The Inhibitory Effect of Celangulin V on the ATP Hydrolytic Activity of the Complex of V-ATPase Subunits A and B in the Midgut of Mythimna separata
Abstract
1. Introduction
2. Results
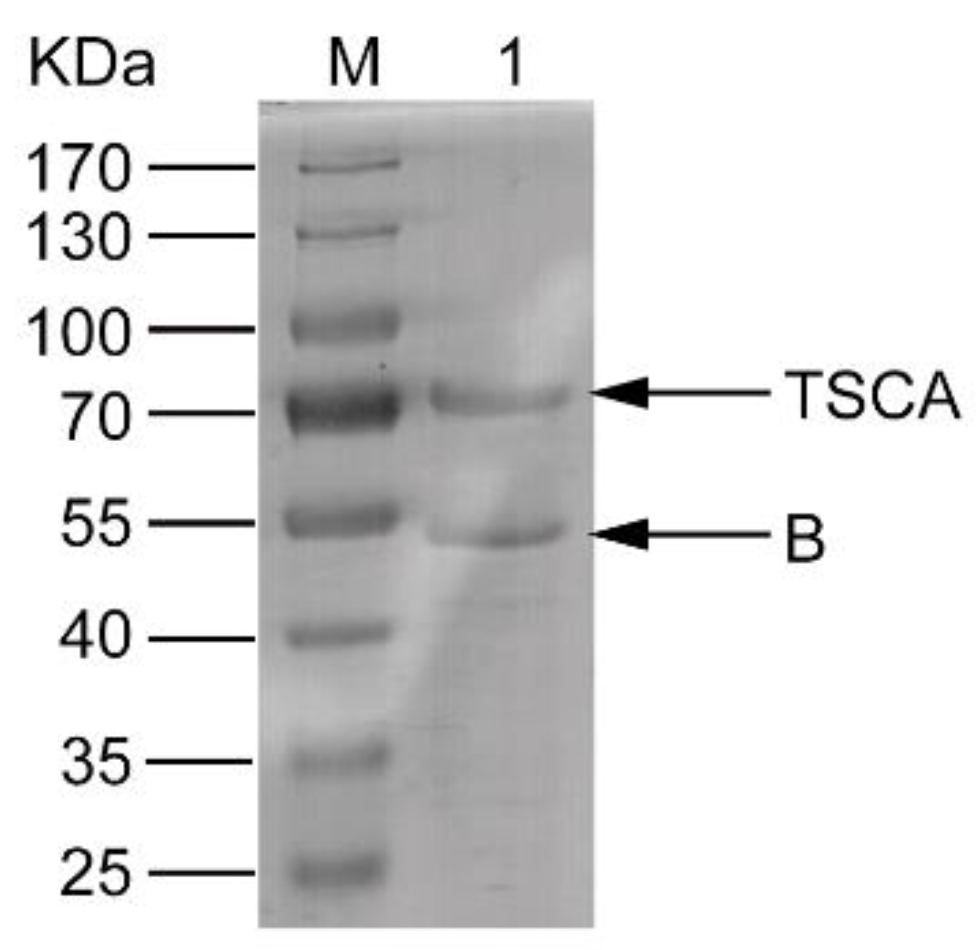
2.1. Expression, Refolding, and Purification of the Complex of the Recombinant Proteins
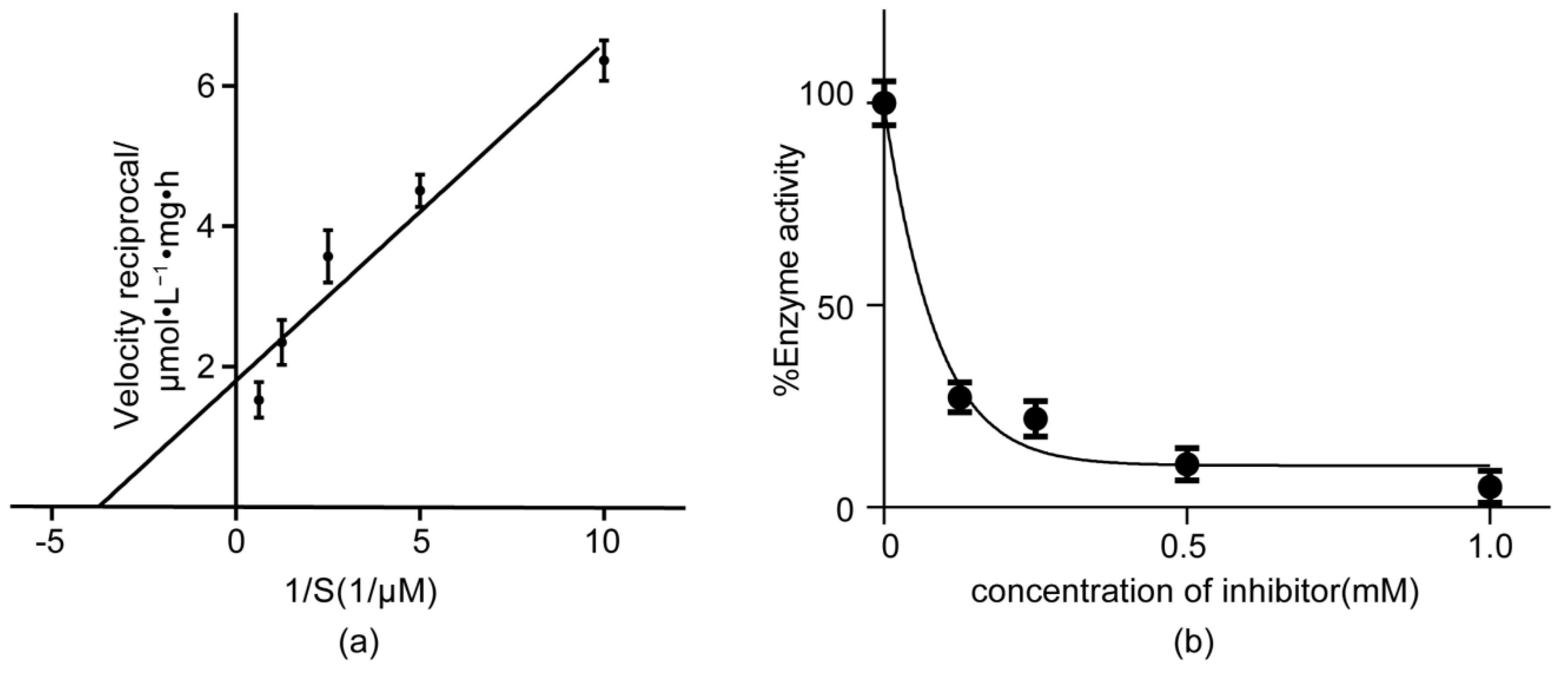
2.2. ATP Hydrolysis Activity Assay of the TSCA–B Complex
2.3. The Inhibitory Activity of CV to ATP Hydrolysis
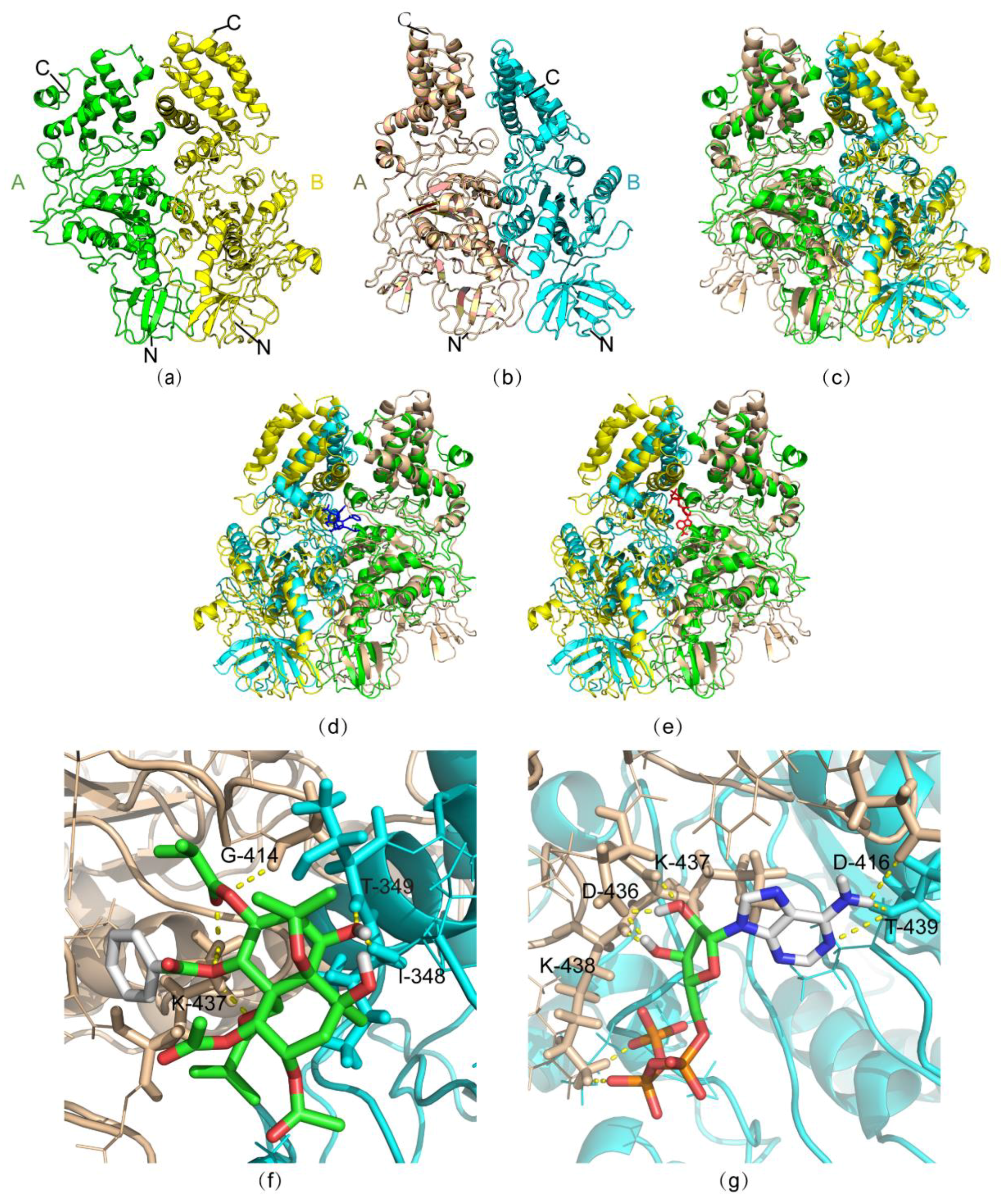
2.4. Molecular Simulation
3. Discussion
4. Materials and Methods
4.1. Plasmid
- Forward primer: CGCATATGAGCAAAAAAGATCAGCTGAAGAAGATCG;
- Reverse mutation: GAGATGACGCTCTTGCCCGCACCGAAGG;
- Forward mutation: CCTTCGGTGCGGGCAAGAGCGTCATCTC;
- Reverse primer: GCAAGCTTGTCCTCCAGGTTACGGAAGG.
4.2. Chemicals
4.3. Expression of TSCA and B Subunits of V-ATPase
4.4. Denaturation and Renaturation of Inclusion Bodies
4.5. ATP Hydrolysis Activity Assays of TSCA–B Complex
4.6. Inhibitory Activity Assays of CV
4.7. Molecular Simulation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, W.J.; Hu, Z.N.; Liu, H.X.; Qi, Z.Y. Insecticidal mechanisms of the major active components from the Chinese bittersweet, Celastrus angulatus and their application. Acta Entomol. Sin. 2005, 5, 770–777. [Google Scholar]
- Wu, W.J.; Ji, Z.Q.; Hu, Z.N.; Zhang, J.W. Advances in research on insecticidal plant Celastrus angulatus: Chemistry of the active components. J. Cent. Chin. Normal Univ. 2005, 1, 50–53. [Google Scholar] [CrossRef]
