A Recurrent Motif: Diversity and Evolution of ShKT Domain Containing Proteins in the Vampire Snail Cumia reticulata
Abstract
1. Introduction
2. Results
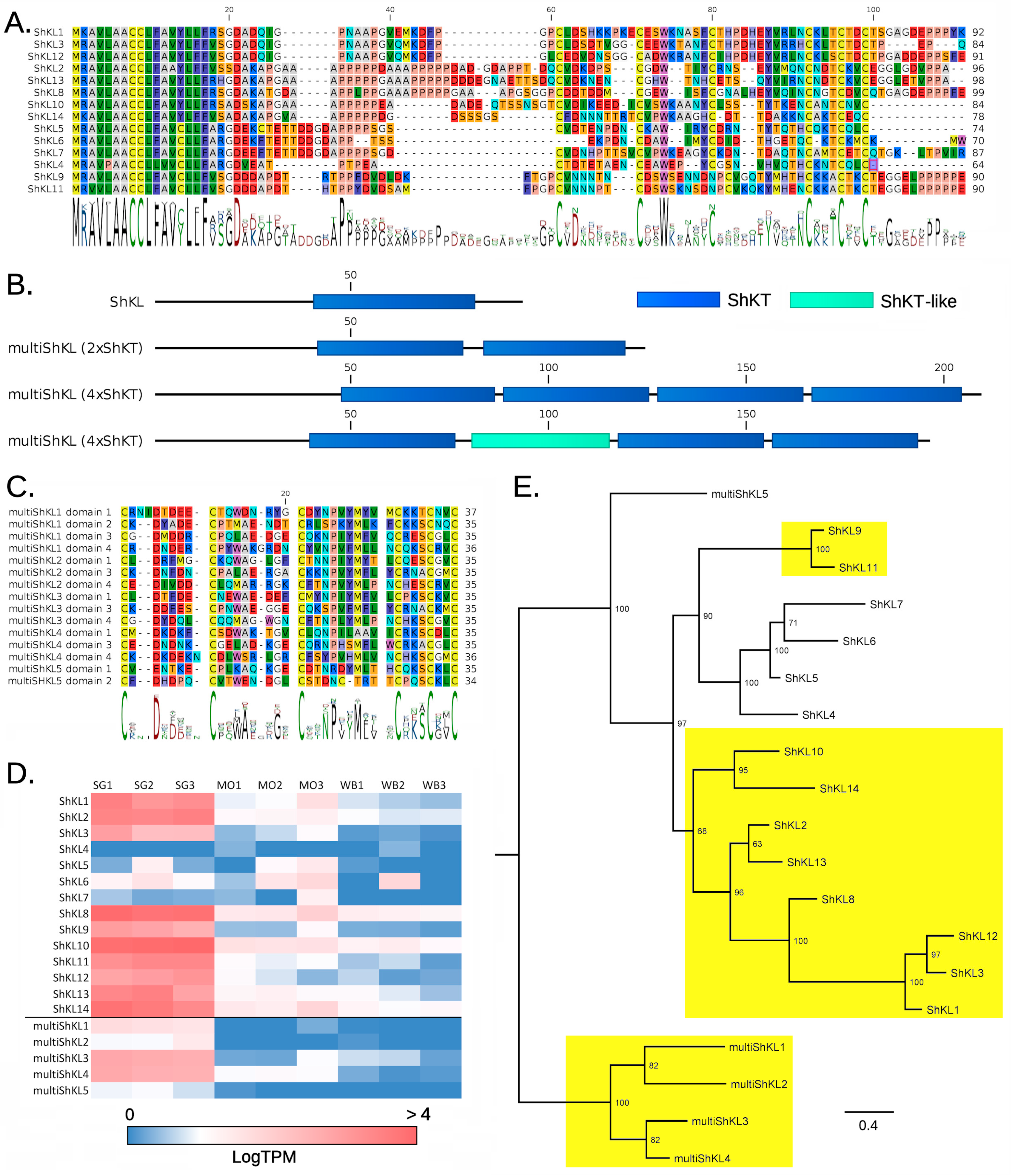
2.1. ShK-Like Proteins
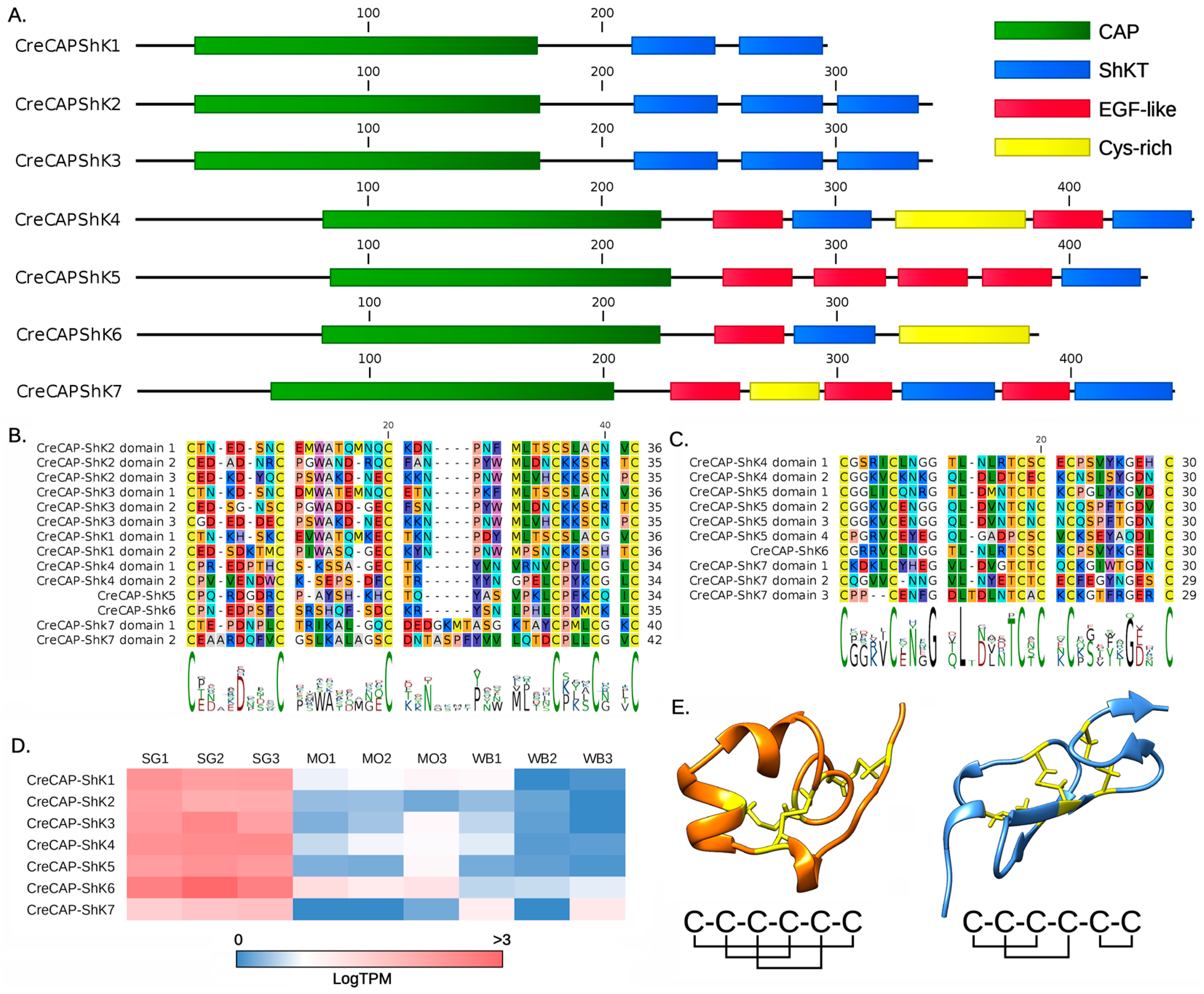
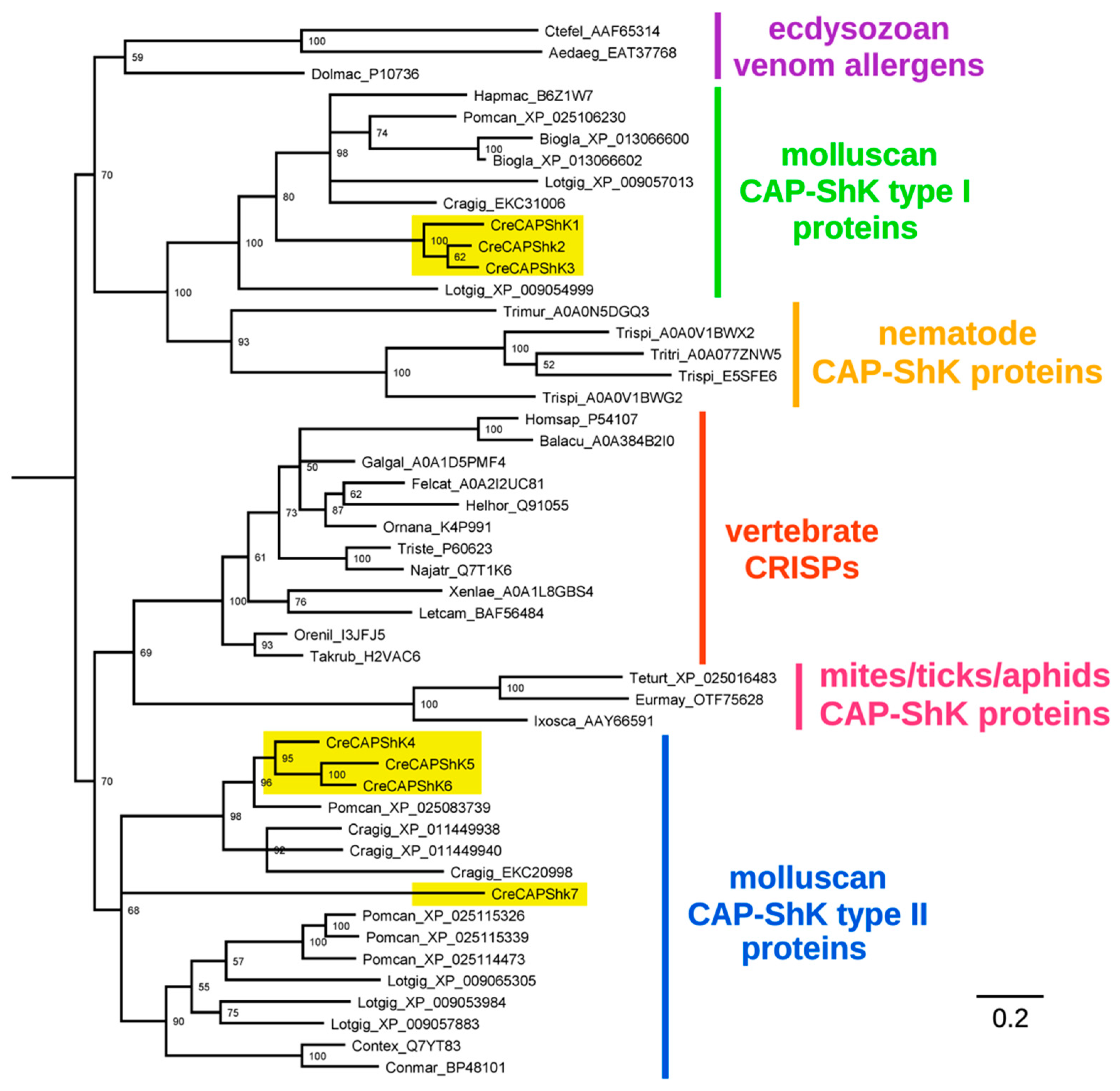
2.2. CAP-ShKT Proteins
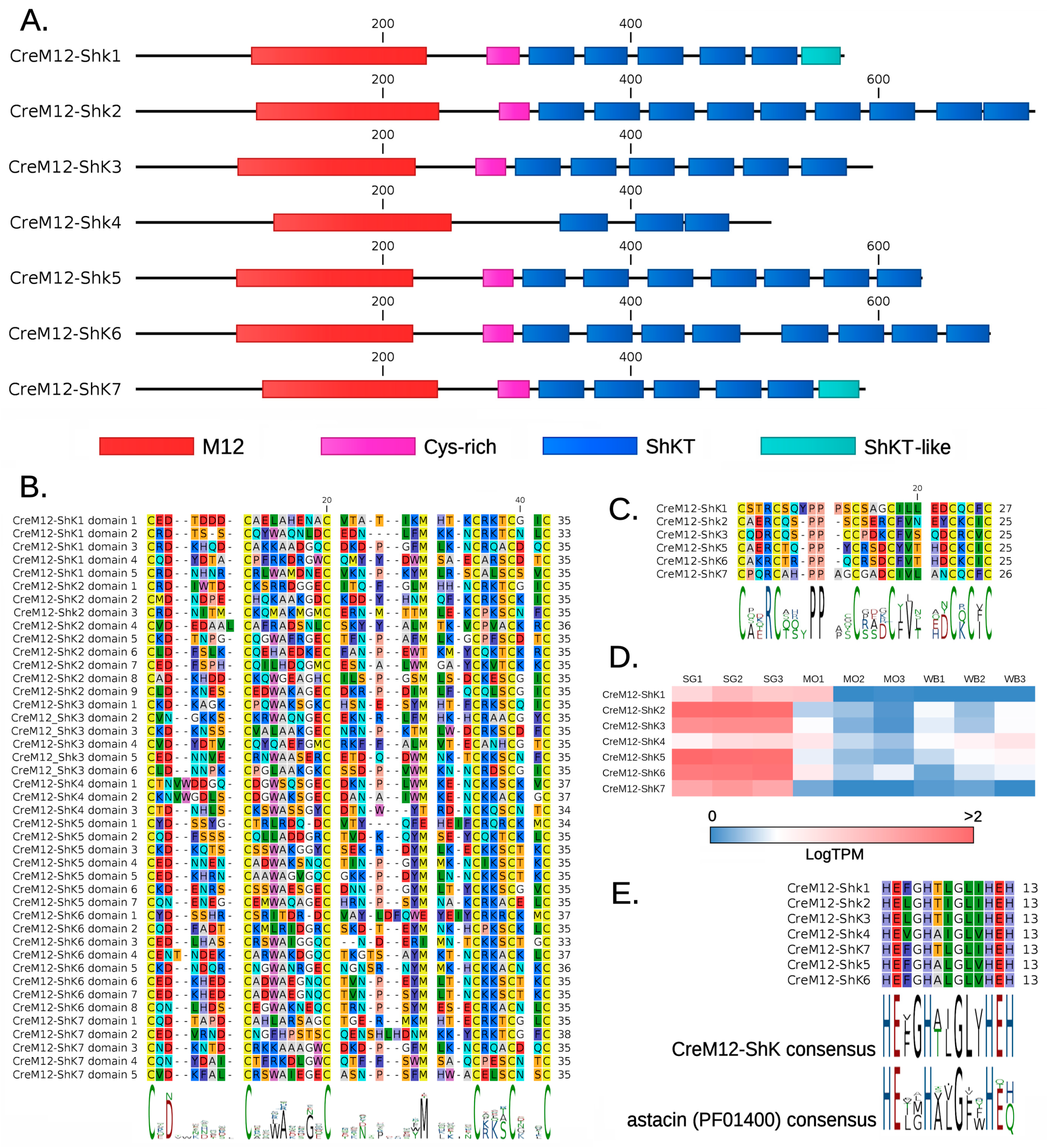
2.3. M12-ShKT Proteins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Identification and Characterization of ShKT Domain Containing Protein Sequences
5.2. Calculation of Gene Expression Values
5.3. Phylogeny of C. reticulata ShKT-Domain Containing Proteins
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castaneda, O.; Sotolongo, V.; Amor, A.M.; Stocklin, R.; Anderson, A.J.; Harvey, A.L.; Engstrom, A.; Wernstedt, C.; Karlsson, E. Characterization of a potassium channel toxin from the Caribbean Sea anemone Stichodactyla helianthus. Toxicon 1995, 33, 603–613. [Google Scholar] [CrossRef]
- Pennington, M.W.; Byrnes, M.E.; Zaydenberg, I.; Khaytin, I.; de Chastonay, J.; Krafte, D.S.; Hill, R.; Mahnir, V.M.; Volberg, W.A.; Gorczyca, W. Chemical synthesis and characterization of ShK toxin: A potent potassium channel inhibitor from a sea anemone. Int. J. Pept. Protein Res. 1995, 46, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Honma, T.; Shiomi, K. Peptide toxins in sea anemones: Structural and functional aspects. Mar. Biotechnol. 2006, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, O.; Harvey, A.L. Discovery and characterization of cnidarian peptide toxins that affect neuronal potassium ion channels. Toxicon 2009, 54, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Orts, D.; Peigneur, S.; Madio, B.; Cassoli, J.; Montandon, G.; Pimenta, A.; Bicudo, J.; Freitas, J.; Zaharenko, A.; Tytgat, J.; et al. Biochemical and Electrophysiological Characterization of Two Sea Anemone Type 1 Potassium Toxins from a Geographically Distant Population of Bunodosoma caissarum. Mar. Drugs 2013, 11, 655–679. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Hasegawa, Y.; Honma, T.; Nagashima, Y.; Shiomi, K. Screening and cDNA Cloning of Kv1 Potassium Channel Toxins in Sea Anemones. Mar. Drugs 2010, 8, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Aneiros, A.; García, I.; Martínez, J.; Harvey, A.L.; Anderson, A.J.; Marshall, D.L.; Engström, Å.; Hellman, U.; Karlsson, E. A potassium channel toxin from the secretion of the sea anemone Bunodosoma granulifera. isolation, amino acid sequence and biological activity. Biochim. Biophys. Acta-Gen. Subj. 1993, 1157, 86–92. [Google Scholar] [CrossRef]
