Structure Elucidation and Biological Evaluation of Maitotoxin-3, a Homologue of Gambierone, from Gambierdiscus belizeanus
Abstract
1. Introduction
2. Results
2.1. Structure Elucidation of Compound 1
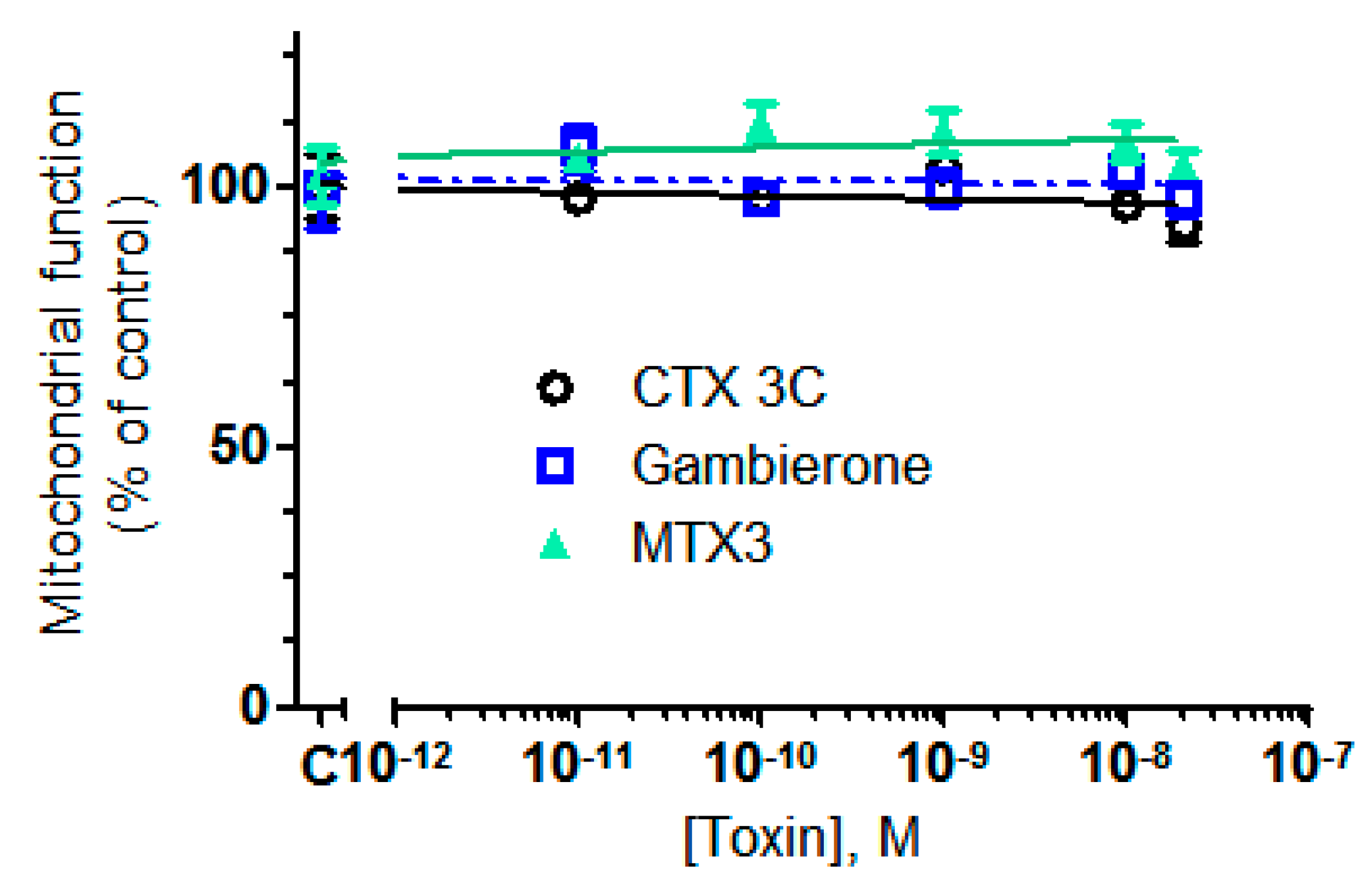
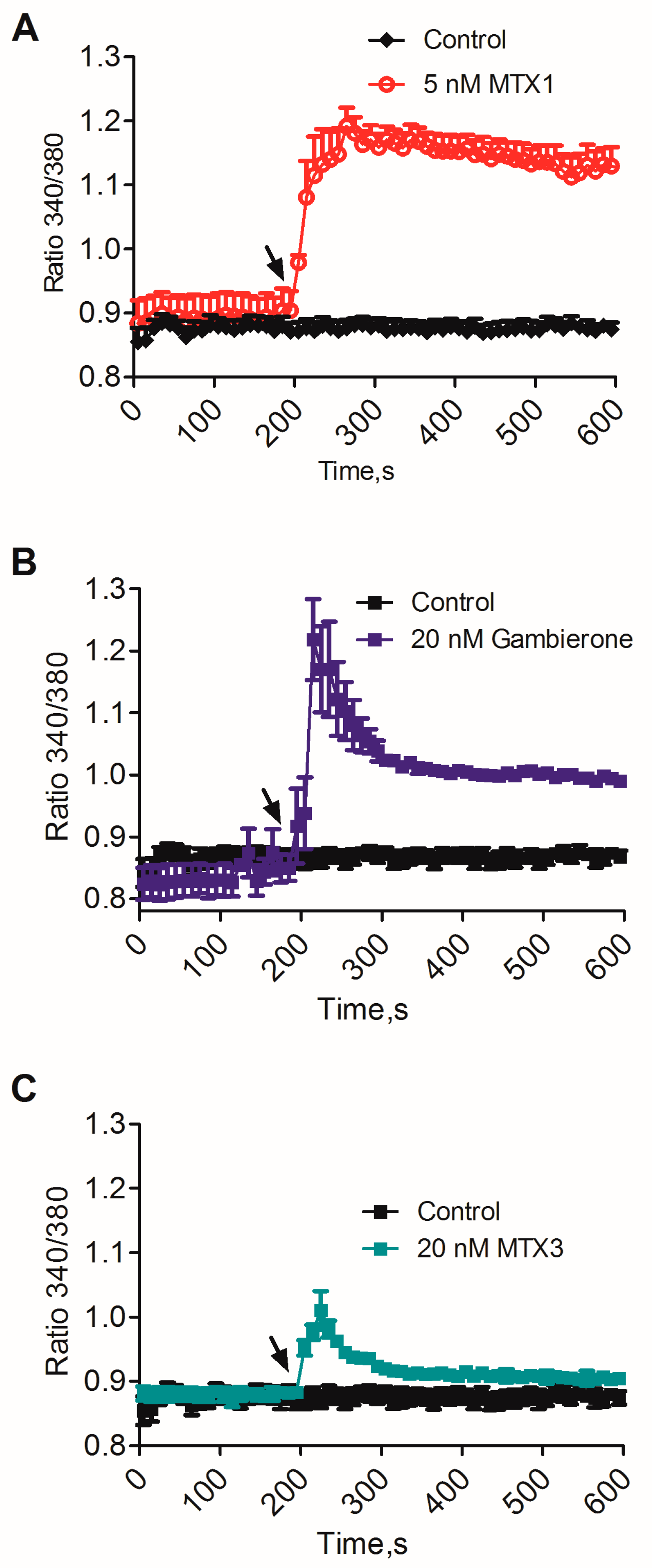
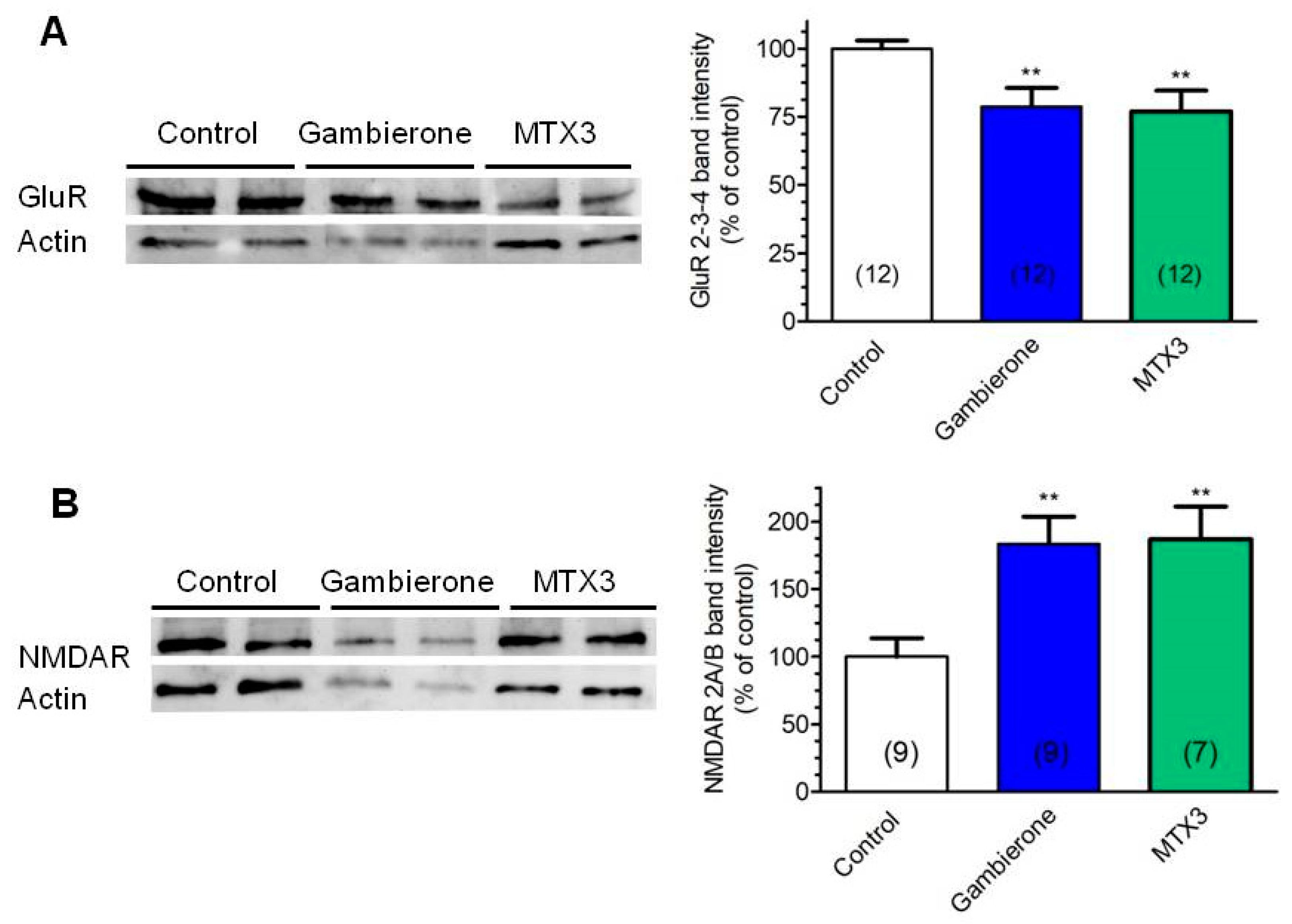
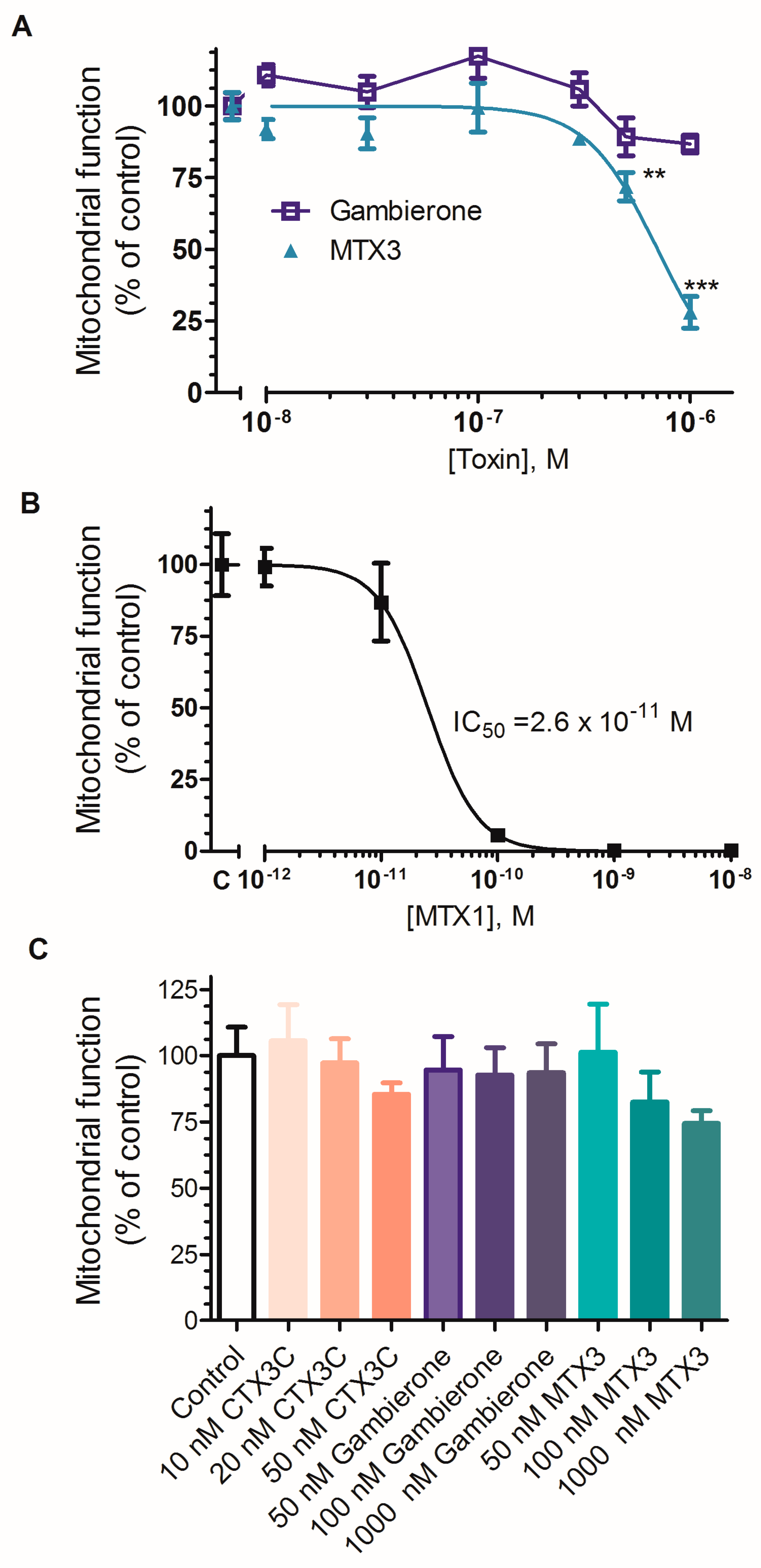
2.2. Biological Activity of MTX3 and Gambierone
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Biological Material
4.3. Cell Culture
4.4. Extraction and Purification
4.4.1. Extraction
4.4.2. Separation and Purification
- Florisil low-pressure column, 45 × 4.5 cm pre-equilibrated with EtOAc/MeOH (9:1). The column was successively eluted with EtOAc/MeOH (9:1, 500 mL), EtOAc/MeOH (8:2, 500 mL), EtOAc/MeOH (7:3, 500 mL) and finally MeOH (500 mL). Ethyl acetate fractions were dried and dissolved in chloroform for the next column.
- Silica low-pressure column, 45 × 4.5 cm pre-equilibrated with CHCl3. The column was successively eluted with CHCl3 (500 mL), CHCl3/MeOH (9:1, 500 mL), CHCl3/MeOH (8:2, 500 mL), CHCl3/MeOH (7:3, 500 mL) and finally MeOH (500 mL). Combined chloroform extracts were evaporated and dissolved in CHCl3/MeOH 2:1.
- Sephadex LH20 low-pressure column, 60 × 2.5 cm pre-equilibrated with CHCl3/MeOH (2:1). Toxins were eluted from 60 mL to 100 mL of CHCl3/MeOH (2:1) with fractions every 3 mL.
