Abstract
Aim: The purpose of this study was to examine the effectiveness of botulinum toxin A (BoNT-A) therapy combined with rehabilitation on motor function in post-stroke patients. Methods: The following sources up to December 31, 2018, were searched from inception for articles in English: Pubmed, Scopus, CINAHL, Embase, PsycINFO, and CENTRAL. Trials using injections of BoNT-A for upper and lower limb rehabilitation were examined. We excluded studies that were not performed for rehabilitation or were not evaluated for motor function. Results: Twenty-six studies were included. In addition to rehabilitation, nine studies used adjuvant treatment to improve spasticity or improve motor function. In the upper limbs, two of 14 articles indicated that significant improvement in upper limb motor function was observed compared to the control group. In the lower limbs, seven of 14 articles indicated that significant improvement in lower limb motor function was observed compared to the control group. Conclusions: The effect of combined with rehabilitation is limited after stroke, and there is not sufficient evidence, but results suggest that BoNT-A may help to improve motor function. In future studies, the establishment of optimal rehabilitation and evaluation times of BoNT-A treatment will be necessary for improving motor function and spasticity.
Keywords:
stroke; botulinum toxin; spasticity; rehabilitation; upper limbs; lower limbs; motor function Key Contribution:
Botulinum toxin therapy combined with rehabilitation for stroke: a systematic review
1. Introduction
Post-stroke patients with upper and lower limb hemiparesis may present with spasticity, which is a symptom of upper motor neuron syndrome [1]. Previous reports have indicated that spasticity is observed in 19% of patients at three months following stroke and in 38% of patients at 12 months following stroke [2,3]. Spasticity occurs after stroke in between 18% and 38% of patients and may interfere with the execution of daily activities, social participation, and quality of life [4]. Spasticity can interfere with the functional recovery of upper limbs, especially actions such as raising arms, the opening and closing of hand and fingers, and holding objects [4]. Therefore, patients may have difficulty maintaining cleanliness and in eating and dressing activities. In lower limbs, spasticity primarily affects walking. An ample range of motion (ROM) and strength is required for walking, and spasticity makes it difficult to adjust the ROM and control muscle contractions [5]. In particular, the continuous contraction of the triceps surae muscle can lead to clonus, which, in turn, may result in an equinovarus foot [5]. An equinovarus foot can result in poor toe clearance during the swing phase of gait and ankle instability during weight bearing [6]. Botulinum toxin A therapy (BoNT-A) temporarily reduces muscle activity by preventing the release of acetylcholine at the neuromuscular injection, resulting in a reduced spasticity and muscle tone [7]. The pharmacological effect of an intramuscular injection of botulinum toxin type A commences at two-to-four days following injection, with an expected peak effect at three weeks, and its efficacy persists six weeks after injection and up to nine and twelve weeks [8,9]. Several open and placebo-controlled studies have reported the efficacy of local botulinum toxin injections in reducing spasticity and have emphasized its ease of use and safety [9,10,11,12]. Recently, there have been reports about the improvement in motor function for post-stroke hemiparesis using BoNT-A [13]. It has been suggested that further improvement of motor function can be expected when using BoNT-A combined with rehabilitation. In an international survey, Bakheit indicated that overall rehabilitation is likely to be more important in producing functional change than a single specific intervention, such as BoNT-A injection [14]. However, no systematic review has examined changes in motor function by BoNT-A combined with rehabilitation, and there have been few reports that have focused on motor function. Recently, we reported a combined treatment program of BoNT-A therapy with multidisciplinary rehabilitation and suggested that this combined treatment was effective for the improvement of motor function in post-stroke patients with upper and lower limb spasticity [15]. In addition, in the lower extremities, these effects are associated with the degree of muscle fibrosis [16]. Furthermore, repeated BoNT-A therapy and rehabilitation may modify not only the lower limb motor function and walking speed but also changes in bracing [17].
The purpose of this study was to review the literature on improvement of upper and lower limb motor function by BoNT-A combined with rehabilitation and to investigate the future direction of research in this field.
2. Results
2.1. Study Selection
After screening 988 citations, 28 potentially relevant studies were identified. After review, 26 articles met the predetermined inclusion criteria (Figure 1). The subjects data and result summary of each study are given in Table 1 and Table 2 and noted below [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Two of the included articles used data from previously published studies. Therefore, these studies were regarded as one article [30,31,38,39].
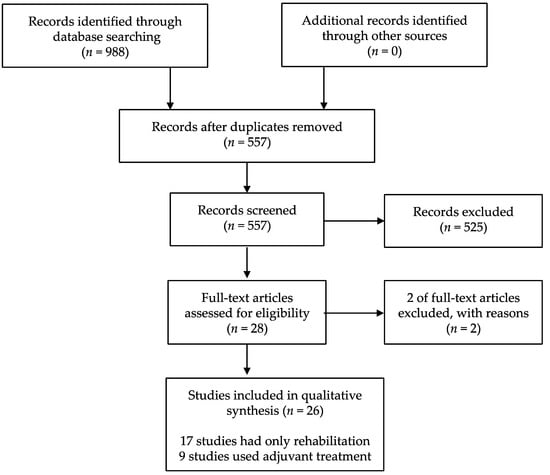
Figure 1.
Study flow diagram.

Table 1.
Study and subject characteristics.

Table 2.
Intervention protocol and result summary.
