The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution
Abstract
:1. Introduction
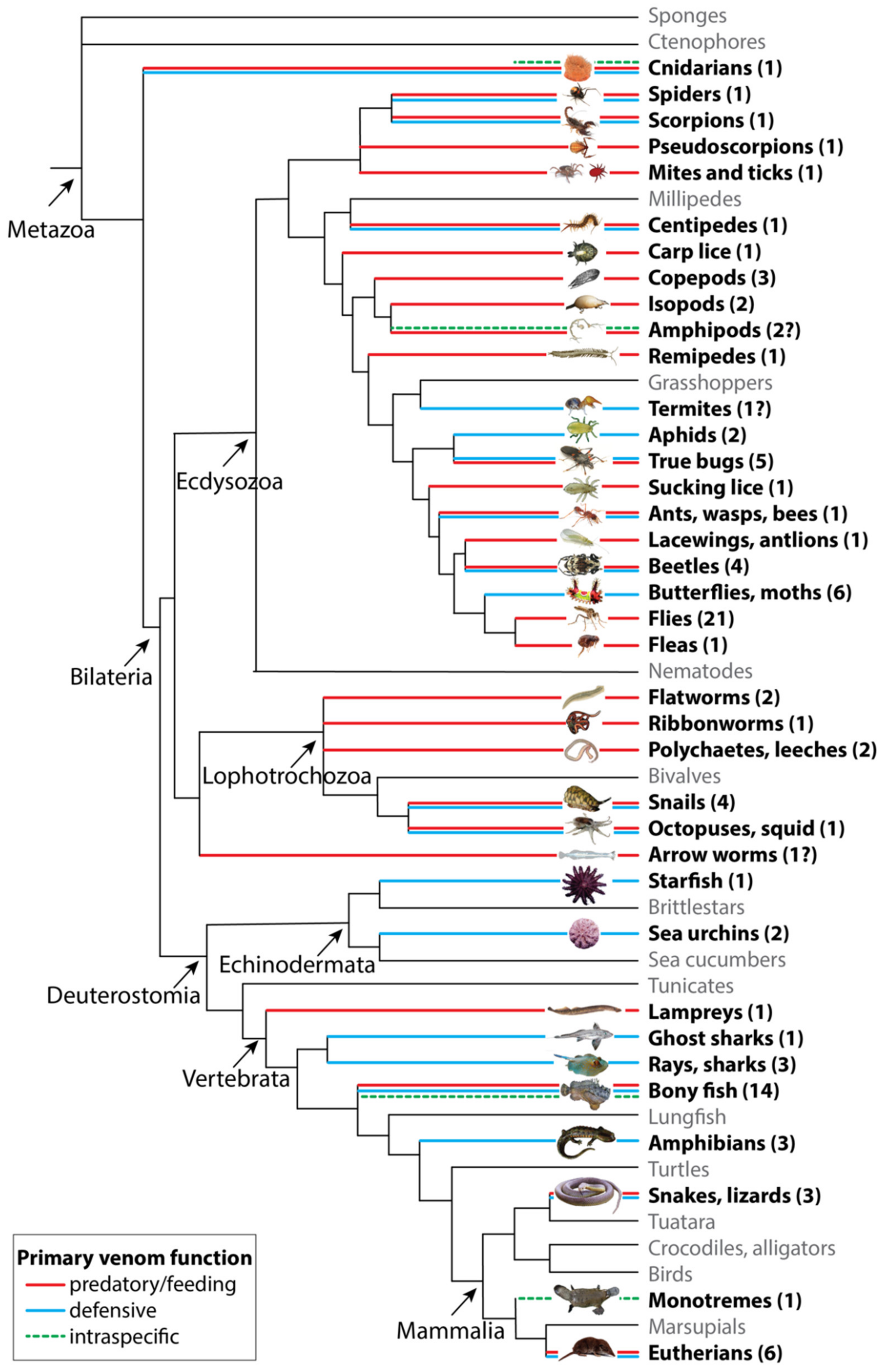
2. The Functional Diversity of Venoms
3. Venom Modulation
3.1. Quantitative Regulation of Venom
3.2. Qualitative Modulation of Venom
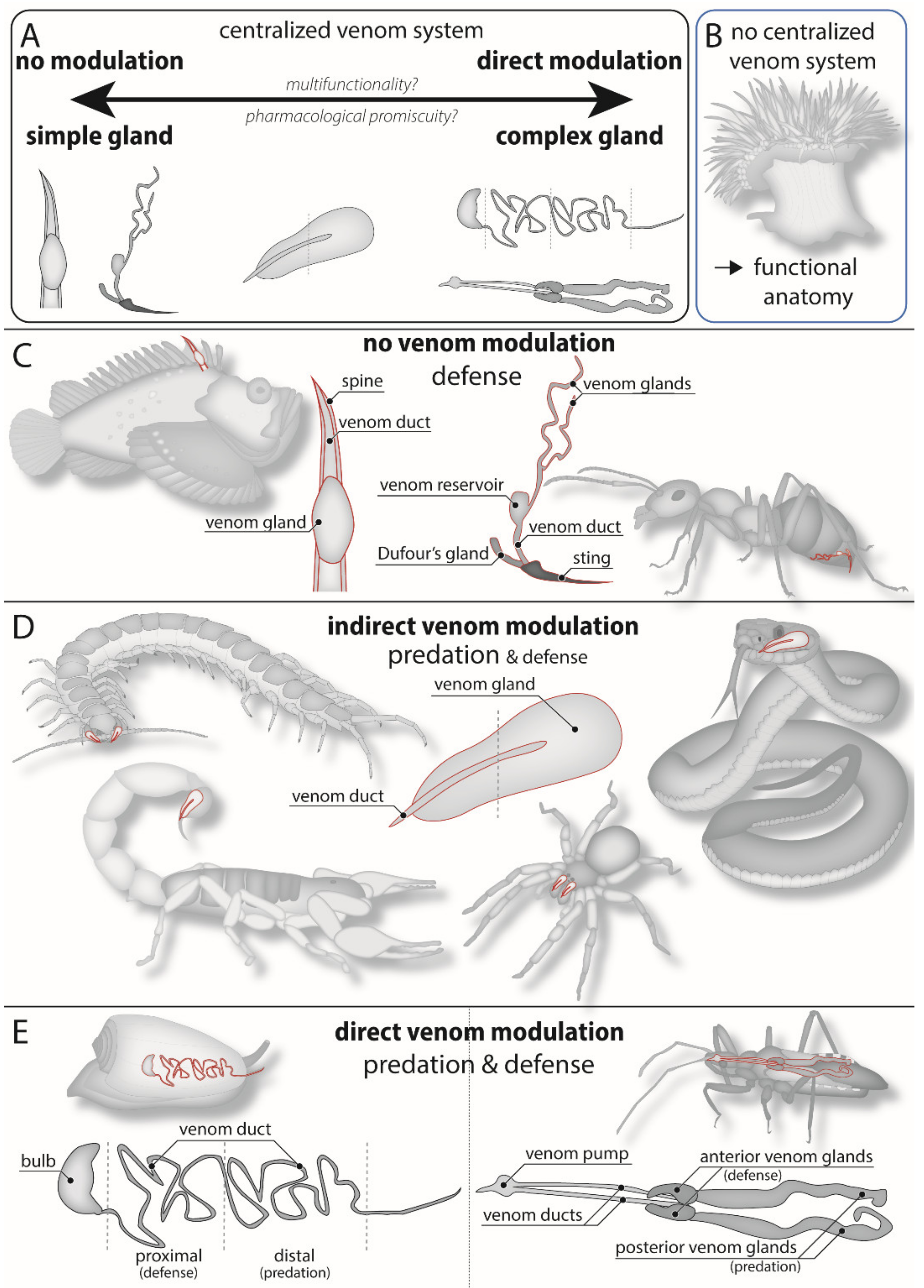
4. Morphological Constraints on Venom Modulation
5. Ecological Function and Venom Complexity
6. Functional Diversity through Toxin Multi-Functionality
6.1. Target Ubiquity
6.2. Target Promiscuity
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fry, B.G.; Koludarov, I.; Jackson, T.N.W.; Holford, M.; Terrat, Y.; Casewell, N.R.; Undheim, E.A.B.; Vetter, I.; Ali, S.A.; Low, D.H.W.; et al. Seeing the Woods for the Trees: Understanding Venom Evolution as a Guide for Biodiscovery. In Venoms to Drugs: Venom as a Source For the Development of Human Therapeutics; King, G.F., Ed.; Royal Society of Chemistry: London, UK, 2015; pp. 1–36. [Google Scholar]
- King, G.F. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Osteen, J.D.; Herzig, V.; Gilchrist, J.; Emrick, J.J.; Zhang, C.; Wang, X.; Castro, J.; Garcia-Caraballo, S.; Grundy, L.; Rychkov, G.Y.; et al. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 2016, 534, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Miljanich, G.P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon 2012, 59, 464–471. [Google Scholar] [CrossRef]
- Qiao, W.; Zhao, L.; Wu, S.; Liu, C.; Guo, L.; Xing, Y.; Zhao, J. SPECT imaging and radionuclide therapy of glioma using (131)I labeled Buthus martensii Karsch chlorotoxin. J. Neurooncol. 2017, 133, 287–295. [Google Scholar] [CrossRef]
- Chassagnon, I.R.; McCarthy, C.A.; Chin, Y.K.; Pineda, S.S.; Keramidas, A.; Mobli, M.; Pham, V.; De Silva, T.M.; Lynch, J.W.; Widdop, R.E.; et al. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2017, 114, 3750–3755. [Google Scholar] [CrossRef]
- Tarcha, E.J.; Olsen, C.M.; Probst, P.; Peckham, D.; Munoz-Elias, E.J.; Kruger, J.G.; Iadonato, S.P. Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial. PLOS One 2017, 12, e0180762. [Google Scholar] [CrossRef]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef]