- Sephadex LH20 low-pressure column, 60 × 2.5 cm pre-equilibrated with MeOH. Toxins were eluted from 60 mL to 100 mL of MeOH with fractions every 3 mL.
- C18 low-pressure column, 60 × 2.5 cm pre-equilibrated with 50 % MeOH and eluted with a stepwise gradient of 50, 60, 70, 80, 90 and 100% of MeOH (150 mL each with fractions every 15 mL). Gambierone eluted from fraction 28 to 43 and MTX3 from 32 to 43. Fractions 28–43 were then combined.
- C18 low-pressure column, 60 × 2.5 cm pre-equilibrated with 35% MeCN and eluted successively with 35, 40, 45, 50 and finally 60% MeCN (150 mL each using 15 mL fractions). Gambierone was eluted in fractions 13 to 17 and MTX3 from 18 to 21.
4.5. Mass Spectrometry
High Resolution Mass Spectrometry
4.6. Evaluation of the Biological Activities
4.6.1. Toxins and Drugs Used
4.6.2. Cortical Human Neural Stem Cell Line CTX0E16 Culture and Differentiation
4.6.3. Neuroblastoma Cell Line
4.6.4. Determination of Cellular Viability
4.6.5. Determination of Cytosolic Calcium Concentration ([Ca2+]c)
4.6.6. Western Blot
4.6.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murray, J.S.; Boundy, M.J.; Selwood, A.I.; Harwood, D.T. Development of an LC–MS/MS method to simultaneously monitor maitotoxins and selected ciguatoxins in algal cultures and P-CTX-1B in fish. Harmful Algae 2018, 80, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Botana, L.M. Toxicological Perspective on Climate Change: Aquatic Toxins. Chem. Res. Toxicol. 2016, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R.W.; Plakas, S.M. Ciguatera: A public health perspective. Toxicon 2010, 56, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Perez-Arellano, J.L.; Luzardo, O.P.; Perez Brito, A.; Hernandez Cabrera, M.; Zumbado, M.; Carranza, C.; Angel-Moreno, A.; Dickey, R.W.; Boada, L.D. Ciguatera fish poisoning, Canary Islands. Emerg. Infect. Dis. 2005, 11, 1981–1982. [Google Scholar] [CrossRef] [PubMed]
- Boada, L.D.; Zumbado, M.; Luzardo, O.P.; Almeida-Gonzalez, M.; Plakas, S.M.; Granade, H.R.; Abraham, A.; Jester, E.L.; Dickey, R.W. Ciguatera fish poisoning on the West Africa Coast: An emerging risk in the Canary Islands (Spain). Toxicon 2010, 56, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Estevez, P.; Castro, D.; Solino, L.; Gouveia, N.; Santos, C.; Rodrigues, S.M.; Leao, J.M.; Gago-Martinez, A. New Insights into the Occurrence and Toxin Profile of Ciguatoxins in Selvagens Islands (Madeira, Portugal). Toxins 2018, 10, 524. [Google Scholar] [CrossRef]
- Otero, P.; Perez, S.; Alfonso, A.; Vale, C.; Rodriguez, P.; Gouveia, N.N.; Gouveia, N.; Delgado, J.; Vale, P.; Hirama, M.; et al. First toxin profile of ciguateric fish in Madeira Arquipelago (Europe). Anal. Chem. 2010, 82, 6032–6039. [Google Scholar] [CrossRef]
- Murata, M.; Yasumoto, T. The structure elucidation and biological activities of high molecular weight algal toxins: Maitotoxin, prymnesins and zooxanthellatoxins. Nat. Prod. Rep. 2000, 17, 293–314. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Purification and characterisation of large and small maitotoxins from cultured Gambierdiscus toxicus. Nat. Toxins 1994, 2, 64–72. [Google Scholar] [CrossRef]
