An Electrochemical Fiveplex Biochip Assay Based on Anti-Idiotypic Antibodies for Fast On-Site Detection of Bioterrorism Relevant Low Molecular Weight Toxins
Abstract
1. Introduction
2. Results
2.1. Fiveplex Biochip Assay Design
2.2. Analytical Characteristics of the Fiveplex Biochip Assay
2.2.1. Assay Specificity
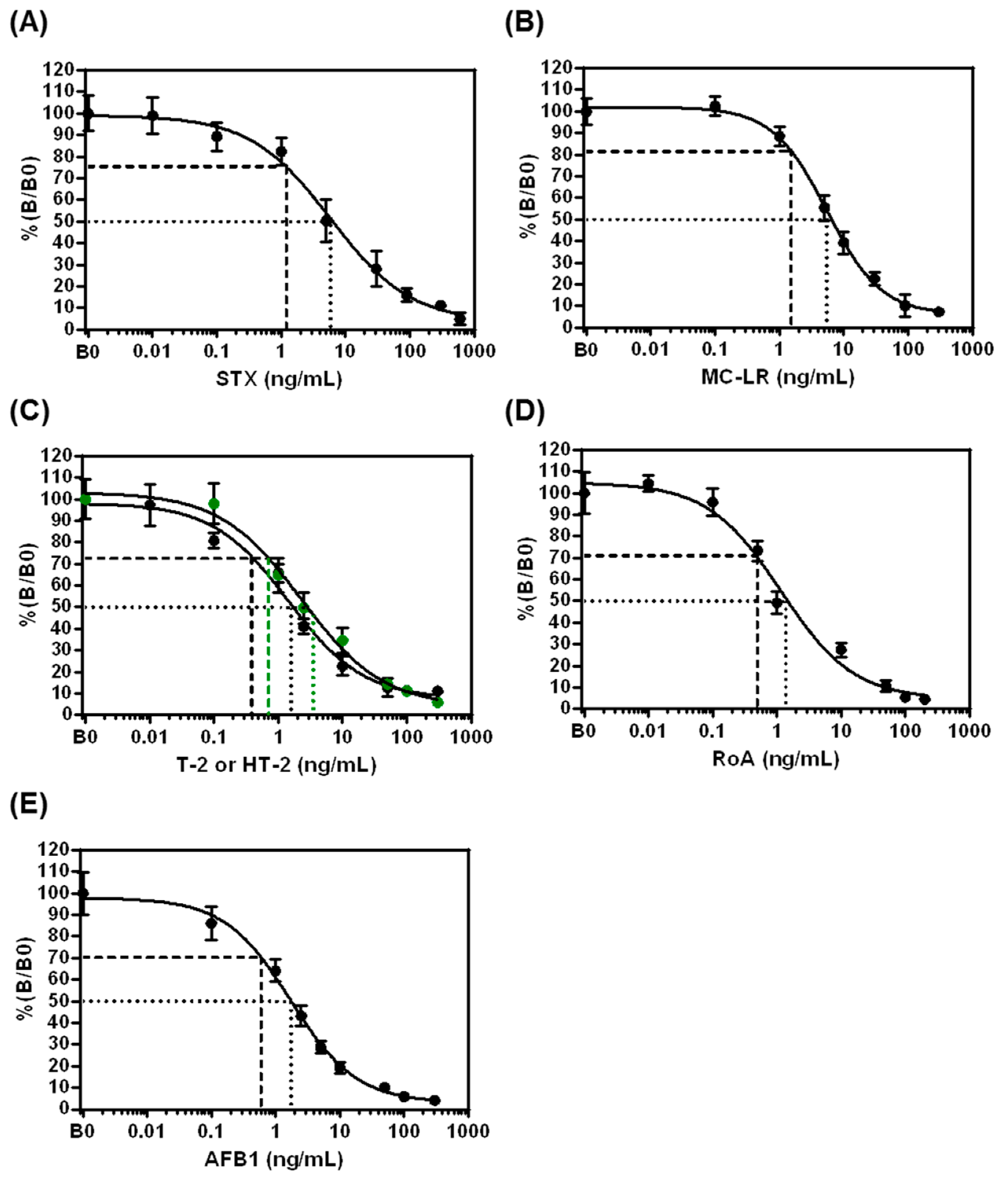
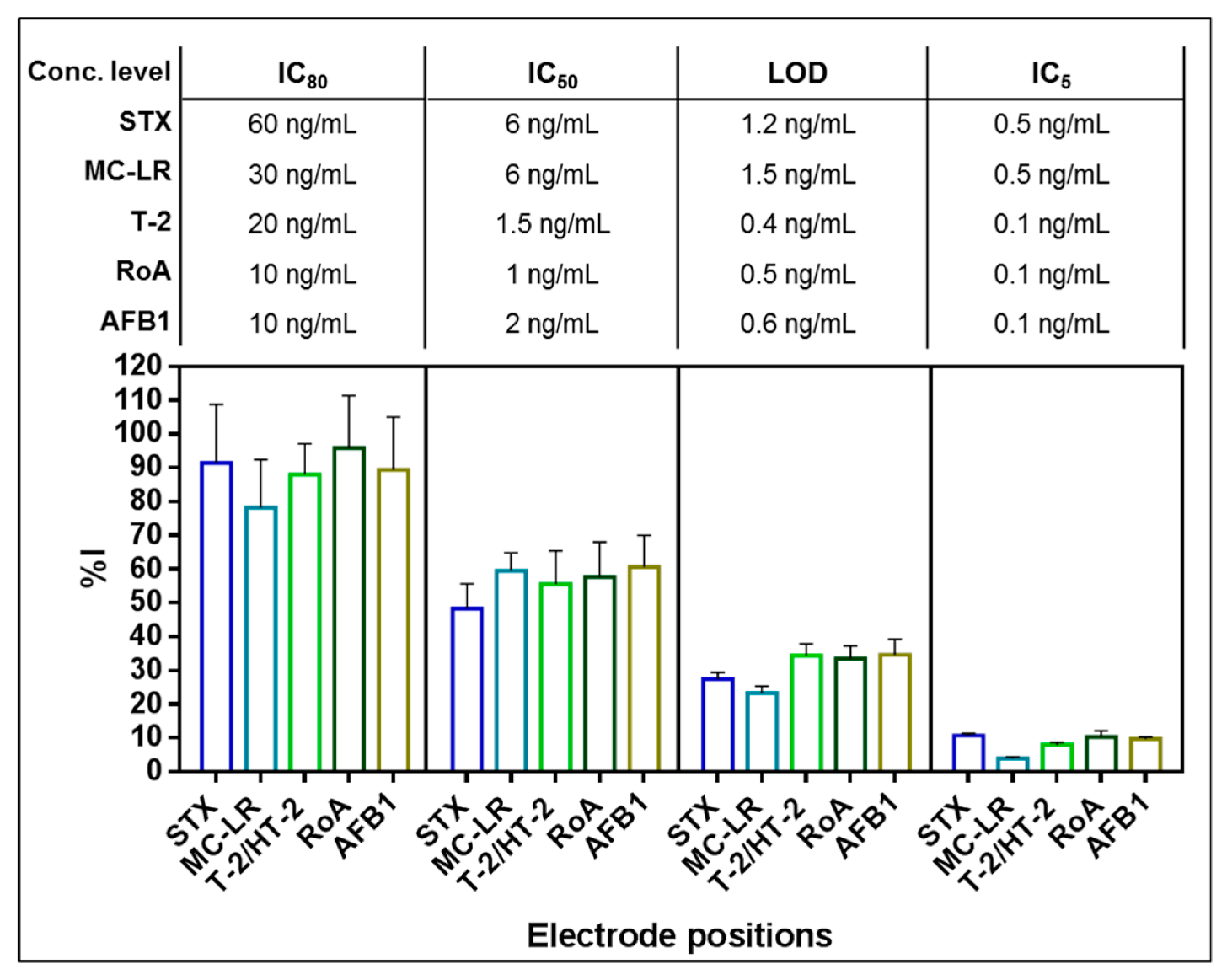
2.2.2. Assay Performance
2.3. Fiveplex Detection of STX, MC-LR, T-2/HT-2, RoA and AFB1 in Buffer and Human Blood Serum
2.3.1. Simultaneous Detection of STX, MC-LR, T-2, RoA and AFB1 in Buffer
2.3.2. Simultaneous Detection of STX, MC-LR, HT-2, RoA and AFB1 in Serum Samples
3. Discussion
- A high level multiplexing biochip to detect multiple potential bioterrorism relevant toxins in a single run.
- An on-site applicability combined with a short assay time (13.4 min).
- A multi-congener detection system by employing toxin group specific mAbs.
- A completely user-safe assay due to waiving of harmful toxin-protein conjugates.
- A straightforward assay workflow through reporter enzyme-conjugated detection mAbs.