- Frazão, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: An overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef]
- Chi, V.; Pennington, M.W.; Norton, R.S.; Tarcha, E.J.; Londono, L.M.; Sims-Fahey, B.; Upadhyay, S.K.; Lakey, J.T.; Iadonato, S.; Wulff, H.; et al. Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases. Toxicon 2012, 59, 529–546. [Google Scholar] [CrossRef]
- Kalman, K.; Pennington, M.W.; Lanigan, M.D.; Nguyen, A.; Rauer, H.; Mahnir, V.; Paschetto, K.; Kem, W.R.; Grissmer, S.; Gutman, G.A.; et al. ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide. J. Biol. Chem. 1998, 273, 32697–32707. [Google Scholar] [CrossRef]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Chang, S.C.; Chauhan, S.; Huq, R.; Tajhya, R.B.; Chhabra, S.; Norton, R.S.; Beeton, C. Development of highly selective Kv1.3-blocking peptides based on the sea anemone peptide ShK. Mar. Drugs 2015, 13, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Harunur Rashid, M.; Tajhya, R.B.; Beeton, C.; Kuyucak, S.; Norton, R.S. A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3. FEBS Lett. 2012, 586, 3996–4001. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Beeton, C.; Galea, C.A.; Smith, B.J.; Chi, V.; Monaghan, K.P.; Garcia, A.; Rangaraju, S.; Giuffrida, A.; Plank, D.; et al. Engineering a stable and selective peptide blocker of the Kv1.3 channel in T lymphocytes. Mol. Pharmacol. 2009, 75, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Heinzelmann, G.; Huq, R.; Tajhya, R.B.; Chang, S.C.; Chhabra, S.; Pennington, M.W.; Beeton, C.; Norton, R.S.; Kuyucak, S. A potent and selective peptide blocker of the Kv1.3 channel: Prediction from free-energy simulations and experimental confirmation. PLoS ONE 2013, 8, e78712. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Huq, R.; Chhabra, S.; Beeton, C.; Pennington, M.W.; Smith, B.J.; Norton, R.S. N-Terminally extended analogues of the K+ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3. FEBS J. 2015, 282, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.K.; Qian, Y.-X.; Liu, B.; Elliott, R.; Aral, J.; Park, C.; Zhang, X.; Stenkilsson, M.; Salyers, K.; Rose, M.; et al. Pharmaceutical Optimization of Peptide Toxins for Ion Channel Targets: Potent, Selective, and Long-Lived Antagonists of Kv1.3. J. Med. Chem. 2015, 58, 6784–6802. [Google Scholar] [CrossRef] [PubMed]