- Gomez, F.; Qiu, D.; Lopes, R.M.; Lin, S. Fukuyoa paulensis gen. et sp. nov., a new genus for the globular species of the dinoflagellate Gambierdiscus (Dinophyceae). PLoS ONE 2015, 10, e0119676. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.J.; Lewis, R.J.; Gillespie, N.C. Toxicity of Australian and French polynesian strains of Gambierdiscus toxicus (Dinophyceae) grown in culture: Characterization of a new type of maitotoxin. Toxicon 1990, 28, 1159–1172. [Google Scholar] [CrossRef]
- Pisapia, F.; Sibat, M.; Herrenknecht, C.; Lhaute, K.; Gaiani, G.; Ferron, P.-J.; Fessard, V.; Fraga, S.; Nascimento, S.M.; Litaker, R.W.; et al. Maitotoxin-4, a Novel MTX Analog Produced by Gambierdiscus excentricus. Mar. Drugs 2017, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Trevino, C.L.; Escobar, L.; Vaca, L.; Morales-Tlalpan, V.; Ocampo, A.Y.; Darszon, A. Maitotoxin: A Unique Pharmacological tool for elucidating Ca2+ dependent mechanims. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; Botana, L., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 503–516. [Google Scholar]
- Vale, C.; Antero, A.; Martín, V. Pharmacology of ciguatoxins. In Phycotoxins, Chemistry and Biochemistry; Alfonso, L.B.A.A., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 23–48. [Google Scholar]
- Nagai, H.; Torigoe, K.; Satake, M.; Murata, M.; Yasumoto, T.; Hirota, H. Gambieric acids: Unprecedented potent antifungal substances isolated from cultures of a marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1992, 114, 1102–1103. [Google Scholar] [CrossRef]
- Watanabe, R.; Uchida, H.; Suzuki, T.; Matsushima, R.; Nagae, M.; Toyohara, Y.; Satake, M.; Oshima, Y.; Inoue, A.; Yasumoto, T. Gambieroxide, a novel epoxy polyether compound from the dinoflagellate Gambierdiscus toxicus GTP2 strain. Tetrahedron 2013, 69, 10299–10303. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. Gambierol: A new toxic polyether compound isolated from the marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1993, 115, 361–362. [Google Scholar] [CrossRef]
- Rodríguez, I.; Genta-Jouve, G.; Alfonso, C.; Calabro, K.; Alonso, E.; Sánchez, J.A.; Alfonso, A.; Thomas, O.P.; Botana, L.M. Gambierone, a Ladder-Shaped Polyether from the Dinoflagellate Gambierdiscus belizeanus. Org. Lett. 2015, 17, 2392–2395. [Google Scholar] [CrossRef]
- Brereton, H.M.; Chen, J.; Rychkov, G.; Harland, M.L.; Barritt, G.J. Maitotoxin activates an endogenous non-selective cation channel and is an effective initiator of the activation of the heterologously expressed hTRPC-1 (transient receptor potential) non-selective cation channel in H4-IIE liver cells. Biochim. Biophys. Acta 2001, 1540, 107–126. [Google Scholar] [CrossRef]
- Flores, P.L.; Rodriguez, E.; Zapata, E.; Carbo, R.; Farias, J.M.; Martinez, M. Maitotoxin Is a Potential Selective Activator of the Endogenous Transient Receptor Potential Canonical Type 1 Channel in Xenopus laevis Oocytes. Mar. Drugs 2017, 15, 198. [Google Scholar] [CrossRef]
- Martin, V.; Vale, C.; Antelo, A.; Hirama, M.; Yamashita, S.; Vieytes, M.R.; Botana, L.M. Differential effects of ciguatoxin and maitotoxin in primary cultures of cortical neurons. Chem. Res. Toxicol. 2014, 27, 1387–1400. [Google Scholar] [CrossRef]
- Soergel, D.G.; Yasumoto, T.; Daly, J.W.; Gusovsky, F. Maitotoxin effects are blocked by SK&F 96365, an inhibitor of receptor-mediated calcium entry. Mol. Pharmacol. 1992, 41, 487–493. [Google Scholar] [PubMed]