- Qi, Z.; Xue, X.; Wu, W.; Zhang, J.; Yang, R. Preparation of monoclonal antibody against Celangulin V and immunolocalization of receptor in the oriental armyworm, Mythimna separata walker (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2006, 54, 7600–7605. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Yang, C.J.; Lian, X.H.; Wu, W.J. Effects of Celangulin V on muscle cells of Mythimna separata. Acta Entomol. Sin. 2003, 4, 417–423. [Google Scholar]
- Liu, H.X.; Dong, Y.X.; Wu, W.J. Effects of Celangulin V on the midgut cells and the digestive enzyme activities of Mythimna separata (Walke) larvae. Acta Entomol. Sin. 1998, 3, 36–40. [Google Scholar]
- Lu, L.; Qi, Z.; Zhang, J.; Wu, W. Separation of Binding Protein of Celangulin V from the Midgut of Mythimna separata Walker by Affinity Chromatography. Toxins (Basel) 2015, 7, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Qi, Z.; Li, Q.; Wu, W. Validation of the Target Protein of Insecticidal Dihydroagarofuran Sesquiterpene Polyesters. Toxins (Basel) 2016, 83, e79. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.; Xi, X.; Liu, L.; Zhao, X.; Wu, W.; Zhang, J. Molecular Insights into the Potential Insecticidal Interaction of beta-Dihydroagarofuran Derivatives with the H Subunit of V-ATPase. Molecules 2017, 22, E1701. [Google Scholar] [CrossRef]
- Maher, M.J.; Akimoto, S.; Iwata, M.; Nagata, K.; Hori, Y.; Yoshida, M.; Yokoyama, S.; Iwata, S.; Yokoyama, K. Crystal structure of A3B3 complex of V-ATPase from Thermus thermophilus. EMBO J. 2009, 28, 3771–3779. [Google Scholar] [CrossRef]
- Sagermann, M.; Stevens, T.H.; Matthews, B.W. Crystal structure of the regulatory subunit H of the V-type ATPase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2001, 98, 7134–7139. [Google Scholar] [CrossRef]