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Methods
5.2.1. Antibody Production
5.2.2. Immobilization of Capture Antibodies on Gold Electrodes of Biochips
5.2.3. Conjugation of Detection Antibodies to β-D-Galactosidase
5.2.4. Instrumentation and Electrochemical Biochip Measurements
5.2.5. Assay Specificity
5.2.6. Assay Sensitivity
5.2.7. Simultaneous Toxin Detection
5.2.8. Analysis of Toxins Spiked in Human Blood Serum
5.2.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vilariño, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human Poisoning from Marine Toxins: Unknowns for Optimal Consumer Protection. Toxins 2018, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Lehane, L. Paralytic shellfish poisoning: A potential public health problem. Med. J. Aust. 2001, 175, 29–31. [Google Scholar] [CrossRef]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef]
- Spoof, L.; Catherine, A. Appendix 3: Tables of Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 526–537. [Google Scholar]
- Jarvis, B.B. Stachybotrys chartarum: A fungus for our time. Phytochemistry 2003, 64, 53–60. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Pitschmann, V.; Hon, Z. Military Importance of Natural Toxins and Their Analogs. Molecules 2016, 21, 556. [Google Scholar] [CrossRef] [PubMed]
- Australia Group Common Control List Handbook Volume II: Biological Weapoons-Related Common Control Lists. Available online: https://australiagroup.net/en/documents/Australia-Group-Common-Control-List-Handbook-Volume-II.pdf (accessed on 30 August 2019).
- Ukwuru, M.U.; Ohaegbu, C.G.M.A. An Overview of Mycotoxin Contamination of Foods and Feeds. J. Biochem. Microb. Toxicol. 2018, 1, 1–11. [Google Scholar]
- Gessner, B.D.; Middaugh, J.P.; Doucette, G.J. Paralytic shellfish poisoning in Kodiak, Alaska. West. J. Med. 1997, 167, 351–353. [Google Scholar]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Brasel, T.L.; Campbell, A.W.; Demers, R.E.; Ferguson, B.S.; Fink, J.; Vojdani, A.; Wilson, S.C.; Straus, D.C. Detection of Trichothecene Mycotoxins in Sera from Individuals Exposed to Stachybotrys chartarum in Indoor Environments. Arch. Environ. Health 2003, 58, 317–323. [Google Scholar] [CrossRef] [PubMed]