2.2. Description of Studies
All trials that were selected for this review were published up to December 2018 and were in English. The 26 studies included a total of 1307 patients that received BoNT-A and rehabilitation therapy with a sample size varying from 15 to 332 patients. Twenty-three articles of this review were randomized controlled trials (RCTs) (two article were cross-over trials). In two of these trials, repeat BoNT-A was performed during the intervention [30,31,35]. In addition to rehabilitation, nine studies used adjuvant treatment to improve spasticity or motor function. There were 12 trials for the upper limbs, 12 trials for the lower limbs, and two trials for the upper and lower limbs. The mean age range of the intervention group was 43.6–67 years, and the mean age range of the control group was 41.2–66.0 years [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. The average time between onset and treatment was 24.2 days to 15.7 years (the units of one study was unclear). In the control group, four trials were of BoNT-A only, seven trials were of placebo injection, and nine trials were of rehabilitation only (robotic training alone was included). Uchiyama et al. (2018) used Group 1 as BoNT-A combined with physical therapy (PT) and occupation therapy (OT), and Group 2 used the first phase as rehabilitation only and the second phase as BoNT-A combined with PT and OT [18]. Pimentel et al. (2014) compared BoNT-A doses at 300 and 1000 U [26]. Lim et al. (2008) administered BoNT-A for shoulder pain and spasticity and compared it to triamcinolone acetonide [33]. Of the studies in which adjuvant treatment was used in combination, three trials were BoNT-A combined with rehabilitation in the control group [37,40,45]. The list of control groups in each study is shown in Table 3.

Table 3.
List of control groups.
2.3. Risk of Bias Assessment
Methodological quality by risk of bias is shown in Table 4 [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Sequence generation was appropriate in 57.6% of the studies. In allocation concealment, 38.4% of the studies were appropriate. In regard to the blinding of participants and personnel, some non-RCT studies were marked high risk [18,25,36]. In addition, in the case where the control group was not placebo injection, it was considered difficult to blind the subjects because the intervention content differed between the intervention group and the control group. Therefore, we marked high risk. In regard to the blinding of outcome assessment, the evaluator was properly blinded in 65.3% of the studies. Regarding the outcome data, many studies properly described the dropout and the completion of trial (88.4%). Regarding selective reporting, the study with no comparison between groups and the control group and the study that indicated the change in the results were marked high risk. In addition, in regard to the injection of BoNT-A, the study without a description of the injection site and injection volume and the study without a description of rehabilitation implementation time were regarded as high risk.

Table 4.
Risk of bias summary.
2.4. Outcome Measure
An overview of assessments of outcomes measure in the intervention is shown in Figure 2. For the assessment of spasticity, the Modified Ashworth Scale (MAS) was the most common. As for motor function evaluation, the Fugl–Meyer Assessment (FMA) was the most adopted for the evaluation of spasticity and motor function evaluation, of which five cases were for upper limb function and two were for lower limb function. The next most adopted was the Action Research Arm Test (ARAT). Regarding walking ability, the six minute walk test (6MWT) was adopted most, followed by walking speed and balance evaluation. The Barthel Index (BI) and the Functional Independence Measure (FIM) were frequently adopted for the activity of daily living. Regarding follow-up, 80.7% of the studies were evaluated at multiple times. Follow-up was evaluated for a minimum of two weeks (26.9%) and a maximum of 120 days after administration. The timing of the first evaluation after intervention and second and subsequent evaluation timing after intervention during follow up are shown in Figure 3. The timing of the first evaluation was most often after four weeks and secondly at two weeks. The timing of evaluation after the first follow-up concentrated on evaluation within 12 weeks and at 24 weeks.
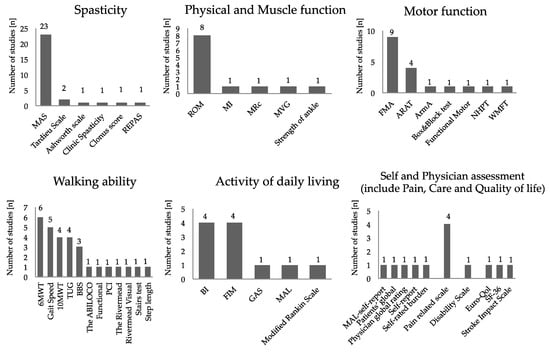
Figure 2.
Overview of outcome measure: The vertical axis represents the number of studies. The horizontal axis represents the evaluation methods that used in each study. Abbreviations: 10MWT, 10 m walk test; 6MWT, six minute walk test; ARAT, Action Research Arm Test; ArmA, the arm activity measure; BBS, Berg Balance Scale; BI, Barthel Index; FIM, Functional Independence Measure; FMA, Fugl–Meyer Assessment; GAS, goal attainment scaling; MAL, the motor activity log; MAS, Modified Ashworth Scale; MI, Motricity Index; MRc, the medical research council scale; MVG, maximum voluntary grip strength; NHPT, Nine Hole Peg Test; PCI, physiological cost index; REPAS, resistance to passive movement Scale; ROM, range of motion; SF-36, MOS 36-Item Short-Form Health Survey; TUG, Timed Up and Go Test; and WMFT, Wolf Motor Function Test.
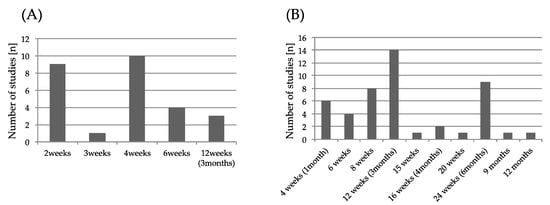
Figure 3.
(A) Initial evaluation timing after botulinum toxin A (BoNT-A) injection. (B) Second and subsequent evaluation timing. The vertical axis represents the number of studies. The horizontal axis represents the evaluation timing after BoNT-A injection.