- Imamura, H.; Funamoto, S.; Yoshida, M.; Yokoyama, K. Reconstitution in vitro of V1 complex of Thermus thermophilus V-ATPase revealed that ATP binding to the A subunit is crucial for V1 formation. J. Biol. Chem. 2006, 281, 38582–38591. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, Y.; Takeda, K.; Kuranaga, T.; Numoto, N.; Miki, K. Origin of Asymmetry at the Intersubunit Interfaces of V1-ATPase from Thermusthermophilus. J. Mol. Biol. 2013, 425, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Guerler, A.; Govindarajoo, B.; Zhang, Y. Mapping Monomeric Threading to Protein–Protein Structure Prediction. J. Chem. Inf. Model. 2013, 53, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Rotkiewicz, P.; Skolnick, J. Fast procedure for reconstruction of full-atom protein models from reduced representations. J. Comput. Chem. 2008, 29, 1460–1465. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Liu, Y.L.; Song, Z.Q. Research advances on the insecticidal plant Celastrus angulatus. Agrochemicals 2006, 3, 148–150, 154. [Google Scholar]
- Wieczorek, H.; Grber, G.; Harvey, W.R.; Huss, M.; Merzendorfer, H.; Zeiske, W. Structure and regulation of insect plasma membrane H(+)V-ATPase. J. Exp. Biol. 2000, 203, 127–135. [Google Scholar]
- Holliday, L.S. Vacuolar H+-ATPase: An essential multitasking enzyme in physiology and pathophysiology. New J. Sci. 2014, 1–22. [Google Scholar] [CrossRef]
- Wieczorek, H.; Beyenbach, K.W.; Huss, M.; Vitavska, O. Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 2009, 212, 1611–1619. [Google Scholar] [CrossRef]