- Kopljar, I.; Labro, A.J.; de Block, T.; Rainier, J.D.; Tytgat, J.; Snyders, D.J. The ladder-shaped polyether toxin gambierol anchors the gating machinery of Kv3.1 channels in the resting state. J. Gen. Physiol. 2013, 141, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Martín, V.; Vale, C.; Hirama, M.; Yamashita, S.; Rubiolo, J.A.; Vieytes, M.R.; Botana, L.M. Synthetic Ciguatoxin CTX 3C Induces a Rapid Imbalance in Neuronal Excitability. Chem. Res. Toxicol. 2015, 28, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.E.; Laczka, O.F.; Harwood, D.T.; Lewis, R.J.; Himaya, S.W.A.; Murray, S.A.; Doblin, M.A. Toxicology of Gambierdiscus spp. (Dinophyceae) from Tropical and Temperate Australian Waters. Mar. Drugs 2018, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Inserra, M.; Vetter, I.; Holland, W.C.; Hardison, D.R.; Tester, P.A.; Litaker, R.W. Rapid Extraction and Identification of Maitotoxin and Ciguatoxin-Like Toxins from Caribbean and Pacific Gambierdiscus Using a New Functional Bioassay. PLoS ONE 2016, 11, e0160006. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Holmes, M.J.; Alewood, P.F.; Jones, A. Ionspray mass spectrometry of ciguatoxin-1, maitotoxin-2 and -3, and related marine polyether toxins. Nat. Toxins 1994, 2, 56–63. [Google Scholar] [CrossRef]
- Munday, R.; Murray, S.; Rhodes, L.L.; Larsson, M.E.; Harwood, D.T. Ciguatoxins and Maitotoxins in Extracts of Sixteen Gambierdiscus Isolates and One Fukuyoa Isolate from the South Pacific and Their Toxicity to Mice by Intraperitoneal and Oral Administration. Mar. Drugs 2017, 15, 208. [Google Scholar] [CrossRef]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.R.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef]
- Boente-Juncal, A.; Mendez, A.G.; Vale, C.; Vieytes, M.R.; Botana, L.M. In Vitro Effects of Chronic Spirolide Treatment on Human Neuronal Stem Cell Differentiation and Cholinergic System Development. ACS Chem. Neurosci. 2018, 9, 1441–1452. [Google Scholar] [CrossRef]
- Boente-Juncal, A.; Vale, C.; Alfonso, A.; Botana, L.M. Synergistic Effect of Transient Receptor Potential Antagonist and Amiloride against Maitotoxin Induced Calcium Increase and Cytotoxicity in Human Neuronal Stem Cells. ACS Chem. Neurosci. 2018, 9, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Danese, A.; Missiroli, S.; Patergnani, S.; Pinton, P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018, 28, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Turrigiano, G.G. All for One But Not One for All: Excitatory Synaptic Scaling and Intrinsic Excitability Are Coregulated by CaMKIV, Whereas Inhibitory Synaptic Scaling Is Under Independent Control. J. Neurosci. 2017, 37, 6778–6785. [Google Scholar] [CrossRef] [PubMed]
- Scheefhals, N.; MacGillavry, H.D. Functional organization of postsynaptic glutamate receptors. Mol. Cell. Neurosci. 2018, 91, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Vale, C.; Rubiolo, J.A.; Roel, M.; Hirama, M.; Yamashita, S.; Vieytes, M.R.; Botana, L.M. Chronic ciguatoxin treatment induces synaptic scaling through voltage gated sodium channels in cortical neurons. Chem. Res. Toxicol. 2015, 28, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Sylvester, P.W. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol. Biol. 2011, 716, 157–168. [Google Scholar] [PubMed]