2.5. Intervention in BoNT-A Therapy
The dose of BoNT-A was the applied dose for each drug. Guided dosing with electromyography (EMG), electrical stimulation (ES), or ultrasonography (US) was performed in 57.6% of the studies. As mentioned above, in two studies, multiple doses were administered in compliance with the dosing interval [30,31,35]. For some studies, although there was mention of botulinum toxin, there was no mention of the dose volume or location or both (30.7%) [19,21,22,23,30,31,32,40,42].
2.6. Rehabilitation
In many studies, the contents of rehabilitation, rehabilitation time, units per day or week, and the total number of sessions were described. The most described training was stretch (42.3%). After that, range of motion (19.2%) and gait training (19.2%) were described frequently. Total sessions varied from study to study and ranged from five to 120 sessions. There were three studies that used ES or functional electrical stimulation (FES) as adjuvant treatment, three studies that used a robot, two studies that did taping and casting, and one study that used constraint-induced movement therapy (CIMT) [36,37,38,39,40,41,42,43,44,45].
2.7. Effect of BoNT-A and Rehabilitation of Motor Function
First, the intervention group had improved spasticity in 23 of 24 studies, except for two studies that did not evaluate spasticity. However, there was no significant difference in improvement in spasticity when compared to the group in which the control group was BoNT-A alone [21,22,36,41]. Moreover, in the studies compared with the group where the control group was placebo injection, three of seven studies were compared among the groups and were considered to have a significant difference [24,27,34]. However, there was also a report showing no significant improvement compared with placebo injection in the comparison between groups [32]. In comparison to triamcinolone acetonide, the MAS did not improve significantly compared to the control group [33]. In regard to adjuvant treatment, in studies where the control group was BoNT-A and regular rehabilitation, there was no significant difference in spasticity among the groups compared [40,45].
Regarding motor function related to the upper extremity, two of the 14 articles that treated the upper extremities indicated that significant improvement in upper extremity function was observed compared to the control group [21,45]. Devier et al. (2017) reported a significant improvement in the FMA compared to the control group (BoNT-A only) after 18–21 and 24–27 weeks [21]. Sun et al. (2010) reported that there was a significant improvement in the ARAT compared to the control group (BoNT-A plus rehabilitation) at three or six months after the intervention [45]. In addition, the motor activity log (MAL) also reported that there was a significant improvement after three or six months. On the other hand, nine of 14 studies showed improvement in upper limb function by the intervention, but there was no significant difference or comparison with the control group [19,20,28,29,30,31,32,37,42]. Wolf et al. (2012) reported that there were no group-by-time interactions for changes in the Wolf Motor Function Test and no treatment difference, although the intervention group could complete more tasks governing proximal joint motions [29]. Meythaler et al. (2009) reported that, compared to the control group (placebo injection), the intervention group showed a slightly enhanced functional status of stroke subjects beyond that obtained with therapy alone at 12 weeks after injection [32]. Suputtitada et al. (2005) indicated a significant decrease in ARAT results at week eight compared with control (placebo injection) in the 1000 U group, but the 500 U group showed improved ARAT results, which peaked at week eight compared to the control group [34]. Pennati et al. (2015) indicated improvement in upper limb function in both groups, but both the FMA and Box and Block test showed better improvement in the control group (rehabilitation only) [42]. In three of 14 studies, there was no significant improvement by intervention [26,27,30,31]. In particular, Demetrios et al. (2014) mentioned that there were no significant differences in change scores for the assessment of upper limbs [25].
Regarding motor function related to the upper extremity, seven of 14 studies that administered BoNT-A to the lower extremity indicated that significant improvement in motor function was observed compared to the control group [22,23,24,36,39,41,43]. Tao et al. (2015) reported that there was a significant difference in step length, cadence, and speed at eight weeks, as well as the FMA and 6MWT compared to the control group (placebo injection) [24]. Fujita et al. (2019) reported that, as compared with the control group (BoNT-A only), walking speed and cadence were significantly increased and the rate of change was significantly greater [36]. In addition, in the intervention group, the step length increased significantly, and an improvement of the asymmetry index of step length was observed. On the other hand, one participant in the intervention group and seven participants in the control group indicated that their walking speed decreased. Johnson et al. (2002, 2004) reported that the physiological cost index (PCI) decreased slightly in the intervention group with improvement in lower extremity function, which was not observed in the control group (rehabilitation only) [38,39]. Though five studies showed improvement in motor function by intervention, there was no significant difference compared with control group or there was no comparison between groups [18,19,26,35,40]. Interestingly, Burbaud et al. (1996) focused not only on changes in lower limb motor function but also on changes in aids. They reported that two patients in the intervention group who previously could not walk independently were able to walk alone with an ordinary walking stick, two patients were able to exchange their tripod stick for an ordinary walking stick, and two other patients no longer needed walking sticks [35]. On the other hand, in two of 14 studies, there was no significant difference between the groups regarding lower limb motor function [25,44].
3. Discussion
This review focused on examining the effectiveness of BoNT-A treatment combined with rehabilitation on motor function in post-stroke patients. Twenty-six studies were reviewed, including 1307 patients. There was a significant effect observed in 23 studies in the intervention group on spasticity [18,19,20,21,22,24,25,26,27,28,29,30,31,32,34,35,36,37,40,41,42,43,44,45]. In regard to upper limb motor function, 11 of 14 cases showed improvement, but two studies showed significant differences in comparison with the control group [19,20,21,25,27,28,29,30,31,32,33,34,37,42,45]. In regard to lower extremity motor function, 12 of 14 cases showed improvement, and seven studies showed a significant difference in comparison to the control group [18,19,22,23,24,25,26,35,36,38,39,40,41,43,44].