- Tarcha, E.J.; Olsen, C.M.; Probst, P.; Peckham, D.; Muñoz-Elías, E.J.; Kruger, J.G.; Iadonato, S.P. Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial. PLoS ONE 2017, 12, e0180762. [Google Scholar] [CrossRef] [PubMed]
- Land, J.; Lintermans, L.L.; Stegeman, C.A.; Muñoz-Elías, E.J.; Tarcha, E.J.; Iadonato, S.P.; Heeringa, P.; Rutgers, A.; Abdulahad, W.H. Kv1.3 Channel Blockade Modulates the Effector Function of B Cells in Granulomatosis with Polyangiitis. Front. Immunol. 2017, 8, 1205. [Google Scholar] [CrossRef]
- Prentis, P.J.; Pavasovic, A.; Norton, R.S. Sea Anemones: Quiet Achievers in the Field of Peptide Toxins. Toxins 2018, 10, 30. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, S.; Khoo, K.K.; Feng, Z.P.; Crossley, G.; Nugent, D.; Khaytin, I.; Chi, V.; Pham, C.; Calabresi, P.; Pennington, M.W.; et al. Potassium channel modulation by a toxin domain in matrix metalloprotease 23. J. Biol. Chem. 2010, 285, 9124–9136. [Google Scholar] [CrossRef] [PubMed]
- Columbus-Shenkar, Y.Y.; Sachkova, M.Y.; Macrander, J.; Fridrich, A.; Modepalli, V.; Reitzel, A.M.; Sunagar, K.; Moran, Y. Dynamics of venom composition across a complex life cycle. Elife 2018, 7, e35014. [Google Scholar] [CrossRef] [PubMed]
- Modica, M.V.; Lombardo, F.; Franchini, P.; Oliverio, M. The venomous cocktail of the vampire snail Colubraria reticulata (Mollusca, Gastropoda). BMC Genom. 2015, 16, 441. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; Reddy, T.; O’Bryan, M.K. The role of cysteine-rich secretory proteins in male fertility. Asian J. Androl. 2011, 13, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Ramazanova, A.S.; Starkov, V.G.; Osipov, A.V.; Ziganshin, R.H.; Filkin, Y.; Tsetlin, V.I.; Utkin, Y.N. Cysteine-rich venom proteins from the snakes of Viperinae subfamily—Molecular cloning and phylogenetic relationship. Toxicon 2008, 53, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Mochca-Morales, J.; Martin, B.M.; Possani, L.D. Isolation and characterization of helothermine, a novel toxin from Heloderma horridum horridum (Mexican beaded lizard) venom. Toxicon 1990, 28, 299–309. [Google Scholar] [CrossRef]
- Ito, N.; Mita, M.; Takahashi, Y.; Matsushima, A.; Watanabe, Y.G.; Hirano, S.; Odani, S. Novel cysteine-rich secretory protein in the buccal gland secretion of the parasitic lamprey, Lethenteron japonicum. Biochem. Biophys. Res. Commun. 2007, 358, 35–40. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef]