- Byard, R.W. Death by food. Forensic Sci. Med. Pathol. 2018, 14, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Rodriguez, F. Genus Gambierdiscus in the Canary Islands (NE Atlantic Ocean) with description of Gambierdiscus silvae sp. nov., a new potentially toxic epiphytic benthic dinoflagellate. Protist 2014, 165, 839–853. [Google Scholar] [CrossRef]
- Lu, X.Z.; Deckey, R.; Jiao, G.L.; Ren, H.F.; Li, M. Caribbean maitotoxin elevates [Ca2+]i and activates non-selective cation channels in HIT-T15 cells. World J. Diabetes 2013, 4, 70–75. [Google Scholar] [CrossRef]
- Meunier, F.A.; Mattei, C.; Molgo, J. Marine toxins potently affecting neurotransmitter release. Prog. Mol. Subcell Biol. 2009, 46, 159–186. [Google Scholar] [PubMed]
- Michio, M.; Nobuaki, M.; Keiichi, K.; Tohru, O. Structural Features of Dinoflagellate Toxins Underlying Biological Activity as Viewed by NMR. Bull. Chem. Soc. Jpn. 2008, 81, 307–319. [Google Scholar]
- Kakizaki, A.; Takahashi, M.; Akagi, H.; Tachikawa, E.; Yamamoto, T.; Taira, E.; Yamakuni, T.; Ohizumi, Y. Ca2+ channel activating action of maitotoxin in cultured brainstem neurons. Eur. J. Pharmacol. 2006, 536, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.G.; Juncal, A.B.; Silva, S.B.L.; Thomas, O.P.; Martin Vazquez, V.; Alfonso, A.; Vieytes, M.R.; Vale, C.; Botana, L.M. The Marine Guanidine Alkaloid Crambescidin 816 Induces Calcium Influx and Cytotoxicity in Primary Cultures of Cortical Neurons through Glutamate Receptors. ACS Chem. Neurosci. 2017, 8, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Roel, M.; Rubiolo, J.A.; Guerra-Varela, J.; Silva, S.B.; Thomas, O.P.; Cabezas-Sainz, P.; Sanchez, L.; Lopez, R.; Botana, L.M. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget 2016, 7, 83071–83087. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D.; Guillard, R.R.L. Factors significant to marine diatom culture. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 113–116. [Google Scholar]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Anderson, G.W.; Deans, P.J.; Taylor, R.D.; Raval, P.; Chen, D.; Lowder, H.; Murkerji, S.; Andreae, L.C.; Williams, B.P.; Srivastava, D.P. Characterisation of neurons derived from a cortical human neural stem cell line CTX0E16. Stem Cell Res. Ther. 2015, 6, 149. [Google Scholar] [CrossRef]
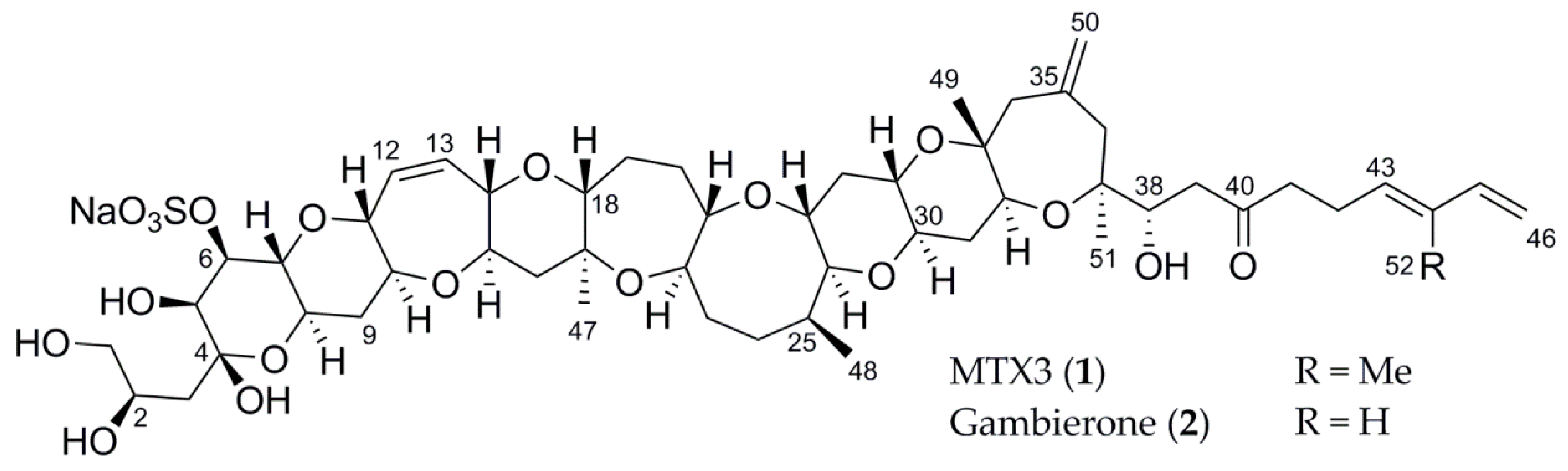
| Atom Number | δH, Mult. (J in Hz) | δC | n° | δH, Mult. (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | 3.47, dd (11.0, 4.5) 3.42, m | 67.8 | 27 | 3.51, m | 77.7 |
| 2 | 4.10, m | 69.8 | 28 | 2.19, m 1.33, m | 39.8 |
| 3 | 2.00, m 1.71, dd (14.5, 10.0) | 39.7 | 29 | 3.12, m | 70.1 |
| 4 | - | 100.7 | 30 | 2.94, m | 78.1 |
| 5 | 4.21, d (3.0) | 73.0 | 31 | 1.89, m 1.54, t (11.5) | 34.9 |
| 6 | 4.70, dd (10.0, 3.0) | 77.8 | 32 | 3.76, dd (8.5, 3.5) | 72.5 |
| 7 | 3.37, m | 77.3 | 33 | - | 77.3 |
| 8 | 3.77, m | 67.9 | 34 | 2.34, d (12.5) 2.15, d (12.5) | 54.1 |
| 9 | 2.17, m 1.58, t (11.5) | 37.9 | 35 | - | 143.5 |
| 10 | 3.35, m | 79.6 | 36 | 2.54, d (14.5) 2.21, d (14.5) | 43.3 |
| 11 | 3.79, m | 82.4 | 37 | - | 79.9 |
| 12 | 5.64, dt (12.5, 2.0) | 132.8 | 38 | 4.06, dt (10.5, 3.0) | 73.1 |
| 13 | 5.75, dt (12.5, 2.0) | 133.0 | 39 | 2.61, m 2.59, m | 45.8 |
| 14 | 3.81, m | 83.0 | 40 | - | 211.6 |