In regard to study design, the control group was set to various types of studies, because many studies did not focus on the improvement of motor function. Previous reports have shown that BoNT-A administration is effective and safe against spasticity [9,10]. Therefore, in studies focusing on the improvement of motor function, setting the control group as placebo injection may not be effective.
In regard to evaluation, both upper and lower limbs were widely used for motor function evaluation in rehabilitation. For the upper limbs, Wolf et al. (2012) mentioned the possibility of proximal improvement. Takekawa et al. (2013) reported improvement in upper extremity function by combining home-based functional training with past BoNT-A injection [46]. Among these, categories A and B improved significantly after one, three, and six months in evaluation by the FMA, and category D improved significantly at three and six months. An improvement of motor function by BoNT-A and rehabilitation may start from the proximal side [46]. Therefore, it may be necessary to carefully observe changes in each category, as well as the total score of motor function evaluation of upper limb function, in order to capture the improvement effect for the upper limbs. For the lower limbs, some studies focused on walking speed and walking durability. In fact, our past study reported that we observed not only improved walking speed but also improved balance using the Timed Up and Go Test (TUG) [15]. According to a review of balance assessment after BoNT-A injection by Phadke et al. (2014), the evidence for balance changes after BoNT-A is weak because of a lack of randomization, control group comparison, objective balance assessment measures, and standard clinical scales [47]. Therefore, in regard to balance, a new systematic review is required in the future. Burbaud et al. (1996) reported not only a change in lower limb motor function but also a change in the aid and reported improvement [35]. A systematic review of gait velocity in randomized controlled trials reported a 0.044 m/s increase (an effect size of 0.193) in gait velocity in the treatment groups, although the number of studies reporting such an improvement was small [48]. In our previous study, we found that the home ambulation group (gait speed < 0.4 m/s) demonstrated a significant change in 10 M gait velocity compared to the limited community ambulation (gait speed = 0.4–0.8 m/s) and full community ambulation (gait speed > 0.8 m/s) groups [15]. Conversely, the full community ambulation group showed a ceiling effect on the post-intervention change. Therefore, there is a limit to the evaluation of improvement of the lower limbs in regard to walking speed only. These results suggest that a multifaceted evaluation focusing on an evaluation classified according to walking speed before intervention, a balance index, and a change of aid is necessary.
Due to the pharmacological activity of BoNT-A, the follow-up period was evaluated at one month and three months, which is the most effective time and the time when the effect disappears, respectively. However, some studies reported a temporary loss of function due to the loss of muscle strength associated with the relief of spasticity by BoNT-A [34,36]. Therefore, multiple evaluations are important in order to grasp the timing when temporary functional decline occurs and the timing when functional improvement is most recognized.
In regard to BoNT-A injection, many studies used methods guided by EMG, ES, or US [20,21,22,23,24,28,33,34,35,36,38,39,40,41,42,43,44]. It has been suggested in past studies that the guided injection method is safer and more accurate than administration by anatomical landmarks, which is usually recommended [49]. BoNT-A also has localized and dose-dependent effects on the injected muscle [50,51]. Therefore, it is necessary to describe the total volume, the dosage volume to each muscle, and the dosage method in detail. Regarding rehabilitation and BoNT-A treatment, a detailed description is required. In this review, the frequency and load of rehabilitation varied. Additionally, it cannot be said that frequent rehabilitation interventions always contribute to the improvement of motor function. Only stretch was shown to be effective against spasticity from past reports [52]. There were also reports of adjuvant treatment, but there were few reports focusing on motor function [36,37,38,39,40,41,42,43,44,45]. In the future, it is necessary to report not only on spasticity with adjuvant treatment but also on the improvement of motor function.
In regard to the risk of bias, the quality of reporting was poor. Though RCTs were used in some studies, there was limited mention of randomization methods. Additionally, for the evaluation, there were insufficient blinds. Because BoNT-A was combined with rehabilitation in this review, both blinding studies were required for the recommended studies. However, many studies were aimed mainly at the effect on spasticity, so few studies were both blinded. In addition, blinding may be difficult at the time of the set up of study design. Regarding the analysis of significant differences in results, some studies did not adequately compare with the control group.
From the above findings, the following items are recommended for conducting future research on the effects of motor function by BoNT-A and rehabilitation:
(i) It is known that BoNT-A is effective for spasticity. Therefore, BoNT-A alone or other rehabilitation is desirable for the control group.
(ii) In the evaluation of upper extremity function, it is recommended to focus not only on the total score of the evaluation but also on changes in sub-score.
(iii) In the evaluation of lower limb function, it is recommended to focus not only on the walking speed but also on changes in balance, changes in walking patterns, and changes in aids.
(iv) It is recommended to follow-up more than once in order to catch the temporary functional decline and the timing that the function improved most.
(v) For the injection of BoNT-A, the guided method is recommended. In addition, it is desirable to describe injection the volume and injection muscle.
(vi) It is desirable to describe details, as much as possible, about the rehabilitation (the time of day, the number of times of week, the total number of times, etc.).
4. Conclusions and Future Perspective
The effect of BoNT-A combined with rehabilitation is limited after stroke and there is not sufficient evidence, but it has been suggested that BoNT-A may help improve motor function. In the future, the establishment of an optimal rehabilitation and the optimal evaluation time in BoNT-A treatment will be a breakthrough in improving both motor function and spasticity.