- De Santis, B.; Debegnach, F.; Sonego, E.; Mazzilli, G.; Buiarelli, F.; Ferri, F.; Giorgi Rossi, P.; Collini, G.; Brera, C. Biomonitoring Data for Assessing Aflatoxins and Ochratoxin A Exposure by Italian Feedstuffs Workers. Toxins 2019, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Marin, S.; Ramos, A.J.; Sanchis, V. Biomonitoring of Fusarium spp. Mycotoxins: Perspectives for an Individual Exposure Assessment Tool. Food Sci. Technol. Int. 2010, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Maizels, M.; Budde, W.L. A LC/MS Method for the Determination of Cyanobacteria Toxins in Water. Anal. Chem. 2004, 76, 1342–1351. [Google Scholar] [CrossRef]
- Shriver-Lake, L.; Liu, J.; Brozozog Lee, P.; Goldman, E.; Dietrich, R.; Märtlbauer, E.; Anderson, G. Integrating scFv into xMAP Assays for the Detection of Marine Toxins. Toxins 2016, 8, 346. [Google Scholar] [CrossRef]
- Li, C.; Wen, K.; Mi, T.; Zhang, X.; Zhang, H.; Zhang, S.; Shen, J.; Wang, Z. A universal multi-wavelength fluorescence polarization immunoassay for multiplexed detection of mycotoxins in maize. Biosens. Bioelectron. 2016, 79, 258–265. [Google Scholar] [CrossRef]
- Dill, K.; Ghindilis, A. Electrochemical Detection on Microarrays. In Microarrays: Preparation, Microfluidics, Detection Methods, and Biological Applications; Dill, K., Liu, R.H., Grodzinski, P., Eds.; Springer: New York, NY, USA, 2009; pp. 25–34. [Google Scholar]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Hintsche, R.; Albers, J.; Bernt, H.; Eder, A. Multiplexing of Microelectrode Arrays in Voltammetric Measurements. Electroanalysis 2000, 12, 660–665. [Google Scholar] [CrossRef]
- Farré, M.; Kantiani, L.; Pérez, S.; Barceló, D.; Barceló, D. Sensors and biosensors in support of EU Directives. Trends Anal. Chem. 2009, 28, 170–185. [Google Scholar] [CrossRef]
- Cui, X.; Jin, M.; Du, P.; Chen, G.; Zhang, C.; Zhang, Y.; Shao, Y.; Wang, J. Development of immunoassays for multi-residue detection of small molecule compounds. Food Agric. Immunol. 2018, 29, 638–652. [Google Scholar] [CrossRef]
- Xiao, H.; Clarke, J.R.; Marquardt, R.R.; Frohlich, A.A. Improved Methods for Conjugating Selected Mycotoxins to Carrier Proteins and Dextran for Immunoassays. J. Agric. Food Chem. 1995, 43, 2092–2097. [Google Scholar] [CrossRef]
- Zejli, H.; Goud, K.Y.; Marty, J.L. An electrochemical aptasensor based on polythiophene-3-carboxylic acid assisted methylene blue for aflatoxin B1 detection. Sens. Biosensing Res. 2019, 25, 100290. [Google Scholar] [CrossRef]
- Sergeyeva, T.; Yarynka, D.; Piletska, E.; Lynnik, R.; Zaporozhets, O.; Brovko, O.; Piletsky, S.; El’skaya, A. Fluorescent sensor systems based on nanostructured polymeric membranes for selective recognition of Aflatoxin B1. Talanta 2017, 175, 101–107. [Google Scholar] [CrossRef]
