De Novo Assessment and Review of Pan-American Pit Viper Anticoagulant and Procoagulant Venom Activities via Kinetomic Analyses
Abstract
:1. Introduction
2. Results
2.1. Anticoagulant Venom Data
2.2. Procoagulant Venom Data
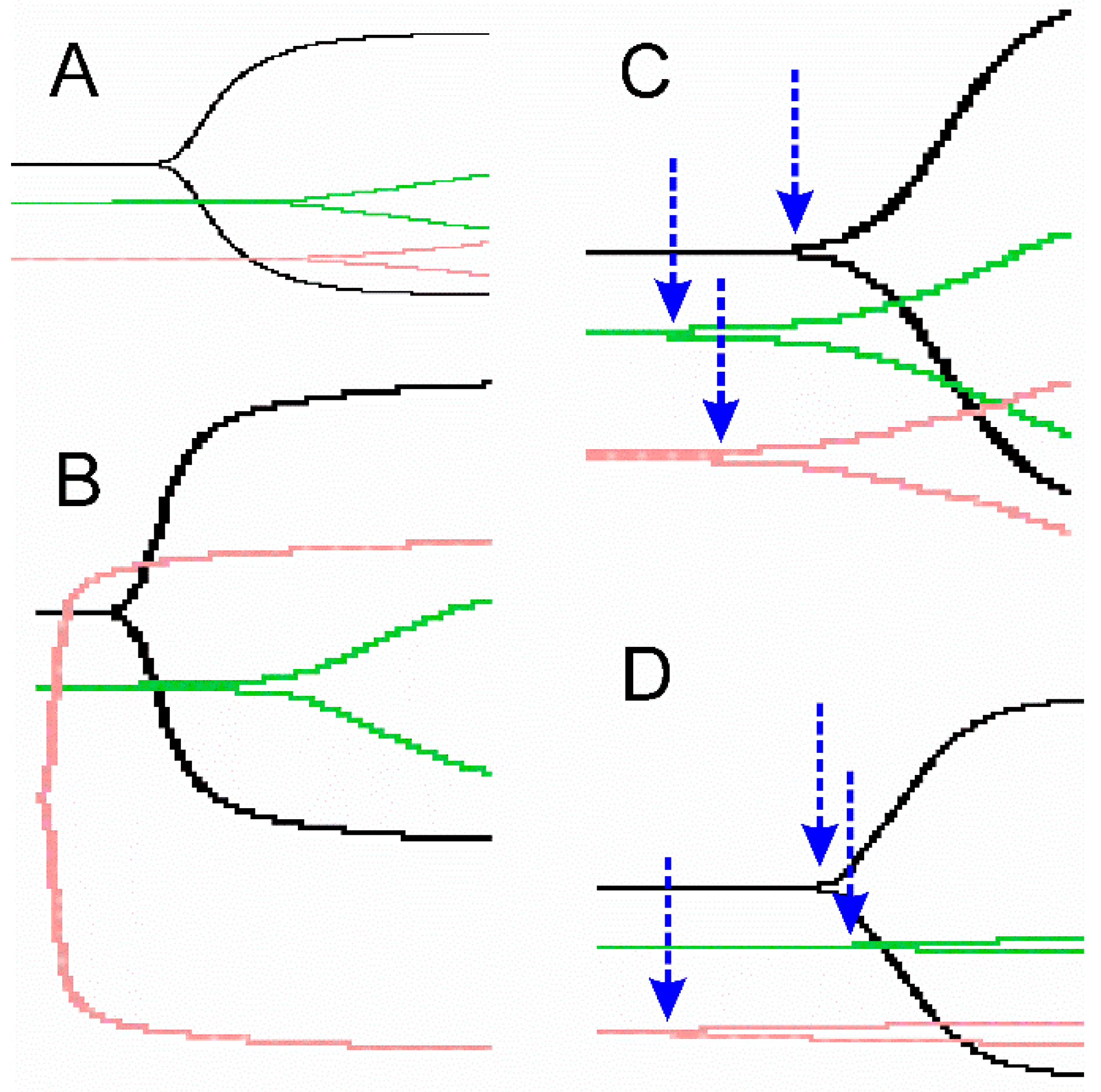
2.3. Representative Thrombelastographic Traces of Anticoagulant and Procoagulant Venoms
2.4. Kinetomic Review of Pan-American Viper Venoms from This and Previous Publications
2.5. Effects of Residual CORM-2 and PHA on Human Plasma Coagulation Kinetics Following Addition of Pre-exposed Venom
3. Discussion
4. Materials and Methods
4.1. Venoms, Chemicals, and Human Plasma
4.2. Thrombelastographic Analyses
4.3. CO Exposures
4.4. PHA Exposures
4.5. Assessment of Residual Concentrations of CORM-2 and PHA on Human Plasma Coagulation Kinetics Following Addition of Pre-exposed Venom
4.6. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Berling, I.; Isbister, G.K. Hematologic effects and complications of snake envenoming. Transfus Med. Rev. 2015, 29, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Sajevic, T.; Leonardi, A.; Križaj, I. Haemostatically active proteins in snake venoms. Toxicon 2011, 57, 627–645. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Cerruti, M.A.; Valencia, O.M.; Amos, Q. Decreased snake venom metalloproteinase effects via inhibition of enzyme and modification of fibrinogen. Biometals 2016, 29, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Frank, N. Role of heme modulation in inhibition of Atheris, Atractaspis, Causus, Cerastes, Echis, and Macrovipera hemotoxic venom activity. Hum. Exp. Toxicol. 2018, 38, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Frank, N.; Matika, R.W. Carbon monoxide inhibits hemotoxic activity of Elapidae venoms: Potential role of heme. Biometals 2018, 31, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Bazzell, C.M. Carbon monoxide attenuates the effects of snake venoms containing metalloproteinases with fibrinogenase or thrombin-like activity on plasmatic coagulation. Med. Chem. Comm. 2016, 7, 1973–1979. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Bazzell, C.M. Carbon monoxide releasing molecule-2 inhibition of snake venom thrombin-like activity: Novel biochemical “brake”? J. Thromb. Thrombolysis. 2017, 43, 203–208. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Frank, N. Differential heme-mediated modulation of Deinagkistrodon, Dispholidus, Protobothrops and Pseudonaja hemotoxic venom activity in human plasma. Biometals 2018. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Frank, N.; Matika, R.W. Effects of Heme Modulation on Ovophis and Trimeresurus Venom Activity in Human Plasma. Toxins 2018, 10, 322. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Losada, P.A. Direct Inhibitory Effects of Carbon Monoxide on Six Venoms Containing Fibrinogenolytic Metalloproteinases. Basic Clin. Pharmacol. Toxicol. 