5. Methods
5.1. Criteria for Considering Studies for Review
5.1.1. Type of Studies
Randomized controlled trials (RCT), non-RCT, cross-over studies, and comparative studies were included in this analysis. Protocols, retrospective studies, and case reports were excluded. Abstracts and non-English language publications were also excluded.
5.1.2. Type of Participants
We included patients with upper and lower limb spasticity after stroke. Patients were >18 years old. Publications included >50% of patients with stroke and 3 or more patients in the cohort.
5.1.3. Type of Interventions and Comparisons
We included studies that performed BoNT-A injection in the upper or lower limbs, with rehabilitation performed after injection, and with assessment of motor function. We excluded studies that did not perform rehabilitation or did not evaluate motor function.
5.1.4. Search Strategy
Searches were performed on the following publication databases: Pubmed, Scopus, CINAHL, Embase, PsycINFO, and CENTRAL. Studies published in English and on or before 31 December 2018 were selected. Selected keywords included stroke, cerebral vascular accident, ischemic stroke, hemorrhagic stroke, botulinum toxin, botulinum toxin therapy, antispastic therapy, rehabilitation, physical therapy, occupational therapy, intensive rehabilitation, multidisciplinary rehabilitation, motor, function, ability, walk, and capacity. Variations of keywords were individualized for each scientific database. The references of all retrieved articles were reviewed to ensure that all relevant articles were included for data synthesis. As an example, the Pubmed search strategy is illustrated in Appendix A.
5.2. Date Collection and Analysis
5.2.1. Selection of Studies
Two authors (T.H. and R.M.) independently reviewed all potential studies for inclusion against the eligibility criteria. They examined the title and abstract, and, where necessary, the full text of studies to assess if they were eligible for inclusion. If they could not reach agreement by discussion, a third author (M.N.) made the final decision concerning eligibility.
5.2.2. Date Extraction
Two authors (T.H. and R.M.) independently used a standard form to extract study characteristics and outcome data from the studies. Discrepancies were checked against the original data. A third author (M.N.) made the final decision in the cases of disagreement.
5.2.3. Assessment of Risk of Bias in the Included Studies
We assessed the methodological quality of selected studies as described in the Cochrane Review Groups [53]. We created a risk of bias table and included description and judgment (low risk of bias, high risk of bias, or unclear risk of bias) for the following domains for each of the included studies: (1) random sequence generation, which is selection bias (biased allocation to interventions) due to the inadequate generation of a randomized sequence; (2) allocation concealment, which is selection bias (biased allocation to interventions) due to the inadequate concealment of allocation prior to assignment; (3) the blinding of participants and personnel, which is performance bias due to knowledge of the allocated interventions by participants and personnel during the study; (4) the blinding of outcome assessment, which is detection bias due to knowledge of the allocated interventions by outcome assessors; (5) incomplete outcome data, which is attrition bias due to the amount, nature or handling of incomplete outcome data; (6) selective reporting, which reporting bias due to selective outcome reporting; and (7) other sources of bias, which are considered bias due to problems not covered elsewhere in the table. Two review authors independently performed quality assessment. Any disagreement between authors arising at any stage was resolved through discussion or through a third author.
Because many trials concerning the effect of BoNT-A for stroke patients have focused on the effect of spasticity and few studies have focused on motor function, in this review, a meta-analysis was not performed. This study was prospectively registered with the PROSPERO database of systematic reviews (CRD42019132145).
Author Contributions
T.H., R.M., M.N., N.Y., H.H. and M.A. conceived and designed this study; T.H., R.M., M.N. extracted and evaluated the data; T.H., R.M., M.N., N.Y., H.H. and M.A. checked the results; T.H. wrote the paper.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
((“stroke”[MeSH Terms] OR “stroke”[All Fields]) OR (“stroke”[MeSH Terms] OR “stroke”[All Fields] OR (“cerebral”[All Fields] AND “vascular”[All Fields] AND “accident”[All Fields]) OR “cerebral vascular accident”[All Fields]) OR ((“ischemia”[MeSH Terms] OR “ischemia”[All Fields] OR “ischemic”[All Fields]) AND (“stroke”[MeSH Terms] OR “stroke”[All Fields])) OR (“intracranial hemorrhages”[MeSH Terms] OR (“intracranial”[All Fields] AND “hemorrhages”[All Fields]) OR “intracranial hemorrhages”[All Fields] OR (“hemorrhagic”[All Fields] AND “stroke”[All Fields]) OR “hemorrhagic stroke”[All Fields])) AND ((“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) OR ((“botulinum toxins”[MeSH Terms] OR (“botulinum”[All Fields] AND “toxins”[All Fields]) OR “botulinum toxins”[All Fields] OR (“botulinum”[All Fields] AND “toxin”[All Fields]) OR “botulinum toxin”[All Fields]) AND (“therapy”[Subheading] OR “therapy”[All Fields] OR “therapeutics”[MeSH Terms] OR “therapeutics”[All Fields])) OR (Antispastic[All Fields] AND (“therapy”[Subheading] OR “therapy”[All Fields] OR “therapeutics”[MeSH Terms] OR “therapeutics”[All Fields]))) AND ((“rehabilitation”[Subheading] OR “rehabilitation”[All Fields] OR “rehabilitation”[MeSH Terms]) OR (“physical therapy modalities”[MeSH Terms] OR (“physical”[All Fields] AND “therapy”[All Fields] AND “modalities”[All Fields]) OR “physical therapy modalities”[All Fields] OR (“physical”[All Fields] AND “therapy”[All Fields]) OR “physical therapy”[All Fields]) OR (“occupational therapy”[MeSH Terms] OR (“occupational”[All Fields] AND “therapy”[All Fields]) OR “occupational therapy”[All Fields]) OR (Intensive[All Fields] AND (“rehabilitation”[Subheading] OR “rehabilitation”[All Fields] OR “rehabilitation”[MeSH Terms])) OR ((“interdisciplinary studies”[MeSH Terms] OR (“interdisciplinary”[All Fields] AND “studies”[All Fields]) OR “interdisciplinary studies”[All Fields] OR “multidisciplinary”[All Fields]) AND (“rehabilitation”[Subheading] OR “rehabilitation”[All Fields] OR “rehabilitation”[MeSH Terms]))) AND ((“physiology”[Subheading] OR “physiology”[All Fields] OR “function”[All Fields] OR “physiology”[MeSH Terms] OR “function”[All Fields]) OR (“aptitude”[MeSH Terms] OR “aptitude”[All Fields] OR “ability”[All Fields]) OR (“walking”[MeSH Terms] OR “walking”[All Fields] OR “walk”[All Fields]) OR “capacity”[All Fields]).