- Smith, J.J.; Undheim, E.A.B. True lies: Using proteomics to assess the accuracy of transcriptome-based venomics in centipedes uncovers false positives and reveals startling intraspecific variation in Scolopendra subspinipes. Toxins 2018, 10, 96. [Google Scholar] [CrossRef]
- Herzig, V.; King, G.F.; Undheim, E.A.B. Can we resolve the taxonomic bias in spider venom research? Toxicon X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Madio, B.; Undheim, E.A.B.; King, G.F. Revisiting venom of the sea anemone Stichodactyla haddoni: Omics techniques reveal the complete toxin arsenal of a well-studied sea anemone genus. J. Proteomics 2017, 166, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Undheim, E.A.; Mobli, M.; King, G.F. Toxin structures as evolutionary tools: Using conserved 3D folds to study the evolution of rapidly evolving peptides. Bioessays 2016, 38, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Quistad, G.B.; Reuter, C.C.; Skinner, W.S.; Dennis, P.A.; Suwanrumpha, S.; Fu, E.W. Paralytic and insecticidal toxins from the funnel web spider, Hololena curta. Toxicon 1991, 29, 329–336. [Google Scholar] [CrossRef]
- Skinner, W.S.; Adams, M.E.; Quistad, G.B.; Kataoka, H.; Cesarin, B.J.; Enderlin, F.E.; Schooley, D.A. Purification and characterization of two classes of neurotoxins from the funnel web spider, Agelenopsis aperta. J. Biol. Chem. 1989, 264, 2150–2155. [Google Scholar]
- Quistad, G.B.; Skinner, W.S. Isolation and sequencing of insecticidal peptides from the primitive hunting spider, Plectreurys tristis (Simon). J. Biol. Chem. 1994, 269, 11098–11101. [Google Scholar]
- Skinner, W.S.; Dennis, P.A.; Li, J.P.; Quistad, G.B. Identification of insecticidal peptides from venom of the trap-door spider, Aptostichus schlingeri (Ctenizidae). Toxicon 1992, 30, 1043–1050. [Google Scholar] [CrossRef]
- Herzig, V.; Ikonomopoulou, M.; Smith, J.J.; Dziemborowicz, S.; Gilchrist, J.; Kuhn-Nentwig, L.; Rezende, F.O.; Moreira, L.A.; Nicholson, G.M.; Bosmans, F.; et al. Molecular basis of the remarkable species selectivity of an insecticidal sodium channel toxin from the African spider Augacephalus ezendami. Sci. Rep. 2016, 6, 29538. [Google Scholar] [CrossRef]
- Bende, N.S.; Dziemborowicz, S.; Mobli, M.; Herzig, V.; Gilchrist, J.; Wagner, J.; Nicholson, G.M.; King, G.F.; Bosmans, F. A distinct sodium channel voltage-sensor locus determines insect selectivity of the spider toxin Dc1a. Nat. Commun. 2014, 5, 1–23. [Google Scholar] [CrossRef]