- Wieczorek, H.; Huss, M.; Merzendorfer, H.; Reineke, S.; Vitavska, O.; Zeiske, W. The insect plasma membrane H+ V-ATPase: Intra-, inter-, and supramolecular aspects. J. Bioenerg. Biomembr. 2003, 35, 359–366. [Google Scholar] [CrossRef]
- Bowman, E.J.; Bowman, B.J. V-ATPases as drug targets. J. Bioenerg. Biomembr. 2005, 37, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xiang, Y.; An, L. Structural bases of physiological functions and roles of the vacuolar H(+)-ATPase. Cell. Signal. 2011, 23, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.8; Schrodinger, LLC: New York, NY, USA, 2015. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Guo, Z.; Xu, H.; Li, T.; Wang, Y.; Tao, H. The Inhibitory Effect of Celangulin V on the ATP Hydrolytic Activity of the Complex of V-ATPase Subunits A and B in the Midgut of Mythimna separata. Toxins 2019, 11, 130. https://doi.org/10.3390/toxins11020130
Ding L, Guo Z, Xu H, Li T, Wang Y, Tao H. The Inhibitory Effect of Celangulin V on the ATP Hydrolytic Activity of the Complex of V-ATPase Subunits A and B in the Midgut of Mythimna separata. Toxins. 2019; 11(2):130. https://doi.org/10.3390/toxins11020130
Chicago/Turabian StyleDing, Liwen, Zongxin Guo, Hang Xu, Tie Li, Yuanyuan Wang, and Hu Tao. 2019. "The Inhibitory Effect of Celangulin V on the ATP Hydrolytic Activity of the Complex of V-ATPase Subunits A and B in the Midgut of Mythimna separata" Toxins 11, no. 2: 130. https://doi.org/10.3390/toxins11020130
APA StyleDing, L., Guo, Z., Xu, H., Li, T., Wang, Y., & Tao, H. (2019). The Inhibitory Effect of Celangulin V on the ATP Hydrolytic Activity of the Complex of V-ATPase Subunits A and B in the Midgut of Mythimna separata. Toxins, 11(2), 130. https://doi.org/10.3390/toxins11020130