- Möhrlen, F.; Hutter, H.; Zwilling, R. The astacin protein family in Caenorhabditis elegans. Eur. J. Biochem. 2003, 270, 4909–4920. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Johnson, J.; Jazwinski, S. Parasitism of sleeping fish by gastropod mollusks in the Colubrariidae and Marginellidae at Kwajalein, Marshall Islands. Festivus 1995, 27, 121–126. [Google Scholar]
- Bouchet, P.; Perrine, D. More gastropods feeding at night on parrotfishes. Bull. Mar. Sci. 1996, 59, 224–228. [Google Scholar]
- Oliverio, M.; Modica, M.V. Relationships of the haematophagous marine snail Colubraria (Rachiglossa: Colubrariidae), within the neogastropod phylogenetic framework. Zool. J. Linn. Soc. 2010, 158, 779–800. [Google Scholar] [CrossRef]
- Gerdol, M.; Cervelli, M.; Oliverio, M.; Modica, M.V. Piercing Fishes: Porin Expansion and Adaptation to Hematophagy in the Vampire Snail Cumia reticulata. Mol. Biol. Evol. 2018, 35, 2654–2668. [Google Scholar] [CrossRef] [PubMed]
- Modica, M.V.; Reinoso Sánchez, J.; Pasquadibisceglie, A.; Oliverio, M.; Mariottini, P.; Cervelli, M. Anti-haemostatic compounds from the vampire snail Cumia reticulata: Molecular cloning and in-silico structure-function analysis. Comput. Biol. Chem. 2018, 75, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Norman, J.A. Tentacles of venom: Toxic protein convergence in the Kingdom Animalia. J. Mol. Evol. 2009, 68, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Chen, L.; Li, Y.; Xie, L.; Zhang, R. Pf-ALMP, a novel astacin-like metalloproteinase with cysteine arrays, is abundant in hemocytes of pearl oyster Pinctada fucata. Biochim. Biophys. Acta Gene Struct. Expr. 2006, 1759, 526–534. [Google Scholar] [CrossRef]
- Marsh, A.G.; Chen, T.T. A divergent cDNA homologue of the c-myc proto-oncogene in the eastern oyster Crassostrea virginica: Implications for Myc evolution. Mol. Mar. Biol. Biotechnol. 1995, 4, 185–192. [Google Scholar]
- Chhabra, S.; Chang, S.C.; Nguyen, H.M.; Huq, R.; Tanner, M.R.; Londono, L.M.; Estrada, R.; Dhawan, V.; Chauhan, S.; Upadhyay, S.K.; et al. Kv1.3 channel-blocking immunomodulatory peptides from parasitic worms: Implications for autoimmune diseases. FASEB J. 2014, 28, 3952–3964. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Villegas-Moreno, J.; Mitchell, M.L.; Csoti, A.; Peigneur, S.; Amero, C.; Pennington, M.W.; Tytgat, J.; Panyi, G.; Norton, R.S. Synthesis, folding, structure and activity of a predicted peptide from the sea anemone Oulactis sp. with an ShKT fold. Toxicon 2018, 150, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Ren, Y.; Wang, H.; Li, S.; Jiang, F.; Yin, L.; Qiao, X.; Zhang, G.; Qian, W.; et al. The genome of the golden apple snail Pomacea canaliculata provides insight into stress tolerance and invasive adaptation. Gigascience 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Simakov, O.; Marletaz, F.; Cho, S.-J.; Edsinger-Gonzales, E.; Havlak, P.; Hellsten, U.; Kuo, D.-H.; Larsson, T.; Lv, J.; Arendt, D.; et al. Insights into bilaterian evolution from three spiralian genomes. Nature 2012, 493, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Adema, C.M.; Hillier, L.W.; Jones, C.S.; Loker, E.S.; Knight, M.; Minx, P.; Oliveira, G.; Raghavan, N.; Shedlock, A.; do Amaral, L.R.; et al. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat. Commun. 2017, 8, 15451. [Google Scholar] [CrossRef] [PubMed]
- Milne, T.J.; Abbenante, G.; Tyndall, J.D.; Halliday, J.; Lewis, R.J. Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J. Biol. Chem. 2003, 278, 31105–31110. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Guo, Z.; Chi, C. Cloning and isolation of a conus cysteine-rich protein homologous to Tex31 but without proteolytic activity. Acta Biochim. Biophys. Sin. (Shanghai) 2008, 40, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R. Hymenoptera Venom Allergens. Clin. Rev. Allergy Immunol. 2006, 30, 109–128. [Google Scholar] [CrossRef]
- Lee, S.; Johnstone, I.; Lee, R.; Opdebeeck, J. Putative salivary allergens of the cat flea, Ctenocephalides felis felis. Vet. Immunol. Immunopathol. 1999, 69, 229–237. [Google Scholar] [CrossRef]
- Darwiche, R.; El Atab, O.; Cottier, S.; Schneiter, R. The function of yeast CAP family proteins in lipid export, mating, and pathogen defense. FEBS Lett. 2018, 592, 1304–1311. [Google Scholar] [CrossRef]
- Ellerman, D.A.; Cohen, D.J.; Da Ros, V.G.; Morgenfeld, M.M.; Busso, D.; Cuasnicú, P.S. Sperm protein “DE” mediates gamete fusion through an evolutionarily conserved site of the CRISP family. Dev. Biol. 2006, 297, 228–237. [Google Scholar] [CrossRef]
- Serrano, R.L.; Kuhn, A.; Hendricks, A.; Helms, J.B.; Sinning, I.; Groves, M.R. Structural Analysis of the Human Golgi-associated Plant Pathogenesis Related Protein GAPR-1 Implicates Dimerization as a Regulatory Mechanism. J. Mol. Biol. 2004, 339, 173–183. [Google Scholar] [CrossRef] [PubMed]