2017, 120, 207–212. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Matika, R.W. Effects of iron and carbon monoxide on Lachesis muta muta venom-mediated degradation of plasmatic coagulation. Hum. Exp. Toxicol. 2017, 36, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Suntravat, M.; Langlais, P.R.; Sánchez, E.E.; Nielsen, V.G. CatroxMP-II: A heme-modulated fibrinogenolytic metalloproteinase isolated from Crotalus atrox venom. Biometals 2018. [Google Scholar] [CrossRef] [PubMed]
- Kuch, U.; Mebs, D.; Gutiérrez, J.M.; Freire, A. Biochemical and biological characterization of Ecuadorian pitviper venoms (genera Bothriechis, Bothriopsis, Bothrops and Lachesis). Toxicon 1996, 34, 714–717. [Google Scholar] [CrossRef]
- Lomonte, B.; Escolano, J.; Fernández, J.; Sanz, L.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J. Proteome Res. 2008, 7, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Retzios, A.D.; Markland, F.S., Jr. Purification, characterization, and fibrinogen cleavage sites of three fibrinolytic enzymes from the venom of Crotalus basiliscus basiliscus. Biochemistry 1992, 31, 4547–4557. [Google Scholar] [CrossRef] [PubMed]
- Retzios, A.D.; Markland, F.S. Fibrinolytic enzymes from the venoms of Agkistrodon contortrix contortrix and Crotalus basiliscus basiliscus: Cleavage site specificity towards the alpha-chain of fibrin. Thromb. Res. 1994, 74, 355–367. [Google Scholar] [CrossRef]
- Gené, J.A.; Roy, A.; Rojas, G.; Gutiérrez, J.M.; Cerdas, L. Comparative study on coagulant, defibrinating, fibrinolytic and fibrinogenolytic activities of Costa Rican crotaline snake venoms and their neutralization by a polyvalent antivenom. Toxicon 1989, 27, 841–848. [Google Scholar] [CrossRef]
- Lomonte, B.; Rey-Suárez, P.; Tsai, W.C.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics of the pit vipers Porthidium nasutum, Porthidium ophryomegas, and Cerrophidion godmani from Costa Rica: Toxicological and taxonomical insights. J. Proteomics. 2012, 75, 1675–1689. [Google Scholar] [CrossRef]
- Chapeaurouge, A.; Reza, M.A.; Mackessy, S.P.; Carvalho, P.C.; Valente, R.H.; Teixeira-Ferreira, A.; Perales, J.; Lin, Q.; Kini, R.M. Interrogating the Venom of the Viperid Snake Sistrurus catenatus edwardsii by a Combined Approach of Electrospray and MALDI Mass Spectrometry. PLoS ONE 2015, 10, e0092091. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Sanz, L.; Chiucchi, J.E.; Farrell, T.M.; Calvete, J.J. Proteomic analysis of ontogenetic and diet-related changes in venom composition of juvenile and adult Dusky Pigmy rattlesnakes (Sistrurus miliarius barbouri). J. Proteomics 2011, 74, 2169–2179. [Google Scholar] [CrossRef]