- Sachkova, M.Y.; Singer, S.A.; Macrander, J.; Reitzel, A.M.; Peigneur, S.; Tytgat, J.; Moran, Y. The birth and death of toxins with distinct functions: A case study in the sea anemone Nematostella. Mol. Biol. Evol. 2019, 36, 2001–2012. [Google Scholar] [CrossRef]
- Zhu, L.; Peigneur, S.; Gao, B.; Zhang, S.; Tytgat, J.; Zhu, S. Target-driven positive selection at hot spots of scorpion toxins uncovers their potential in design of insecticides. Mol. Biol. Evol. 2016, 33, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wuster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Giribet, G.; Edgecombe, G.D. The phylogeny and evolutionary history of arthropods. Curr. Biol. 2019, 29, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, K. Evolutionary context of venom in animals. In Evolution of Venomous Animals and Their Toxins; Gopalakrishnakone, P., Malhotra, A., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Temple-Smith, P.D. Seasonal Breeding Biology of the Platypus, Ornithorhynchus anatinus (Shaw, 1799), with Special Reference to the Male; The Australian National University: Canberra, ACT, Australia, 1973. [Google Scholar]
- Sentenská, L.; Graber, F.; Richard, M.; Kropf, C. Sexual dimorphism in venom gland morphology in a sexually stinging scorpion. Biol. J. Linn. Soc. 2017, 122, 429–443. [Google Scholar] [CrossRef]
- Lebrun, E.G.; Jones, N.T.; Gilbert, L.E. Chemical warfare among invaders: A detoxification interaction facilitates an ant invasion. Science 2014, 343, 1014–1017. [Google Scholar] [CrossRef]
- Primon-Barros, M.; José Macedo, A. Animal venom peptides: Potential for new antimicrobial agents. Curr. Top. Med. Chem. 2017, 17, 1119–1156. [Google Scholar] [CrossRef]
- Dufton, M.J. Venomous mammals. Pharmacol. Ther. 1992, 53, 199–215. [Google Scholar] [CrossRef]
- Williams, F.X. Life history studies of Pepsis and Hemipepsis wasps in California (hymenoptera, Pomoilidae). Ann. Entomol. Soc. Am. 1956, 49, 447–466. [Google Scholar] [CrossRef]
- Petrunkewitch, A. Tarantula versus tarantula-hawk: A study in instinct. J. Exp. Zool. 1926, 45, 367–397. [Google Scholar] [CrossRef]
