Resour. 2003, 16, 347–352. [Google Scholar] [CrossRef]
- Haberkorn, H.; Tran, D.; Massabuau, J.-C.; Ciret, P.; Savar, V.; Soudant, P. Relationship between valve activity, microalgae concentration in the water and toxin accumulation in the digestive gland of the Pacific oyster Crassostrea gigas exposed to Alexandrium minutum. Mar. Pollut. Bull. 2011, 62, 1191–1197. [Google Scholar] [CrossRef]
- Lagos, N.; Compagnon, D.; Seguel, M.; Oshima, Y. Paralytic shellfish toxin composition: A quantitative analysis in Chilean mussels and dinoflagellate. In Harmful Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovermental Oceanographic Comission of UNESCO: Paris, France, 1996; pp. 121–124. [Google Scholar]
- Krock, B.; Seguel, C.; Cembella, A. Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae 2007, 6, 734–744. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Cembella, A.D. Fate of gonyautoxins in surfclams, Spisula solidissima, grazing upon toxigenic Alexandrium. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Lavoisier, Intercept Ltd: Paris, France, 1995; pp. 413–418. [Google Scholar]
- Bricelj, V.M.; Lee, J.H.; Cembella, A.D. Influence of dinoflagellate cell toxicity on uptake and loss of paralytic shellfish toxins in the northern quahog Mercenaria mercenaria. Mar. Ecol. Prog. Ser. 1991, 74, 33–46. [Google Scholar] [CrossRef]
- Sullivan, J.J. Paralytic Shellfish Poisoning: Analytical and Biochemical Investigations; University of Washington: Seattle, WA, USA, 1982. [Google Scholar]
- Montojo, U.M.; Romero, M.L.; Borja, V.M.; Sato, S. Comparative PSP toxin accumulation in bivalves, Paphia undulata and Perna viridis in Sorsogon Bay, Philippines. In Proceedings of the Seventh International Conference on Molluscan Shellfish Safety; Lassus, P., Ed.; Ifremer: Nantes, France, 2010; pp. 49–55. [Google Scholar]
- Contreras, A.M.; Marsden, I.D.; Munro, M.H.G. Physiological effects and biotransformation of PSP toxins in the New Zealand scallop, Pecten novaezelandiae. J. Shellfish Res. 2012, 31, 1151–1159. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Lee, J.M.; Cembella, A.D.; Anderson, D.M. Uptake kinetics of paralytic shellfish toxins from the dinoflagellate Alexandrium fundyense in the mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 1990, 63, 177–188. [Google Scholar] [CrossRef]
- Ichimi, K.; Suzuki, T.; Yamasaki, M. Non-selective retention of PSP toxins by the mussel Mytilus galloprovincialis feb with the toxic dinoflagellate Alexandrium tamarense. Toxicon 2001, 39, 1917–1921. [Google Scholar] [CrossRef]
- Blanco, J.; Reyero, M.; Franco, J. Kinetics of accumulation and transformation of paralytic shellfish toxins in the blue mussel Mytilus galloprovincialis. Toxicon 2003, 42, 777–784. [Google Scholar] [CrossRef]
- Medina-Elizalde, J.; García-Mendoza, E.; Turner, A.D.; Sánchez-Bravo, Y.A.; Murillo-Martínez, R. Transformation and depuration of paralytic shellfish toxins in the Geoduck clam Panopea globosa from the Northern Gulf of California. Front. Mar. Sci. 2018, 5, 335. [Google Scholar] [CrossRef]
- Sullivan, J.J.; Iwaoka, W.T.; Liston, J. Enzymatic transformation of PSP toxins in the littleneck clam (Protothaca staminea). Biochem. Biophys. Res. Commun. 1983, 114, 465–472. [Google Scholar] [CrossRef]
- Turner, A.D.; Lewis, A.M.; O’Neil, A.; Hatfield, R.G. Transformation of paralytic shellfish poisoning toxins in UK surf clams (Spisula solida) for targeted production of reference materials. Toxicon 2013, 65, 41–58. [Google Scholar] [CrossRef]
- Haberkorn, H.; Lambert, C.; Le Goïc, N.; Moal, J.; Suquet, M.; Guéguen, M.; Sunila, I.; Soudant, P. Effects of Alexandrium minutum exposure on nutrition-related processes and reproductive output in oysters Crassostrea gigas. Harmful Algae 2010, 9, 427–439. [Google Scholar] [CrossRef]
- Estrada, N.; de Jesús Romero, M.; Campa-Córdova, A.; Luna, A.; Ascencio, F. Effects of the toxic dinoflagellate, Gymnodinium catenatum on hydrolytic and antioxidant enzymes, in tissues of the giant lions-paw scallop Nodipecten subnodosus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 502–510. [Google Scholar] [CrossRef]
- Galimany, E.; Sunila, I.; Hégaret, H.; Ramón, M.; Wikfors, G.H. Pathology and immune response of the blue mussel (Mytilus edulis L.) after an exposure to the harmful dinoflagellate Prorocentrum minimum. Harmful Algae 2008, 7, 630–638. [Google Scholar] [CrossRef]
- Hégaret, H.; da Silva, P.; Sunila, I.; Dixon, M.S.; Alix, J.; Shumway, S.E.; Wikfors, G.H.; Soudant, P. Perkinsosis in the Manila clam Ruditapes philippinarum affects responses to the harmful-alga, Prorocentrum minimum. J. Exp. Mar. Biol. Ecol. 2009, 371, 112–120. [Google Scholar] [CrossRef]