- Komori, Y.; Nikai, T.; Ohara, A.; Yagihashi, S.; Sugihara, H. Effect of bilineobin, a thrombin-like proteinase from the venom of common cantil (Agkistrodon bilineatus). Toxicon 1993, 31, 257–270. [Google Scholar] [CrossRef]
- Gabrijelcic, D.; Drujan, B.; Gubensek, F. Coagulant proteinase from Bothrops colombiensis venom. Toxicon 1982, 20, 275–278. [Google Scholar] [CrossRef]
- Girón, M.E.; Salazar, A.M.; Aguilar, I.; Pérez, J.C.; Sánchez, E.E.; Arocha-Piñango, C.L.; Rodríguez-Acosta, A.; Guerrero, B. Hemorrhagic, coagulant and fibrino(geno)lytic activities of crude venom and fractions from mapanare (Bothrops colombiensis) snakes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Girón, M.E.; Guerrero, B.; Salazar, A.M.; Sánchez, E.E.; Alvarez, M.; Rodríguez-Acosta, A. Functional characterization of fibrinolytic metalloproteinases (colombienases) isolated from Bothrops colombiensis venom. Toxicon 2013, 74, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Machado, L.; Pla, D.; Sanz, L.; Jorge, R.J.B.; Leitão-De-Araújo, M.; Alves, M.L.M.; Alvares, D.J.; De Miranda, J.; Nowatzki, J.; de Morais-Zani, K.; et al. Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J. Proteomics. 2016, 135, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Perchuć, A.M.; Menin, L.; Stocklin, R.; Buhler, B.; Schoni, R. The potential of Bothrops moojeni venom in the field of hemostasis. Established use and new insights. Pathophysiol. Haemost. Thromb. 2005, 34, 241–245. [Google Scholar] [CrossRef] [PubMed]
- De Morais, N.C.; Neves Mamede, C.C.; Fonseca, K.C.; De Queiroz, M.R.; Gomes-Filho, S.A.; Santos-Filho, N.A.; Bordon Kde, C.; Beletti, M.E.; Sampaio, S.V.; Arantes, E.C.; et al. Isolation and characterization of moojenin, an acid-active, anticoagulant metalloproteinase from Bothrops moojeni venom. Toxicon 2012, 60, 1251–1258. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, L.M.; Ullah, A.; Masood, R.; Zelanis, A.; Spencer, P.J.; Serrano, S.M.; Arni, R.K. Rapid purification of serine proteinases from Bothrops alternatus and Bothrops moojeni venoms. Toxicon 2013, 76, 282–290. [Google Scholar] [CrossRef]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.; Gillett, A.; Del-Rei, T.H.M.; Chalkidis, H.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef]
- Durban, J.; Sanz, L.; Trevisan-Silva, D.; Neri-Castro, E.; Alagón, A.; Calvete, J.J. Integrated Venomics and Venom Gland Transcriptome Analysis of Juvenile and Adult Mexican Rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus Revealed miRNA-modulated Ontogenetic Shifts. J. Proteome Res. 2017, 16, 3370–3390. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Boyer, L.V.; Redford, D.T.; Ford, P. Thrombelastographic characterization of the thrombin-like activity of Crotalus simus and Bothrops asper venom. Blood Coagul. Fibrinolysis. 