- Arvidson, R.; Kaiser, M.; Lee, S.S.; Urenda, J.P.; Dail, C.; Mohammed, H.; Nolan, C.; Pan, S.; Stajich, J.E.; Libersat, F.; et al. Parasitoid jewel wasp mounts multipronged neurochemical attack to hijack a host brain. Mol. Cell. Proteomics 2019, 18, 99–114. [Google Scholar] [CrossRef]
- Moreau, S.J.M.; Vinchon, S.; Cherqui, A.; Prévost, G. Components of Asobara venoms and their effects on host. In Advances in Parasitology; Prevost, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 70, pp. 217–232. [Google Scholar]
- Danneels, E.L.; Rivers, D.B.; de Graaf, D.C. Venom proteins of the parasitoid wasp Nasonia vitripennis: Recent discovery of an untapped pharmacopee. Toxins 2010, 2, 494–516. [Google Scholar] [CrossRef] [PubMed]
- Martinson, E.O.; Werren, J.H. Venom is beneficial but not essential for development and survival of Nasonia. Ecol. Entomol. 2018, 43, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Cascardi, J.; Young, B.A.; Husic, H.D.; Sherma, J. Protein variation in the venom spat by the red spitting cobra, Naja pallida (Reptilia Serpentes). Toxicon 1999, 37, 1271–1279. [Google Scholar] [CrossRef]
- Jeanne, R.L.; Keeping, M.G. Venom spraying in Parachartergus colobopterus: A novel defensive behavior in a social wasp (Hymenoptera: Vespidae). J. Insect Behav. 1995, 8, 433–442. [Google Scholar] [CrossRef]
- Nisani, Z.; Hayes, W.K. Venom-spraying behavior of the scorpion Parabuthus transvaalicus (Arachnida: Buthidae). Behav. Process. 2015, 115, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.S. Insect assassins. Sci. Am. 1960, 202, 72–79. [Google Scholar] [CrossRef]
- Fink, L.S. Venom spitting by the green lynx spider. J. Arachnol. 1984, 12, 372–373. [Google Scholar]
- Suter, R.B.; Stratton, G.E. Spitting performance parameters and their biomechanical implications in the spitting spider, Scytodes thoracica. J. Insect Sci. 2009, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Moran, Y.; Genikhovich, G.; Gordon, D.; Wienkoop, S.; Zenkert, C.; Ozbek, S.; Technau, U.; Gurevitz, M. Neurotoxin localization to ectodermal gland cells uncovers an alternative mechanism of venom delivery in sea anemones. Proc. Biol. Sci. 2012, 279, 1351–1358. [Google Scholar] [CrossRef] [Green Version]
- Basulto, A.; Perez, V.M.; Noa, Y.; Varela, C.; Otero, A.J.; Pico, M.C. Immunohistochemical targeting of sea anemone cytolysins on tentacles, mesenteric filaments and isolated nematocysts of Stichodactyla helianthus. J. Exp. Zool. 2006, 305, 253–258. [Google Scholar] [CrossRef]