- Li, H.; Wei, X.; Gu, C.; Su, K.; Wan, H.; Hu, N.; Wang, P. A Dual Functional Cardiomyocyte-based Hybrid-biosensor for the Detection of Diarrhetic Shellfish Poisoning and Paralytic Shellfish Poisoning Toxins. Anal. Sci. 2018, 34, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Benito-Pena, E.; Barderas, R.; Sauer, U.; González Andrade, M.; Moreno-Bondi, M.C. Microarray-Based Immunoassay with Synthetic Mimotopes for the Detection of Fumonisin B1. Anal. Chem. 2017, 89, 6216–6223. [Google Scholar] [CrossRef]
- Hou, S.; Ma, Z.; Meng, H.; Xu, Y.; He, Q. Ultrasensitive and green electrochemical immunosensor for mycotoxin ochratoxin A based on phage displayed mimotope peptide. Talanta 2019, 194, 919–924. [Google Scholar] [CrossRef]
- He, J.; Fan, M.; Liang, Y.; Liu, X. Application of Anti-idiotype Antibody in Small Molecules Immunoassay. Chin. J. Anal. Chem. 2010, 38, 1366–1370. [Google Scholar] [CrossRef]
- Jerne, N.K. Towards a network theory of the immune system. Ann. Immunol. (Paris) 1974, 125c, 373–389. [Google Scholar] [PubMed]
- Shu, M.; Xu, Y.; Wang, D.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Qiu, Y.; Ji, Y.; Wang, X. Anti-idiotypic nanobody: A strategy for development of sensitive and green immunoassay for Fumonisin B1. Talanta 2015, 143, 388–393. [Google Scholar] [CrossRef]
- Shu, M.; Xu, Y.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Fu, J.; Gee, S.J.; Hammock, B.D. Anti-idiotypic nanobody-alkaline phosphatase fusion proteins: Development of a one-step competitive enzyme immunoassay for fumonisin B1 detection in cereal. Anal. Chim. Acta 2016, 924, 53–59. [Google Scholar] [CrossRef]
- Guan, D.; Li, P.; Cui, Y.; Zhang, Q.; Zhang, W. A competitive immunoassay with a surrogate calibrator curve for aflatoxin M1 in milk. Anal. Chim. Acta 2011, 703, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Pöhlmann, C.; Dietrich, R.; Märtlbauer, E.; Elßner, T. Electrochemical Biochip Assays Based on Anti-idiotypic Antibodies for Rapid and Automated On-Site Detection of Low Molecular Weight Toxins. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Pöhlmann, C.; Elßner, T. Field-Based Multiplex Detection of Biothreat Agents. In Proceedings of the Scientific International Conference on CBRNe (SICC), Rome, Italy, 22–24 May 2017; pp. 31–39. [Google Scholar]