2017, 28, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G. Carbon monoxide inhibits the anticoagulant activity of phospholipase A2 purified from Crotalus adamanteus venom. J. Thromb. Thrombolysis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Gurley, W.Q.; Burch, T.M. Impact of factor XIII on coagulation kinetics and clot strength determined by thrombelastography. Anesth. Analg. 2004, 99, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Redford, D.T.; Boyle, P.K. Effect of iron and carbon monoxide on fibrinogenase-like degradation of plasmatic coagulation by venoms of six Agkistrodon species. Basic Clin. Pharmacol. Toxicol. 2016, 118, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G. Iron and carbon monoxide prevent degradation of plasmatic coagulation by thrombin-like activity in rattlesnake venom. Hum. Exp Toxicol. 2016, 35, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Redford, D.T.; Boyle, P.K. Effect of iron and carbon monoxide on fibrinogenase-like degradation of plasmatic coagulation by venoms of four Crotalus species. Blood Coagul. Fibrinolysis. 2017, 28, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Audu, P.; Cankovic, L.; Lyerly, R.T., III; Steenwyk, B.L.; Armstead, V.; Powell, G. Qualitative thrombelastographic detection of tissue factor in human plasma. Anesth. Analg. 2007, 104, 59–64. [Google Scholar] [CrossRef]
- Comer, J.M.; Zhang, L. Experimental Methods for Studying Cellular Heme Signaling. Cells 2018, 7, 47. [Google Scholar] [CrossRef]
- Motterlini, R.; Clark, J.E.; Foresti, R.; Sarathchandra, P.; Mann, B.E.; Green, C.J. Carbon monoxide-releasing molecules: Characterization of biochemical and vascular activities. Circ. Res. 2002, 90, E17–E24. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am. J. Physiol. Cell Physiol. 2017, 312, C302–C313. [Google Scholar] [CrossRef]
- Pryor, W.A.; Houk, K.N.; Foote, C.S.; Fukuto, J.M.; Ignarro, L.J.; Squadrito, G.L.; Davies, K.J. Free radical biology and medicine: It’s a gas, man! Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R491–R511. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.E.; Wade, R.S.; Belser, N.O. Conversion of oxyhemoglobin to methemoglobin by organic and inorganic reductants. Biochemistry 1978, 17, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Arkebauer, M.R.; Vosseller, K. Redox-based thrombelastographic method to detect carboxyhemefibrinogen-mediated hypercoagulability. Blood Coagul. Fibrinolysis. 2011, 22, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Stein, A.B.; Wu, W.J.; Tan, W.; Zhu, X.; Li, Q.H.; Dawn, B.; Motterlini, R.; Bolli, R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1649–H1653. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Hafner, D.T.; Steinbrenner, E.B. Tobacco smoke-induced hypercoagulation in human plasma: Role of carbon monoxide. Blood Coagul. Fibrinolysis. 2013, 24, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G. Crotalus atrox venom exposed to carbon monoxide has decreased fibrinogenolytic activity in vivo in rabbits. Basic Clin. Pharmacol. Toxicol. 2018, 122, 82–86. [Google Scholar] [CrossRef]