- Beckmann, A.; Ozbek, S. The nematocyst: A molecular map of the cnidarian stinging organelle. Int. J. Dev. Biol. 2012, 56, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Surm, J.M.; Smith, H.L.; Madio, B.; Undheim, E.A.B.; King, G.F.; Hamilton, B.R.; van der Burg, C.A.; Pavasovic, A.; Prentis, P.J. A process of convergent amplification and tissue-specific expression dominates the evolution of toxin and toxin-like genes in sea anemones. Mol. Ecol. 2019, 28, 2272–2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrander, J.; Brugler, M.R.; Daly, M. A RNA-seq approach to identify putative toxins from acrorhagi in aggressive and non-aggressive Anthopleura elegantissima polyps. BMC Genomics 2015, 16, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madio, B.; Peigneur, S.; Chin, Y.K.Y.; Hamilton, B.R.; Henriques, S.T.; Smith, J.J.; Cristofori-Armstrong, B.; Dekan, Z.; Boughton, B.A.; Alewood, P.F.; et al. PHAB toxins: A unique family of predatory sea anemone toxins evolving via intra-gene concerted evolution defines a new peptide fold. Cell. Mol. Life Sci. 2018, 75, 4511–4524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Columbus-Shenkar, Y.Y.; Sachkova, M.Y.; Macrander, J.; Fridrich, A.; Modepalli, V.; Reitzel, A.M.; Sunagar, K.; Moran, Y. Dynamics of venom composition across a complex life cycle. eLife 2018, 7, e35014. [Google Scholar] [CrossRef]
- Morgenstern, D.; King, G.F. The venom optimization hypothesis revisited. Toxicon 2013, 63, 120–128. [Google Scholar] [CrossRef]
- Whittington, C.M.; Belov, K. Tracing monotreme venom evolution in the genomics era. Toxins 2014, 6, 1260–1273. [Google Scholar] [CrossRef] [Green Version]
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient venom systems: A review on Cnidaria toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef] [Green Version]
- Nekaris, K.A.-I.; Weldon, A.; Imron, M.A.; Maynard, K.Q.; Nijman, V.; Poindexter, S.A.; Morcatty, T.Q. Venom in furs: Facial masks as aposematic signals in a venomous mammal. Toxins 2019, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Rode-Margono, J.E.; Nekaris, K.A. Cabinet of curiosities: venom systems and their ecological function in mammals, with a focus on primates. Toxins 2015, 7, 2639–2658. [Google Scholar] [CrossRef] [Green Version]