- Nebling, E.; Grunwald, T.; Albers, J.; Schäfer, P.; Hintsche, R. Electrical Detection of Viral DNA Using Ultramicroelectrode Arrays. Anal. Chem. 2004, 76, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Märtlbauer, E.; Gareis, M.; Terplan, G. Enzyme immunoassay for the macrocyclic trichothecene roridin A: Production, properties, and use of rabbit antibodies. Appl. Environ. Microbiol. 1988, 54, 225–230. [Google Scholar]
- Hack, R.; Märtlbauer, E.; Terplan, G. Production and characterization of monoclonal antibodies to the macrocyclic trichothecene roridin A. Appl. Environ. Microbiol. 1988, 54, 2328–2330. [Google Scholar]
- DeGrasse, S.; Rivera, V.; Roach, J.; White, K.; Callahan, J.; Couture, D.; Simone, K.; Peredy, T.; Poli, M. Paralytic shellfish toxins in clinical matrices: Extension of AOAC official method 2005.06 to human urine and serum and application to a 2007 case study in Maine. Deep Sea Res. Part Ii Top. Stud. Oceanogr. 2014, 103, 368–375. [Google Scholar] [CrossRef]
- Kamala, A.; Shirima, C.; Jani, B.; Bakari, M.; Sillo, H.; Rusibamayila, N.; Saeger, S.D.; Kimanya, M.; Gong, Y.Y.; Simba, A.; et al. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 2018, 11, 311–320. [Google Scholar] [CrossRef]
- Peltomaa, R.; Agudo-Maestro, I.; Más, V.; Barderas, R.; Benito-Peña, E.; Moreno-Bondi, M.C. Development and comparison of mimotope-based immunoassays for the analysis of fumonisin B1. Anal. Bioanal. Chem. 2019, 1–11. [Google Scholar] [CrossRef]
- Zhao, F.; Tian, Y.; Shen, Q.; Liu, R.; Shi, R.; Wang, H.; Yang, Z. A novel nanobody and mimotope based immunoassay for rapid analysis of aflatoxin B1. Talanta 2019, 195, 55–61. [Google Scholar] [CrossRef]
- Soares, R.R.G.; Santos, D.R.; Pinto, I.F.; Azevedo, A.M.; Aires-Barros, M.R.; Chu, V.; Conde, J.P. Multiplexed microfluidic fluorescence immunoassay with photodiode array signal acquisition for sub-minute and point-of-need detection of mycotoxins. Lab Chip 2018, 18, 1569–1580. [Google Scholar] [CrossRef]
- Jodra, A.; López, M.Á.; Escarpa, A. Disposable and reliable electrochemical magnetoimmunosensor for Fumonisins simplified determination in maize-based foodstuffs. Biosens. Bioelectron. 2015, 64, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Bickman, S.R.; Campbell, K.; Elliott, C.; Murphy, C.; O’Kennedy, R.; Papst, P.; Lochhead, M.J. An Innovative Portable Biosensor System for the Rapid Detection of Freshwater Cyanobacterial Algal Bloom Toxins. Environ. Sci. Technol. 2018, 52, 11691–11698. [Google Scholar] [CrossRef] [PubMed]
- Maguire, I.; Fitzgerald, J.; Heery, B.; Nwankire, C.; O’Kennedy, R.; Ducree, J.; Regan, F. Novel Microfluidic Analytical Sensing Platform for the Simultaneous Detection of Three Algal Toxins in Water. Acs Omega 2018, 3, 6624–6634. [Google Scholar] [CrossRef] [PubMed]