| Species | Common Name | Location |
|---|---|---|
| Bothriechis schlegelii [13,14] | Eyelash Palm Pit Viper | Central and South America |
| Crotalus basiliscus [15,16] | Mexican West Coast Rattlesnake | West Coast of Mexico |
| Crotalus cerastes cercobombus | Sonoran Desert Sidewinder | Mexico; Southwest Arizona, USA |
| Crotalus organus abyssus | Grand Canyon Rattlesnake | Northern Arizona, USA |
| Porthidium nasutum [17,18] | Rainforest Hognosed Pit Viper | Central and South America |
| Sistrurus catenatus edwardsii [19] | Desert Massasauga | North and Central America |
| Sistrurus miliarius barbourii [20] | Southeastern Pygmy Rattlesnake | Southeastern USA |
| Species | Common Name | Location |
|---|---|---|
| Agkistrodon bilineatus [21] | Common Cantil | El Salvador, Guatemala, Mexico |
| Atropoides olmec | Olmecan Pit Viper | Guatemala, Mexico |
| Bothrops colombiensis [22,23,24] | Common Lancehead | Republic of Columbia |
| Bothrops jararaca, S and SE [25,29] | Jararaca | Argentina, Brazil, Paraguay |
| Bothrops moojeni [26,27,28] | Brazilian Lancehead | Brazil, Paraguay |
| Crotalus simus tzabcan [30,31] | Central American Rattlesnake | Central America |
| Abbreviation | Definition |
|---|---|
| CO | carbon monoxide |
| CORM-2 | tricarbonyldichlororuthenium (II) dimer, a CO releasing molecule |
| DMSO | dimethyl sulfoxide |
| FXIII | factor XIII |
| iRM | inactive releasing molecule |
| metheme | a heme moiety that has its iron center in the Fe+3 rather than the Fe+2 state |
| MRTG | maximum rate of thrombus generation (dynes/cm2/second) |
| PBS | phosphate buffered saline that is calcium free |
| PHA | O-phenylhydroxylamine |
| TMRTG | time to maximum rate of thrombus generation (minutes) |
| TTG | total thrombus generation (dynes/cm2) |
| SP | split point, time from the start of the test to the split of the trace (minutes) |
| Parameter | Control | V | V/CO | V/iRM | V/PHA |
|---|---|---|---|---|---|
| Bothriechis schlegelii (0.15 µg/mL) | |||||
| TMRTG | 11.7 ± 1.2 | 26.6 ± 2.9 * | 14.9 ± 1.7 *† | 16.5 ± 1.7 *† | 23.1 ± 3.8 |
| MRTG | 3.6 ± 0.6 | 0.8 ± 0.3 * | 2.5 ± 0.3 *† | 2.0 ± 0.9 *† | 1.1 ± 0.4 |
| TTG | 203 ± 10 | 67 ± 32 * | 196 ± 14 † | 162 ± 66 † | 116 ± 56 |
| Crotalus basiliscus (1.0 µg/mL) | |||||
| TMRTG | 11.7 ± 1.2 | 22.7 ± 4.8 * | 13.3 ± 1.1 † | 21.4 ± 4.1 *‡ | 21.8 ± 4.8 |
| MRTG | 3.6 ± 0.6 | 0.3 ± 0.5 * | 2.8 ± 0.3 *† | 0.9 ± 0.8 *‡ | 0.6 ± 0.7 |
| TTG | 203 ± 10 | 33 ± 60 * | 196 ± 6 † | 96 ± 73 *‡ | 60 ± 73 |