- van Galen, J.; Olrichs, N.K.; Schouten, A.; Serrano, R.L.; Nolte-’t Hoen, E.N.M.; Eerland, R.; Kaloyanova, D.; Gros, P.; Helms, J.B. Interaction of GAPR-1 with lipid bilayers is regulated by alternative homodimerization. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2175–2183. [Google Scholar] [CrossRef]
- Olrichs, K.N.; Helms, J.B. Novel insights into the function of the conserved domain of the CAP superfamily of proteins. AIMS Biophys. 2016, 3, 232–246. [Google Scholar] [CrossRef]
- Xu, X.; Francischetti, I.M.B.; Lai, R.; Ribeiro, J.M.C.; Andersen, J.F. Structure of protein having inhibitory disintegrin and leukotriene scavenging functions contained in single domain. J. Biol. Chem. 2012, 287, 10967–10976. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Honma, T.; Ide, M.; Nagashima, Y.; Ishida, M.; Chino, M. An epidermal growth factor-like toxin and two sodium channel toxins from the sea anemone Stichodactyla gigantea. Toxicon 2003, 41, 229–236. [Google Scholar] [CrossRef]
- Honma, T.; Kawahata, S.; Ishida, M.; Nagai, H.; Nagashima, Y.; Shiomi, K. Novel peptide toxins from the sea anemone Stichodactyla haddoni. Peptides 2008, 29, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Macrander, J.; Broe, M.; Daly, M. Tissue-specific venom composition and differential gene expression in sea anemones. Genome Biol. Evol. 2016, 8, 2358–2375. [Google Scholar] [CrossRef]
- von Reumont, B.M.; Campbell, L.I.; Richter, S.; Hering, L.; Sykes, D.; Hetmank, J.; Jenner, R.A.; Bleidorn, C. A Polychaete’s powerful punch: Venom gland transcriptomics of Glycera reveals a complex cocktail of toxin homologs. Genome Biol. Evol. 2014, 6, 2406–2423. [Google Scholar] [CrossRef]
- Cuypers, E.; Peigneur, S.; Debaveye, S.; Shiomi, K.; Tytgat, J. TRPV1 Channel as New Target for Marine Toxins: Example of Gigantoxin I, a Sea Anemone Toxin Acting Via Modulation of the PLA2 Pathway. Acta Chim. Slov. 2011, 58, 735–741. [Google Scholar]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef]
- Kumpfmüller, G.; Rybakine, V.; Takahashi, T.; Fujisawa, T.; Bosch, T.C. Identification of an astacin matrix metalloprotease as target gene for Hydra foot activator peptides. Dev. Genes Evol. 1999, 209, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Gröger, H.; Schmid, V.; Spring, J. A toxin homology domain in an astacin-like metalloproteinase of the jellyfish Podocoryne carnea with a dual role in digestion and development. Dev. Genes Evol. 1998, 208, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hunt, V.L.; Tsai, I.J.; Coghlan, A.; Reid, A.J.; Holroyd, N.; Foth, B.J.; Tracey, A.; Cotton, J.A.; Stanley, E.J.; Beasley, H.; et al. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nat. Genet. 2016, 48, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Struck, T.H.; Wey-Fabrizius, A.R.; Golombek, A.; Hering, L.; Weigert, A.; Bleidorn, C.; Klebow, S.; Iakovenko, N.; Hausdorf, B.; Petersen, M.; et al. Platyzoan Paraphyly Based on Phylogenomic Data Supports a Noncoelomate Ancestry of Spiralia. Mol. Biol. Evol. 2014, 31, 1833–1849. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.-L.; Chan, R.K.Y.; Qiu, J.-W. The Potential of the Invasive Snail Pomacea canaliculata as a Predator of Various Life-Stages of Five Species of Freshwater Snails. Malacologia 2009, 51, 343–356. [Google Scholar] [CrossRef]
- Turzańska, K. Arion slugs as nest predators of small passerine species—A review. J. Avian Biol. 2016, 48, 455–458. [Google Scholar] [CrossRef]
- Trevisan-Silva, D.; Gremski, L.H.; Chaim, O.M.; da Silveira, R.B.; Meissner, G.O.; Mangili, O.C.; Barbaro, K.C.; Gremski, W.; Veiga, S.S.; Senff-Ribeiro, A. Astacin-like metalloproteases are a gene family of toxins present in the venom of different species of the brown spider (genus Loxosceles). Biochimie 2010, 92, 21–32. [Google Scholar] [CrossRef]
- Andrade, M.A.; Petosa, C.; O’Donoghue, S.I.; Müller, C.W.; Bork, P. Comparison of ARM and HEAT protein repeats. J. Mol. Biol. 2001, 309, 1–18. [Google Scholar] [CrossRef]
- Andrade, M.A.; Perez-Iratxeta, C.; Ponting, C.P. Protein Repeats: Structures, Functions, and Evolution. J. Struct. Biol. 2001, 134, 17–31. [Google Scholar] [CrossRef]
- Looman, C.; Åbrink, M.; Mark, C.; Hellman, L. KRAB Zinc Finger Proteins: An Analysis of the Molecular Mechanisms Governing Their Increase in Numbers and Complexity During Evolution. Mol. Biol. Evol. 2002, 19, 2118–2130. [Google Scholar] [CrossRef]
- McLachlan, A.D. Repeated folding pattern in copper–zinc superoxide dismutase. Nature 1980, 285, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Lespinet, O.; Wolf, Y.I.; Koonin, E.V.; Aravind, L. The role of lineage-specific gene family expansion in the evolution of eukaryotes. Genome Res. 2002, 12, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Koyanagi, R.; Gyoja, F.; Kanda, M.; Hisata, K.; Fujie, M.; Goto, H.; Yamasaki, S.; Nagai, K.; Morino, Y.; et