References
- Mayer, N.H. Clinicophysiologic concepts of spasticity and motor dysfunction in adults with an upper motoneuron lesion. Muscle Nerve Suppl. 1997, 6, S1–S13. [Google Scholar] [CrossRef]
- Sommerfeld, D.K.; Eek, E.U.; Svensson, A.K.; Holmqvist, L.W.; von Arbin, M.H. Spasticity after stroke: Its occurrence and association with motor impairments and activity limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Leathley, M.J.; Gregson, J.M.; Moore, A.P.; Smith, T.L.; Sharma, A.K. Prevalence of spasticity post stroke. Clin. Rehabil. 2002, 16, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Schinwelsk, M.; Sławek, J. Prevalence of spasticity following stroke and its impact on quality of life with emphasis on disability in activities of daily living. Systematic review. Neurol. Neurochir. 2010, 44, 404–411. [Google Scholar] [CrossRef]
- Kimura, A.; Abo, M.; Kawate, N.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M. Efficacy and Safety of Botulinum Toxin Type A in treating Lower Limb Spasticity in Post-stroke Patients: A Multicenter, Double-blind, Placebo-controlled Trial followed by an Open-label Trial. Jpn. J. Rehabil. Med. 2010, 47, 626–636. [Google Scholar] [CrossRef]
- Tanikawa, H.; Kagaya, H.; Saitoh, E.; Ozaki, K.; Hirano, S.; Itoh, N.; Yamada, J.; Kanada, Y. Efficacy of Botulinum Toxin A Treatment for Pes Varus during Gait. J. Stroke Cerebrovasc. Dis. 2015, 24, 2416–2422. [Google Scholar] [CrossRef]
- Brin, M.F. Botulinum toxin: Chemistry, pharmacology, toxicity and immunology. Muscle Nerve Suppl. 1997, 6, 146–168. [Google Scholar] [CrossRef]
- Boyd, R.N.; Pliatsios, V.; Starr, R.; Wolfe, R.; Graham, H.K. Biomechanical transformation of the gastroc-soleus muscle with botulinum toxin A in children with cerebral palsy. Dev. Med. Child Neurol. 2000, 42, 32–41. [Google Scholar] [CrossRef]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum Neurotoxin for Post-Stroke Spasticity in Adults: A Systematic Review. Mov. Disord. Soc. 2009, 24, 801–812. [Google Scholar] [CrossRef]
- Rosales, R.L.; Chua-Yap, A.S. Evidence-based systematic review on the efficacy and safety of botulinum toxin-A therapy in post-stroke spasticity. J. Neural Transm. 2008, 115, 617–623. [Google Scholar] [CrossRef]
- Naumann, M.; Albanese, A.; Heinen, F.; Molenaers, G.; Relja, M. Safety and efficacy of botulinum toxin type A following long-term use. Eur. J. Neurol. 2006, 13, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.F.; Brashear, A.; Elovic, E.; Kassicieh, D.; Marciniak, C.; Liu, J.; Turkel, C. Repeated dosing of botulinum toxin type A for upper limb spasticity following stroke. Neurology 2004, 63, 1971–1973. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Gracies, J.M.; Graham, H.K.; Miyasaki, J.M.; Naumann, M.; Russman, B.; Simpson, L.L.; So, Y. Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review) Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 1691–1698. [Google Scholar] [PubMed]
- Bakheit, A.M.; Zakine, B.; Maisonobe, P.; Aymard, C.; Fhedoroff, K.; Hefter, H.; Jacinto, J.; Jost, W.H.; Molteni, F.; Stam, H.; et al. The profile of patients and current practice of treatment of upper limb muscle spasticity with botulinum toxin type A: An international survey. Int. J. Rehabil. Res. 2010, 33, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Abo, M.; Hara, H.; Kobayashi, K.; Shimamoto, Y.; Samizo, Y.; Sasaki, N.; Yamada, N.; Niimi, M. Effects of botulinum toxin A therapy and multidisciplinary rehabilitation on upper and lower limb spasticity in post-stroke patients. Int. J. Neurosci. 2017, 127, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Abo, M.; Hara, H.; Kobayashi, K.; Shimamoto, Y.; Shibata, Y.; Sasaki, N.; Yamada, N.; Niimi, M. Effects of botulinum toxin A therapy and multidisciplinary rehabilitation on lower limb spasticity classified by spastic muscle echo intensity in post-stroke patients. Int. J. Neurosci. 2018, 128, 412–420. [Google Scholar] [CrossRef]
- Hara, T.; Abo, M.; Hara, H.; Sasaki, N.; Yamada, N.; Niimi, M.; Shimamoto, Y. The Effect of Repeated Botulinum Toxin A Therapy Combined with Intensive Rehabilitation on Lower Limb Spasticity in Post-Stroke Patients. Toxins (Basel) 2018, 31, 10. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Koyama, T.; Wada, Y.; Katsutani, M.; Kodama, N.; Domen, K. Botulinum Toxin Type A Treatment Combined with Intensive Rehabilitation for Gait Poststroke: A Preliminary. Study. J. Stroke Cerebrovasc. Dis. 2018, 27, 1975–1986. [Google Scholar] [CrossRef]
- Prazeres, A.; Lira, M.; Aguiar, P.; Monteiro, L.; Vilasbôas, Í.; Melo, A. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurol. Int. 2018, 10, 7385. [Google Scholar] [CrossRef]