| Crotalus cerastes cercobombus (2.0 µg/mL) | |||||
| TMRTG | 11.8 ± 0.7 | 19.8 ± 4.0 * | 12.5 ± 1.9 † | 21.6 ± 4.2 *‡ | 12.9 ± 1.1 † |
| MRTG | 2.8 ± 0.5 | 1.4 ± 0.9 * | 2.6 ± 0.4 † | 1.4 ± 0.6 *‡ | 3.2 ± 0.5 † |
| TTG | 176 ± 13 | 123 ± 65 | 178 ± 9 | 119 ± 52 | 191 ± 30 † |
| Crotalus organus abyssus (0.5 µg/mL) | |||||
| TMRTG | 11.7 ± 1.2 | 23.2 ± 5.3 * | 14.6 ± 2.7 † | 13.7 ± 3.3 † | 21.2 ± 5.5 |
| MRTG | 3.6 ± 0.6 | 0.6 ± 0.6 * | 3.2 ± 0.6 † | 2.9 ± 0.6 *† | 0.6 ± 0.6 |
| TTG | 203 ± 10 | 72 ± 79 * | 196 ± 17 † | 188 ± 15 † | 68 ± 57 |
| Porthidium nasutum (1.0 µg/mL) | |||||
| TMRTG | 11.8 ± 0.7 | 25.4 ± 6.4 * | 12.9 ± 3.1 † | 22.8 ± 4.1 *‡ | 29.0 ± 1.5 |
| MRTG | 2.8 ± 0.5 | 0.6 ± 0.9 * | 3.4 ± 1.7 † | 1.0 ± 0.7 *‡ | 0.1 ± 0.1 |
| TTG | 176 ± 13 | 57 ± 87 * | 187 ± 37 † | 107 ± 68 | 5 ± 9 |
| Sistrurus catenatus edwardsii (0.5 µg/mL) | |||||
| TMRTG | 11.7 ± 1.2 | 21.0 ± 7.7 * | 15.0 ± 5.3 * | 26.7 ± 4.6 *‡ | 25.9 ± 6.3 |
| MRTG | 3.6 ± 0.6 | 0.6 ± 0.6 * | 2.8 ± 1.0 *† | 0.1 ± 0.1 *‡ | 0.1 ± 0.1 |
| TTG | 203 ± 10 | 59 ± 68 * | 186 ± 17 † | 2 ± 4 *†‡ | 3 ± 4 |
| Sistrurus miliarius barbourii (0.25 µg/mL) | |||||
| TMRTG | 11.7 ± 1.2 | 26.6 ± 3.9 * | 13.8 ± 3.2 † | 24.0 ± 5.8 *‡ | 29.1 ± 2.3 |
| MRTG | 3.6 ± 0.6 | 0.5 ± 0.5 * | 3.2 ± 0.9 † | 0.6 ± 0.4 *‡ | 0.0 ± 0.0 |
| TTG | 203 ± 10 | 42 ± 67 * | 210 ± 19 † | 66 ± 69 *‡ | 1 ± 2 |
| Parameter | V | V/CO | V/iRM | V/PHA |
|---|---|---|---|---|
| Agkistrodon bilineatus (5 µg/mL) | ||||
| TMRTG | 6.3 ± 0.4 | 12.9 ± 1.0 * | 6.6 ± 0.3 † | 14.9 ± 0.2 * |
| MRTG | 2.7 ± 0.1 | 3.6 ± 0.6 * | 2.5 ± 0.2 † | 0.0 ± 0.0 * |
| TTG | 144 ± 4 | 136 ± 37 | 143 ± 7 | 0 ± 0 * |
| Atropoides olmec (10 µg/mL) | ||||
| TMRTG | 13.5 ± 1.1 | 12.6 ± 0.6 | 13.8 ± 0.9 | 13.2 ± 1.5 |
| MRTG | 0.8 ± 0.4 | 2.5 ± 0.3 * | 0.7 ± 0.2 † | 0.9 ± 0.5 |
| TTG | 51 ± 25 | 121 ± 15 * | 45 ± 13 † | 50 ± 30 |
| Bothrops colombiensis (2 µg/mL) | ||||
| TMRTG | 1.9 ± 0.1 | 5.0 ± 0.2 * | 2.1 ± 0.2 † | 10.6 ± 1.5 * |
| MRTG | 9.5 ± 1.0 | 3.3 ± 0.5 * | 8.7 ± 1.3 † | 0.1 ± 0.0 * |
| TTG | 207 ± 10 | 143 ± 17 * | 208 ± 15 † | 2 ± 0 * |
| Bothrops jararaca, S (2 µg/mL) | ||||
| TMRTG | 1.0 ± 0.1 | 2.3 ± 0.2 * | 1.3 ± 0.2 *† | 9.2 ± 4.3 * |
| MRTG | 12.0 ± 0.8 | 9.6 ± 1.5 * | 12.0 ± 0.9 | 2.8 ± 2.6 * |
| TTG | 178 ± 9 | 196 ± 19 | 195 ± 9 | 81 ± 63 * |
| Bothrops jararaca, SE (2 µg/mL) | ||||
| TMRTG | 1.6 ± 0.1 | 3.9 ± 0.4 * | 1.7 ± 0.1 † | 10.3 ± 1.1 * |
| MRTG | 11.8 ± 2.1 | 6.2 ± 1.2 * | 11.6 ± 1.4 † | 4.6 ± 0.8 * |
| TTG | 204 ± 32 | 175 ± 24 | 197 ± 18 | 158 ± 10 * |
| Bothrops moojeni (2 µg/mL) | ||||
| TMRTG | 1.2 ± 0.1 | 3.7 ± 0.7 * | 1.2 ± 0.0 † | 11.2 ± 0.6 * |
| MRTG | 11.2 ± 1.5 | 6.5 ± 1.5 * | 10.8 ± 1.5 † | 1.0 ± 0.2 * |
| TTG | 167 ± 27 | 179 ± 23 | 153 ± 17 | 62 ± 16 * |
| Crotalus simus tzabcan (3 µg/mL) | ||||
| TMRTG | 6.0 ± 1.5 | 15.1 ± 2.2 * | 12.3 ± 7.9 * | 10.4 ± 2.5 * |