- Galimany, E.; Sunila, I.; Hégaret, H.; Ramón, M.; Wikfors, G.H. Experimental exposure of the blue mussel (Mytilus edulis, L.) to the toxic dinoflagellate Alexandrium fundyense: Histopathology, immune responses, and recovery. Harmful Algae 2008, 7, 702–711. [Google Scholar] [CrossRef]
- Abi-Khalil, C.; Lopez-Joven, C.; Abadie, E.; Savar, V.; Amzil, Z.; Laabir, M.; Rolland, J.L. Exposure to the paralytic shellfish toxin producer Alexandrium catenella increases the susceptibility of the oyster Crassostrea gigas to pathogenic vibrios. Toxins 2016, 8, 24. [Google Scholar] [CrossRef]
- López, A.Z.; Cárdenas, L.; González, M.T. Metazoan symbionts of the yellow clam, Mesodesma donacium (Bivalvia), in Southern Chile: Geographical variations. J. Parasitol. 2014, 100, 797–804. [Google Scholar] [CrossRef]
- MacQuarrie, S.P.; Bricelj, V.M. Behavioral and physiological responses to PSP toxins in Mya arenaria populations in relation to previous exposure to red tides. Mar. Ecol. Prog. Ser. 2008, 366, 59–74. [Google Scholar] [CrossRef]
- Phillips, J.M.; Bricelj, V.M.; Mitch, M.; Cerrato, R.M.; MacQuarrie, S.; Connell, L.B. Biogeography of resistance to paralytic shellfish toxins in softshell clam, Mya arenaria (L.), populations along the Atlantic coast of North America. Aquat. Toxicol. 2018, 202, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Bricelj, V.M.; MacQuarrie, S.P.; Doane, J.A.E.; Connell, L.B. Evidence of selection for resistance to paralytic shellfish toxins during the early life history of soft-shell clam, Mya arenaria, populations. Limmology Oceanogr. 2010, 55, 2463–2475. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Connell, L.; Konoki, K.; Macquarrie, S.P.; Scheuer, T.; Catterall, W.A.; Trainer, V.L. Sodium channel mutation leading to saxitoxin resistance in Clams increases risk of PSP. Nature 2005, 434, 763–767. [Google Scholar] [CrossRef]
- Anonymous. AOAC Official Method 959.08. Paralytic shellfish poison. Biological method. Final action. In AOAC Official Methods for Analysis; Truckses, M.W., Ed.; AOAC International: Gaithersburg, MD, USA, 2008; pp. 79–80. [Google Scholar]
- van de Riet, J.; Gibbs, R.S.; Muggah, P.M.; Rourke, W.A.; MacNeil, J.D.; Quilliam, M.A. Liquid Chromatography Post-Column Oxidation (PCOX) method for the determination of Paralytic Shellfish Toxins in mussels, clams, and scallops: Collaborative study. J. Aoac Int. 2011, 94, 1154–1176. [Google Scholar] [PubMed]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.P.; Domenico, A.D.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Scientific opinion of the panel on contaminants in the food chain on a request from the european commission of marine biotoxins in shellfish—saxitoxin group. EFSA J. 2010, 8, 1–76. [Google Scholar]
- Shaw, B.L.; Battle, H.I. The gross microscopic anatomy of the digestive tract of the oyster Crassostrea virginica (Gmelin). Can. J. Zool. 1957, 35, 325–347. [Google Scholar] [CrossRef]
- Howard, D.W.; Lewis, E.J.; Keller, B.J.; Smith, C.S. Histological Techniques for Marine Bivalve Mollusks and Crustaceans; NOAA Technical Memorandum NOS NCCOC: Oxford, UK, 2004. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, G.; Díaz, P.A.; Godoy, M.; Araya, M.; Ganuza, I.; Pino, R.; Álvarez, F.; Rengel, J.; Hernández, C.; Uribe, E.; et al. Paralytic Shellfish Toxins in Surf Clams Mesodesma donacium during a Large Bloom of Alexandrium catenella Dinoflagellates Associated to an Intense Shellfish Mass Mortality. Toxins 2019, 11, 188. https://doi.org/10.3390/toxins11040188
Álvarez G, Díaz PA, Godoy M, Araya M, Ganuza I, Pino R, Álvarez F, Rengel J, Hernández C, Uribe E, et al. Paralytic Shellfish Toxins in Surf Clams Mesodesma donacium during a Large Bloom of Alexandrium catenella Dinoflagellates Associated to an Intense Shellfish Mass Mortality. Toxins. 2019; 11(4):188. https://doi.org/10.3390/toxins11040188
Chicago/Turabian StyleÁlvarez, Gonzalo, Patricio A. Díaz, Marcos Godoy, Michael Araya, Iranzu Ganuza, Roberto Pino, Francisco Álvarez, José Rengel, Cristina Hernández, Eduardo Uribe, and et al. 2019. "Paralytic Shellfish Toxins in Surf Clams Mesodesma donacium during a Large Bloom of Alexandrium catenella Dinoflagellates Associated to an Intense Shellfish Mass Mortality" Toxins 11, no. 4: 188. https://doi.org/10.3390/toxins11040188
APA StyleÁlvarez, G., Díaz, P. A., Godoy, M., Araya, M., Ganuza, I., Pino, R., Álvarez, F., Rengel, J., Hernández, C., Uribe, E., & Blanco, J. (2019). Paralytic Shellfish Toxins in Surf Clams Mesodesma donacium during a Large Bloom of Alexandrium catenella Dinoflagellates Associated to an Intense Shellfish Mass Mortality. Toxins, 11(4), 188. https://doi.org/10.3390/toxins11040188