- Umar, M.; Masood, T.; Badshah, M. Effect of botulinum toxin A&task-specific training on upper limb function in post-stroke focaldystonia. J. Pak. Med. Assoc. 2018, 68, 526–531. [Google Scholar]
- Devier, D.; Harnar, J.; Lopez, L.; Brashesr, A.; Graham, G. Rehabilitation plus OnabotulinumtoxinA Improves Motor Function over OnabotulinumtoxinA Alone in Post-Stroke Upper Limb Spasticity: A Single-Blind, Randomized Trial. Toxins (Basel) 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Zory, R.; Sauthier, A.; Bonnyaud, C.; Pradon, D.; Bensmail, D. Effect of rehabilitation and botulinum toxin injection on gait in chronic stroke patients: A randomized controlled study. J. Rehabil. Med. 2015, 47, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.D.; Zhang, G.B.; Chen, H.X.; Wang, W.; Song, J.H.; Fu, D.G. Color Doppler ultrasound-guided botulinum toxin type A injection combined with an ankle footbrace for treating lower limb spasticity after a stroke. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 406–411. [Google Scholar] [PubMed]
- Tao, W.; Yan, D.; Li, J.H.; Shi, Z.H. Gait improvement by low-dose botulinum toxin A injection treatment of the lower limbs in subacute stroke patients. J. Phys. Ther. Sci. 2015, 27, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Demetrios, M.; Gorelik, A.; Louie, J.; Brand, C.; Baguley, I.J.; Khan, F. Outcomes of ambulatory rehabilitation programmes following botulinum toxin for spasticity in adults with stroke. J. Rehabil. Med. 2014, 46, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, L.H.; Alencar, F.J.; Rodrigues, L.R.; Sousa, F.C.; Teles, J.B. Effects of botulinum toxin type A for spastic foot in post-stroke patients enrolled in a rehabilitationprogram. Arq. Neuropsiquiatr. 2014, 72, 28–32. [Google Scholar] [CrossRef]
- Rosales, R.L.; Kong, K.H.; Goh, K.J.; Kumthornthip, W.; Mok, V.C.; Delgado-De Los Santos, M.M.; Chua, K.S.; Abdullah, S.J.; Zakine, B.; Maisonobe, P.; et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: A randomized controlled trial. Neurorehabil. Neural Repair 2012, 26, 812–821. [Google Scholar] [CrossRef]
- Hesse, S.; Mach, H.; Fröhlich, S.; Behrend, S.; Werner, C.; Melzer, I. An early botulinum toxin A treatment in subacute stroke patients may prevent a disabling fingerflexor stiffness six months later: A randomized controlled trial. Clin. Rehabil. 2012, 26, 237–245. [Google Scholar] [CrossRef]
- Wolf, S.L.; Milton, S.B.; Reiss, A.; Easley, K.A.; Shenvi, N.V.; Clark, P.C. Further assessment to determine the additive effect of botulinum toxin type A on an upperextremity exercise program to enhance function among individuals with chronic stroke but extensor capability. Arch. Phys. Med. Rehabil. 2012, 93, 578–587. [Google Scholar] [CrossRef]
- Shaw, L.C.; Price, C.I.; van Wijck, F.M.; Shackley, P.; Steen, N.; Barnes, M.P.; Ford, G.A.; Graham, L.A.; Rodgers, H. BoTULS Investigators. Botulinum Toxin for the Upper Limb after Stroke (BoTULS) Trial: Effect on impairment, activitylimitation, and pain. Stroke 2011, 42, 1371–1379. [Google Scholar] [CrossRef]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L. BoTULS investigators. BoTULS: A multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol. Assess. 2010, 14, 1–113. [Google Scholar] [CrossRef] [PubMed]
- Meythaler, J.M.; Vogtle, L.; Brunner, R.C. A preliminary assessment of the benefits of the addition of botulinum toxin a to a conventionaltherapy program on the function of people with longstanding stroke. Arch. Phys. Med. Rehabil. 2009, 90, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Koh, J.H.; Paik, N.J. Intramuscular botulinum toxin-A reduces hemiplegic shoulder pain: A randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke 2008, 39, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Suputtitada, A.; Suwanwela, N.C. The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil. Rehabil. 2005, 27, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Burbaud, P.; Wiart, L.; Dubos, J.L.; Gaujard, E.; Debelleix, X.; Joseph, P.A.; Mazaux, J.M.; Bioulac, B.; Barat, M.; Lagueny, A. A randomised, double blind, placebo controlled trial of botulinum toxin in the treatment of spasticfoot in hemiparetic patients. J. Neurol. Neurosurg. Psychiatry 1996, 61, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Miaki, H.; Hori, H.; Kobayashi, Y.; Nakagawa, T. How effective is physicaltherapy for gait muscle activity in hemiparetic patients who receive botulinum toxin injections? Eur. J. Phys. Rehabil. Med. 2019, 55, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Skidmore, E.R.; Niyonkuru, C.; Chang, C.L.; Huber, L.M.; Munin, M.C. Cyclic functional electrical stimulation does not enhance gains in hand grasp function when used as an adjunct to onabotulinumtoxinA and task practice therapy: A single-blind, randomized controlled pilot study. Arch. Phys. Med. Rehabil. 2010, 91, 679–686. [Google Scholar] [CrossRef]