- Gayk, L. Entwicklung und Anwendung auf Antiidiotypischen Antikörpern Basierender Verfahren zum Nachweis von Aflatoxinen und T-2 Toxin. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2019. [Google Scholar]
- Szkola, A.; Moura Linares, E.; Worbs, S.; Dorner, B.G.; Dietrich, R.; Märtlbauer, E.; Niessner, R.; Seidel, M. Rapid and simultaneous detection of ricin, Staphylococcal enterotoxin B and saxitoxin by chemiluminescence-based microarray immunoassay. Analyst 2014, 139, 5885–5892. [Google Scholar] [CrossRef] [PubMed]
- Elsholz, B.; Wörl, R.; Blohm, L.; Albers, J.; Feucht, H.; Grunwald, T.; Jürgen, B.; Schweder, T.; Hintsche, R. Automated Detection and Quantitation of Bacterial RNA by Using Electrical Microarrays. Anal. Chem. 2006, 78, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
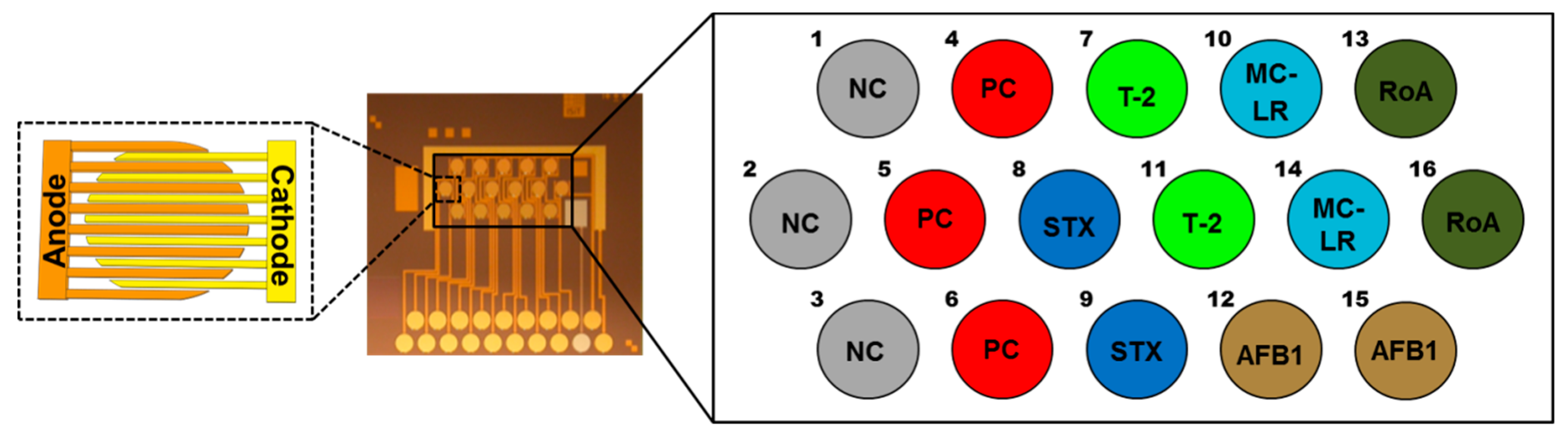
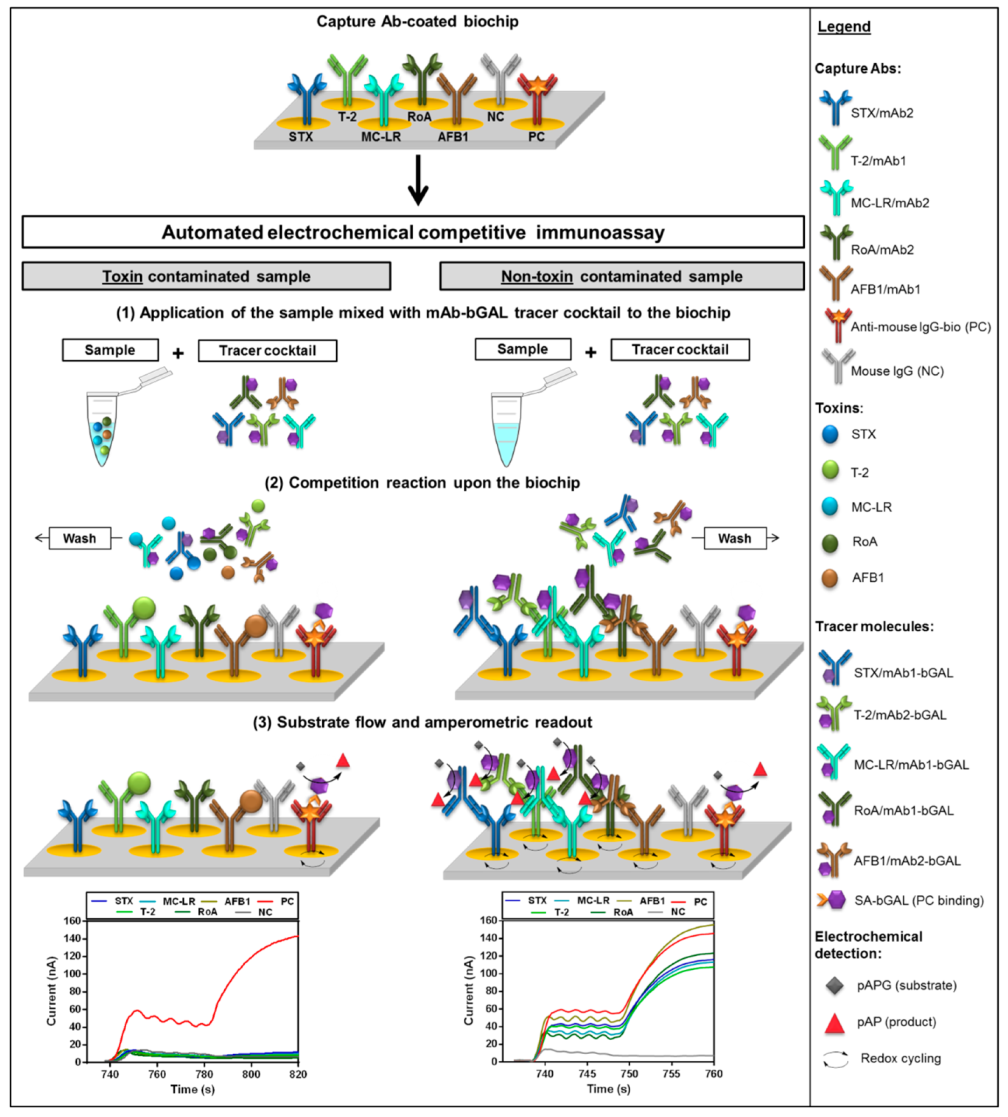
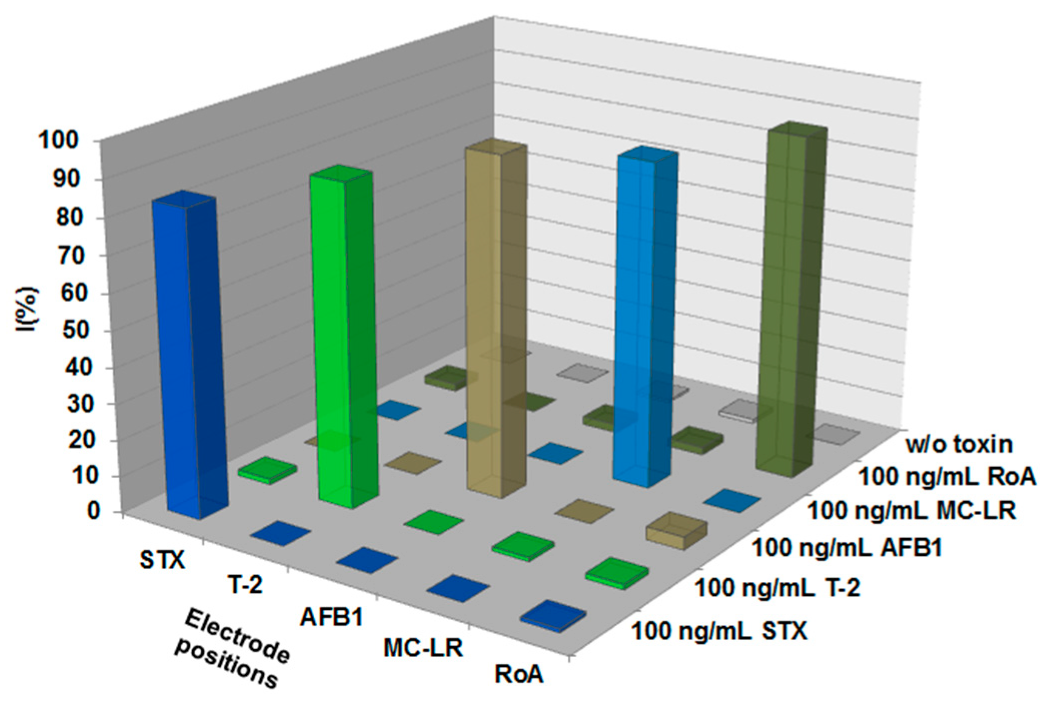
| Toxins | Target Electrode Positions for: | |||||
|---|---|---|---|---|---|---|
| Group | Congener | STX | MC-LR | T-2 | RoA | AFB1 |
| PSP toxin | STX | +++ | − | − | − | − |
| NEO | − | − | − | − | − | |
| GTX-1/-4 | − | − | − | − | − | |
| dc-STX | ++ | − | − | − | − | |
| GTX-2/-3 | +++ | − | − | − | − | |
| GTX-5 | ++ | − | − | − | − | |
| dc-NEO | − | − | − | − | − | |
| dc-GTX-2/-3 | +++ | − | − | − | − | |
| C1/C2 | − | − | − | − | − | |
| MC | MC-LR | − | +++ | − | − | − |
| [DAsp3]MC-LR | − | +++ | − | − | − | |
| MC-RR | − | +++ | − | − | − | |
| MC-YR | − | +++ | − | − | − | |
| MC-LA | − | + | − | − | − | |
| MC-LY | − | + | − | − | − | |
| MC-LW | − | + | − | − | − | |
| Nodularin | Nodularin-R | − | +++ | − | − | − |
| Type A trichothecene | T-2 | − | − | +++ | − | − |
| HT-2 | − | − | +++ | − | − | |
| T-2 triol | − | − | − | − | − | |
| T-2 tetraol | − | − | − | − | − | |
| Verrucarol | − | − | − | − | − | |
| Type D trichothecene | RoA | − | − | − | +++ | − |
| RoE | − | − | − | +++ | − | |
| SatH | − | − | − | ++ | − | |
| VerA | − | − | − | +++ | − | |
| Aflatoxin | AFB1 | − | − | − | − | +++ |
| AFM1 | − | − | − | − | +++ | |
| AFG1 | − | − | − | − | +++ | |
| AFB2 | − | − | − | − | ++ | |
| AFG2 | − | − | − | − | ++ | |
| Toxin | Sensitivity (ng/mL) | Reproducibility (Inter-Chip %CV) | |||
|---|---|---|---|---|---|
| LOD | IC50 | IC30–IC80 | B0 | BIC50 | |
| STX | 1.2 | 5.9 | 1.8–56.4 | 9.9 | 10.4 |
| MC-LR | 1.5 | 5.5 | 2.8–31.1 | 9.2 | 10.2 |