| MRTG | 0.1 ± 0.0 | 2.3 ± 0.5 * | 0.1 ± 0.1 † | 0.1 ± 0.0 |
| TTG | 7 ± 1 | 155 ± 31 * | 6 ± 3 † | 93 ± 19 † |
| Species | Activity | [µg/mL] | CO | PHA |
|---|---|---|---|---|
| North American Snakes | ||||
| Agkistrodon contortrix contortrix | A | 10 | − | NT |
| Agkistrodon contortrix laticinctus | A | 30 | + | NT |
| Agkistrodon contortrix pictigaster | A | 11 | NT | NT |
| Agkistrodon contortrix mokasen | A | 8 | + | NT |
| Agkistrodon piscivorus leucostoma | A | 5 | + | NT |
| Agkistrodon piscivorus piscivorus | A | 5 | + | NT |
| Crotalus adamanteus | TLA | 5 | + | NT |
| Crotalus atrox | A | 2 | + | NT |
| Crotalus cerastes cercobombus | A | 2 | + | + |
| Crotalus horridus horridus | TLA/TGA | 5 | + | NT |
| Crotalus organus abyssus | A | 0.5 | ? | − |
| Crotalus oreganus cerberus | A | 2 | + | − |
| Crotalus oreganus helleri | TLA | 10 | NT | NT |
| Crotalus oreganus oreganus | A | 2 | + | NT |
| Crotalus ruber ruber | A | 10 | NT | NT |
| Crotalus viridis viridis | A | 10 | NT | NT |
| Sistrurus catenatus edwardsii | A | 0.5 | + | − |
| Sistrurus miliarius barbourii | A | 0.25 | + | − |
| Central American Snakes | ||||
| Agkistrodon bilineatus | TGA | 5 | + | + |
| Atropoides olmec | TLA | 10 | + | − |
| Bothriechis schlegelii | A | 0.15 | ? | − |
| Crotalus basiliscus | A | 1 | + | − |
| Crotalus simus simus | TLA | 2 | NT | NT |
| Crotalus simus tzabcan | TLA | 2 | + | + |
| Porthidium nasutum | A | 1 | + | − |
| South American Snakes | ||||
| Bothrops asper | TGA | 2 | + | NT |
| Bothrops atrox | TGA | 20 | NT | NT |
| Bothrops colombiensis | TGA | 2 | + | + |
| Bothrops jararaca | TGA | 2 | + | + |
| Bothrops moojeni | TGA | 2 | + | + |
| Bothrops neuwiedi | TGA | 20 | NT | NT |
| Lachesis muta muta | A/TLA | 2 | + | NT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, V.G.; Frank, N.; Afshar, S. De Novo Assessment and Review of Pan-American Pit Viper Anticoagulant and Procoagulant Venom Activities via Kinetomic Analyses. Toxins 2019, 11, 94. https://doi.org/10.3390/toxins11020094
Nielsen VG, Frank N, Afshar S. De Novo Assessment and Review of Pan-American Pit Viper Anticoagulant and Procoagulant Venom Activities via Kinetomic Analyses. Toxins. 2019; 11(2):94. https://doi.org/10.3390/toxins11020094
Chicago/Turabian StyleNielsen, Vance G., Nathaniel Frank, and Sam Afshar. 2019. "De Novo Assessment and Review of Pan-American Pit Viper Anticoagulant and Procoagulant Venom Activities via Kinetomic Analyses" Toxins 11, no. 2: 94. https://doi.org/10.3390/toxins11020094
APA StyleNielsen, V. G., Frank, N., & Afshar, S. (2019). De Novo Assessment and Review of Pan-American Pit Viper Anticoagulant and Procoagulant Venom Activities via Kinetomic Analyses. Toxins, 11(2), 94. https://doi.org/10.3390/toxins11020094