al. Bivalve-specific gene expansion in the pearl oyster genome: Implications of adaptation to a sessile lifestyle. Zool. Lett. 2016, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Guo, X.; Litman, G.W.; Dishaw, L.J.; Zhang, G. Massive expansion and functional divergence of innate immune genes in a protostome. Sci. Rep. 2015, 5, 8693. [Google Scholar] [CrossRef] [PubMed]
- Björklund, Å.K.; Ekman, D.; Elofsson, A. Expansion of Protein Domain Repeats. PLoS Comput. Biol. 2006, 2, e114. [Google Scholar] [CrossRef]
- Patthy, L. Genome evolution and the evolution of exon-shuffling—A review. Gene 1999, 238, 103–114. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. Advantages of combined transmembrane topology and signal peptide prediction-the Phobius web server. Nucleic Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Finn, R.D.; Attwood, T.K.; Babbitt, P.C.; Bateman, A.; Bork, P.; Bridge, A.J.; Chang, H.-Y.; Dosztányi, Z.; El-Gebali, S.; Fraser, M.; et al. InterPro in 2017-beyond protein family and domain annotations. Nucleic Acids Res. 2017, 45, D190–D199. [Google Scholar] [CrossRef]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Goldman, N. A General Empirical Model of Protein Evolution Derived from Multiple Protein Families Using a Maximum-Likelihood Approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerdol, M.; Cervelli, M.; Mariottini, P.; Oliverio, M.; Dutertre, S.; Modica, M.V. A Recurrent Motif: Diversity and Evolution of ShKT Domain Containing Proteins in the Vampire Snail Cumia reticulata. Toxins 2019, 11, 106. https://doi.org/10.3390/toxins11020106
Gerdol M, Cervelli M, Mariottini P, Oliverio M, Dutertre S, Modica MV. A Recurrent Motif: Diversity and Evolution of ShKT Domain Containing Proteins in the Vampire Snail Cumia reticulata. Toxins. 2019; 11(2):106. https://doi.org/10.3390/toxins11020106
Chicago/Turabian StyleGerdol, Marco, Manuela Cervelli, Paolo Mariottini, Marco Oliverio, Sébastien Dutertre, and Maria Vittoria Modica. 2019. "A Recurrent Motif: Diversity and Evolution of ShKT Domain Containing Proteins in the Vampire Snail Cumia reticulata" Toxins 11, no. 2: 106. https://doi.org/10.3390/toxins11020106
APA StyleGerdol, M., Cervelli, M., Mariottini, P., Oliverio, M., Dutertre, S., & Modica, M. V. (2019). A Recurrent Motif: Diversity and Evolution of ShKT Domain Containing Proteins in the Vampire Snail Cumia reticulata. Toxins, 11(2), 106. https://doi.org/10.3390/toxins11020106