| 15 | 3.44, dd (5.0, 2.0) | 79.9 | 41 | 2.59, m | 43.9 |
| 16 | 1.99, m 1.49, t (12.0) | 47.4 | 42 | 2.39, q (7.0) | 23.3 |
| 17 | - | 76.6 | 43 | 5.45, t (7.5) | 132.3 |
| 18 | 3.01, dt (11.0, 2.5) | 86.8 | 44 | - | 136.0 |
| 19 | 1.79, m 1.62, m | 25.4 | 45 | 6.33, dt (17.5, 10.5) | 142.6 |
| 20 | 1.95, m 1.80, m | 34.2 | 46 | 5.09, d (17.5) 4.90, d (11.0) | 111.2 |
| 21 | 3.53, m | 87.4 | 47 | 1.20, s | 16.4 |
| 22 | 3.54, m | 75.7 | 48 | 1.00, d (7.5) | 13.5 |
| 23 | 1.82, m 1.64, m | 32.8 | 49 | 1.19, s | 16.9 |
| 24 | 1.92, m 1.78, m | 29.5 | 50 | 4.98, br s 4.85 a | 118.6 |
| 25 | 2.19, m | 35.6 | 51 | 1.13, s | 20.7 |
| 26 | 3.11, m | 85.9 | 52 | 1.74, s | 11.7 |
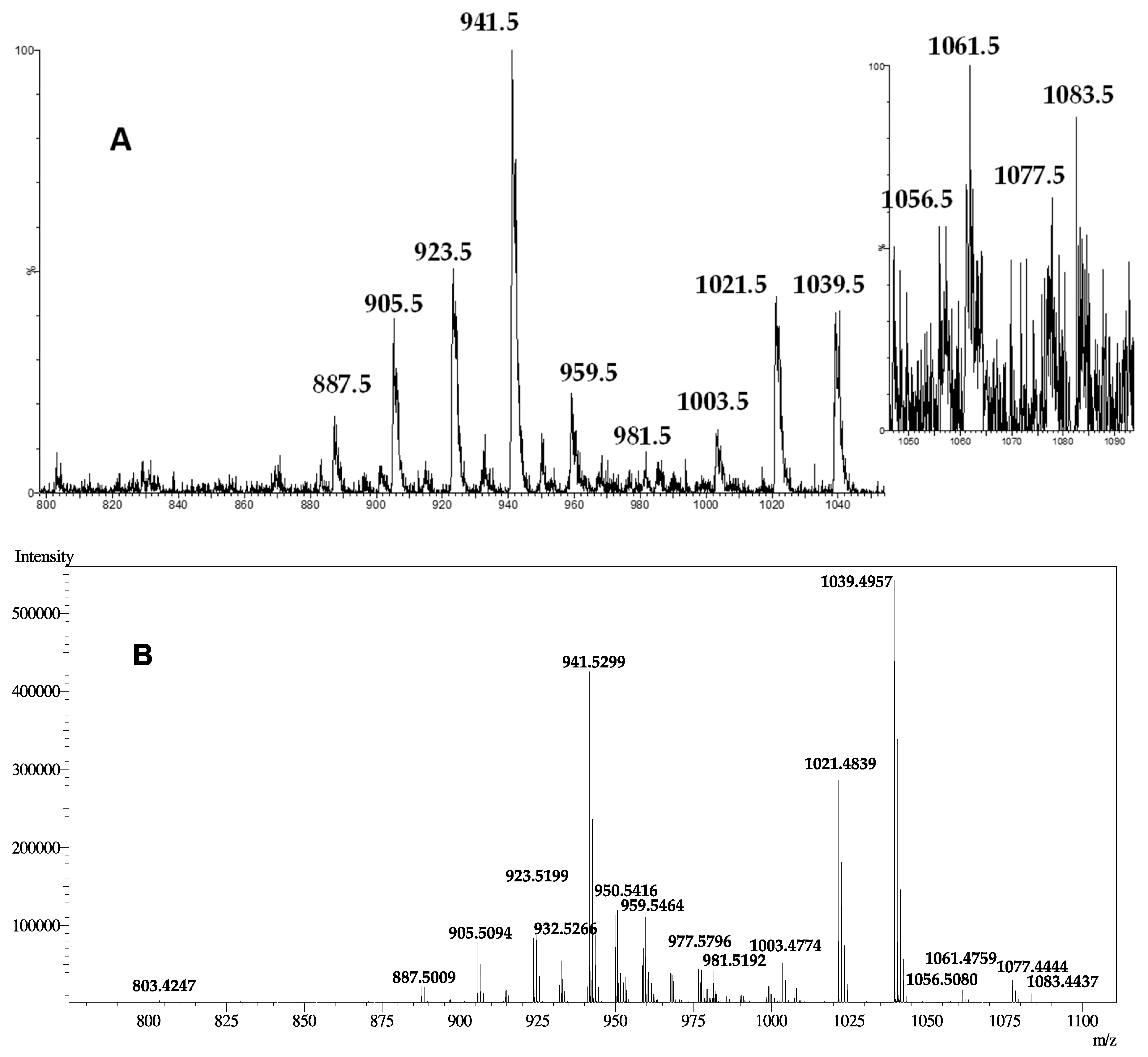
| Peaks | Ion Species and Fragments Reported in ESI(+)-LRMS for: | |
|---|---|---|
| Compound 1 | MTX3 by Lewis et al. [29] | |
| 1099.5 | nd | [M-H+2Na+K]+ |
| 1083.5 | [M-H+2Na]+ | [M-2H+3Na]+ |
| 1077.5 | [M+K]+ | [M-H+Na+K]+ |
| 1061.5 | [M+Na]+ | [M-H+2Na]+ |
| 1056.5 | [M+NH4]+ | nd |
| 1055.5 | nd | [M+K]+ |
| 1039.5 | [M+H]+ | [M+Na]+ |
| 1021.5 | [M+H-H2O]+ | [M+Na-H2O]+ |
| 1003.5 | [M+H-2H2O]+ | [M+Na-2H2O]+ |
| 996.5 | nd | [M-H+Na+K-SO3]+ |
| 981.5 | [M+Na-SO3]+ | [M-H+2Na-SO3]+ |
| 963.5 | nd | [M-H+2Na-H2O-SO3]+ |
| 959.5 | [M+H-SO3]+ | [M+Na-SO3]+ |
| 941.5 | [M+H-H2SO4]+ | [M+Na-H2O-SO3]+ |
| 923.5 | [M+H-H2O-H2SO4]+ | [M+Na-2H2O-SO3]+ |
| 905.5 | [M+H-3H2O-SO3]+ | [M+Na-3H2O-SO3]+ |
| 887.5 | [M+H-4H2O-SO3]+ | [M+Na-4H2O-SO3]+ |
| Compound | Maitotoxin-3 | Gambierone | ||||||
|---|---|---|---|---|---|---|---|---|
| ESI Mode | Parent Ion | Cone/V | Fragment Ion | Coll/ev | Parent Ion | Cone/V | Fragment Ion | Coll/ev |
| + | 1039.5 | 30 | 233.1 | 30 | 1025.5 | 30 | 219.1 | 30 |
| 277.1 | 60 | 277.1 | 60 | |||||
| 803.4 | 30 | 803.4 | 30 | |||||
| 941.5 | 30 | 927.5 | 30 | |||||
| 1021.5 | 4 | 1007.5 | 4 | |||||
| − | 899.6 | 70 | 96.8 | 60 | 899.6 | 70 | 96.8 | 60 |
| 1037.5 | 70 | 96.8 | 60 | 1023.5 | 70 | 96.8 | 60 | |
| 899.6 | 60 | 899.6 | 60 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boente-Juncal, A.; Álvarez, M.; Antelo, Á.; Rodríguez, I.; Calabro, K.; Vale, C.; Thomas, O.P.; Botana, L.M. Structure Elucidation and Biological Evaluation of Maitotoxin-3, a Homologue of Gambierone, from Gambierdiscus belizeanus. Toxins 2019, 11, 79. https://doi.org/10.3390/toxins11020079
Boente-Juncal A, Álvarez M, Antelo Á, Rodríguez I, Calabro K, Vale C, Thomas OP, Botana LM. Structure Elucidation and Biological Evaluation of Maitotoxin-3, a Homologue of Gambierone, from Gambierdiscus belizeanus. Toxins. 2019; 11(2):79. https://doi.org/10.3390/toxins11020079
Chicago/Turabian StyleBoente-Juncal, Andrea, Mercedes Álvarez, Álvaro Antelo, Inés Rodríguez, Kevin Calabro, Carmen Vale, Olivier P. Thomas, and Luis M. Botana. 2019. "Structure Elucidation and Biological Evaluation of Maitotoxin-3, a Homologue of Gambierone, from Gambierdiscus belizeanus" Toxins 11, no. 2: 79. https://doi.org/10.3390/toxins11020079
APA StyleBoente-Juncal, A., Álvarez, M., Antelo, Á., Rodríguez, I., Calabro, K., Vale, C., Thomas, O. P., & Botana, L. M. (2019). Structure Elucidation and Biological Evaluation of Maitotoxin-3, a Homologue of Gambierone, from Gambierdiscus belizeanus. Toxins, 11(2), 79. https://doi.org/10.3390/toxins11020079