| T-2 | 0.4 | 1.4 | 0.5–17.4 | 10.1 | 9.2 |
| HT-2 | 0.7 | 2.4 | 0.8–25.6 | 11.6 | |
| RoA | 0.5 | 1.2 | 0.5–10.8 | 9.6 | 10.0 |
| AFB1 | 0.6 | 1.7 | 0.6–10.5 | 11.2 | 8.2 |
| Toxin | Spiking Concentration (ng/mL) | Assay Concentration (ng/mL) | %(B/B0)Serum * (% ± SD) | Recovery Rate (% ± SD) | Correctly Identified (%) |
|---|---|---|---|---|---|
| STX | 0 | 0 | 96.7 ± 13.9 | - | 100 |
| 6 | 3 | 60.6 ± 6.5 | 113.7 ± 24.5 | 100 | |
| 20 | 10 | 41.7 ± 8.8 | 103.2 ± 16.11 | 100 | |
| 200 | 20 | 32.2 ± 3.6 | 112.6 ± 26.1 | 100 | |
| MC-LR | 0 | 0 | 110.9 ± 19.8 | - | 100 |
| 6 | 3 | 78.7 ± 6.5 | 78.8 ± 22.7 | 80 | |
| 20 | 10 | 46.9 ± 10.6 | 93.9 ± 24.9 | 100 | |
| 200 | 20 | 26.3 ± 4.8 | 98.2 ± 22.5 | 100 | |
| HT-2 | 0 | 0 | 117.3 ± 20.4 | - | 100 |
| 4 | 2 | 67.5 ± 4.9 | 51.8 ± 17.6 | 100 | |
| 10 | 5 | 54.3 ± 10.6 | 60.6 ± 8.9 | 100 | |
| 100 | 10 | 26.3 ± 3.5 | 79.2 ± 24.3 | 100 | |
| RoA | 0 | 0 | 101.4 ± 18.9 | - | 100 |
| 4 | 2 | 38.9 ± 3.8 | 87.9 ± 6.9 | 100 | |
| 10 | 5 | 24.7 ± 2.2 | 100.7 ± 13.4 | 100 | |
| 100 | 10 | 16.7 ± 1.7 | 95.6 ± 18.2 | 100 | |
| AFB1 | 0 | 0 | 109.6 ± 17.1 | - | 100 |
| 4 | 2 | 48.7 ± 6.3 | 101.1 ± 31.1 | 100 | |
| 10 | 5 | 25.6 ± 5.1 | 112.9 ± 24.6 | 100 | |
| 100 | 10 | 17.4 ± 2.9 | 114.8 ± 18.43 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, K.; Pöhlmann, C.; Dietrich, R.; Märtlbauer, E.; Elßner, T. An Electrochemical Fiveplex Biochip Assay Based on Anti-Idiotypic Antibodies for Fast On-Site Detection of Bioterrorism Relevant Low Molecular Weight Toxins. Toxins 2019, 11, 696. https://doi.org/10.3390/toxins11120696
Schulz K, Pöhlmann C, Dietrich R, Märtlbauer E, Elßner T. An Electrochemical Fiveplex Biochip Assay Based on Anti-Idiotypic Antibodies for Fast On-Site Detection of Bioterrorism Relevant Low Molecular Weight Toxins. Toxins. 2019; 11(12):696. https://doi.org/10.3390/toxins11120696
Chicago/Turabian StyleSchulz, Katharina, Christopher Pöhlmann, Richard Dietrich, Erwin Märtlbauer, and Thomas Elßner. 2019. "An Electrochemical Fiveplex Biochip Assay Based on Anti-Idiotypic Antibodies for Fast On-Site Detection of Bioterrorism Relevant Low Molecular Weight Toxins" Toxins 11, no. 12: 696. https://doi.org/10.3390/toxins11120696
APA StyleSchulz, K., Pöhlmann, C., Dietrich, R., Märtlbauer, E., & Elßner, T. (2019). An Electrochemical Fiveplex Biochip Assay Based on Anti-Idiotypic Antibodies for Fast On-Site Detection of Bioterrorism Relevant Low Molecular Weight Toxins. Toxins, 11(12), 696. https://doi.org/10.3390/toxins11120696