- Johnson, C.A.; Wood, D.E.; Swain, I.D.; Tromans, A.M.; Strike, P.; Burridge, J.H. A pilot study to investigate the combined use of botulinum neurotoxin type a and functionalelectrical stimulation, with physiotherapy, in the treatment of spastic dropped foot in subacutestroke. Artif. Organs 2002, 26, 263–266. [Google Scholar] [CrossRef]
- Johnson, C.A.; Burridge, J.H.; Strike, P.W.; Wood, D.E.; Swain, I.D. The effect of combined use of botulinum toxin type A and functional electric stimulation in the treatment of spastic drop foot after stroke: A preliminary investigation. Arch. Phys. Med. Rehabil. 2004, 85, 902–909. [Google Scholar] [CrossRef]
- Erbil, D.; Tugba, G.; Murat, T.H.; Melike, A.; Merve, A.; Cagla, K.; Mehmetali, Ç.C.; Akay, Ö.; Nigar, D. Effects of robot-assisted gait training in chronic stroke patients treated by botulinum toxin-a: A pivotal study. Physiother. Res. Int. 2018, 23, e1718. [Google Scholar] [CrossRef]
- Picelli, A.; Bacciga, M.; Melotti, C.L.A.; Marchina, E.; Verzini, E.; Ferrari, F.; Pontillo, A.; Corradi, J.; Tamburin, S.; Saltuari, L.; et al. Combined effects of robot-assisted gait training and botulinum toxin type A on spastic equinus foot in patients with chronic stroke: A pilot, single blind, randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 759–766. [Google Scholar] [PubMed]
- Pennati, G.V.; Da Re, C.; Messineo, I.; Bonaiuti, D. How could robotic training and botolinum toxin be combined in chronic post stroke upper limbspasticity? A pilot study. Eur. J. Phys. Rehabil. Med. 2015, 51, 381–387. [Google Scholar] [PubMed]
- Carda, S.; Invernizzi, M.; Baricich, A.; Cisari, C. Casting, taping or stretching after botulinum toxin type A for spastic equinus foot: A single-blindrandomized trial on adult stroke patients. Clin. Rehabil. 2011, 25, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Karadag-Saygi, E.; Cubukcu-Aydoseli, K.; Kablan, N.; Ofluoglu, D. The role of kinesiotaping combined with botulinum toxin to reduce plantar flexors spasticity afterstroke. Top. Stroke Rehabil. 2010, 17, 318–322. [Google Scholar] [CrossRef]
- Sun, S.F.; Hsu, C.W.; Sun, H.P.; Hwang, C.W.; Yang, C.L.; Wang, J.L. Combined botulinum toxin type A with modified constraint-induced movement therapy for chronicstroke patients with upper extremity spasticity: A randomized controlled study. Neurorehabil. Neural Repair 2010, 24, 34–41. [Google Scholar] [CrossRef]
- Takekawa, T.; Abo, M.; Ebihara, K.; Taguchi, K.; Sase, Y.; Kakuda, W. Long-term effects of injection of botulinum toxin type A combined with home-based functional training for post-stroke patients with spastic upper limb hemiparesis. Acta Neurol. Belg. 2013, 113, 469–475. [Google Scholar] [CrossRef]
- Phadke, C.P.; Ismail, F.; Boulias, C.; Gage, W.; Mochizuki, G. The impact of post-stroke spasticity and botulinum toxin on standing balance: A systematic review. Expert Rev. Neurother. 2014, 14, 319–327. [Google Scholar] [CrossRef]
- Foley, N.; Murie-Fernandez, M.; Speechley, M.; Salter, K.; Sequeira, K.; Teasell, R. Does the treatment of spastic equinovarus deformity following stroke with botulinum toxin increase gait velocity? A systematic review and meta-analysis. Eur. J. Neurol. 2010, 17, 1419–1427. [Google Scholar] [CrossRef]
- Picelli, A.; Lobba, D.; Midiri, A.; Prandi, P.; Melotti, C.; Baldessarelli, S.; Smania, N. Botulinum toxin injection into the forearm muscles for wrist and fingers spastic overactivity in adults with chronic stroke: A randomized controlled trial comparing three injection techniques. Clin. Rehabil. 2014, 28, 232–242. [Google Scholar] [CrossRef]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M. GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke lower limb spasticity: A multicenter, double-blind, placebo-controlled trial. J. Neurol. 2010, 257, 1330–1337. [Google Scholar] [CrossRef]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M. GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke upper limb spasticity. Curr. Med. Res. Opin. 2010, 26, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, B.Z.; Lannin, N.A.; Cusick, A.; Harvey, L.A.; Rawicki, B. Rehabilitation therapies after botulinum toxin-A injection to manage limb spasticity: A systematic review. Phys. Ther. 2014, 94, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Lundh, A.; Gøtzsche, P.C. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med. Res. Methodol. 2008, 8, 22. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).