- Moreau, S.; Asgari, S. Venom proteins from parasitoid wasps and their biological functions. Toxins 2015, 7, 2385–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouiaei, M.; Sunagar, K.; Federman Gross, A.; Scheib, H.; Alewood, P.F.; Moran, Y.; Fry, B.G. Evolution of an ancient venom: Recognition of a novel family of cnidarian toxins and the common evolutionary origin of sodium and potassium neurotoxins in sea anemone. Mol. Biol. Evol. 2015, 32, 1598–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, A.A.; Madio, B.; Jin, J.; Undheim, E.A.B.; Fry, B.G.; King, G.F. Melt with this kiss: Paralyzing and liquefying venom of the assassin bug Pristhesancus plagipennis (Hemiptera: Reduviidae). Mol. Cell. Proteomics 2017, 16, 552–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Undheim, E.A.; Fry, B.G.; King, G.F. Centipede venom: recent discoveries and current state of knowledge. Toxins 2015, 7, 679–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Reumont, B.M.; Undheim, E.A.B.; Jauss, R.T.; Jenner, R.A. Venomics of remipede crustaceans reveals novel peptide diversity and illuminates the venom’s biological role. Toxins 2017, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Mackessy, S.P.; Saviola, A.J. Understanding biological roles of venoms among the Caenophidia: The importance of rear-fanged snakes. Integr. Comp. Biol. 2016, 56, 1004–1021. [Google Scholar] [CrossRef]
- Helmark, S.; Garm, A. Gonadal cnidocytes in the cubozoan Tripedalia cystophora Conant, 1897 (Cnidaria: Cubozoa). J. Morphol. 2019, 280, 1530–1536. [Google Scholar] [CrossRef]
- Babonis, L.S.; Martindale, M.Q.; Ryan, J.F. Do novel genes drive morphological novelty? An investigation of the nematosomes in the sea anemone Nematostella vectensis. BMC Evol. Biol. 2016, 16, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhao, M.; Rotgans, B.A.; Ni, G.; Dean, J.F.; Nahrung, H.F.; Cummins, S.F. Proteomic analysis of the venom and venom sac of the woodwasp, Sirex noctilio— Towards understanding its biological impact. J. Proteomics 2016, 146, 195–206. [Google Scholar] [CrossRef]
- Frederickson, M.E.; Greene, M.J.; Gordon, D.M. Devil’s garden’s bedevilled by ants. Nature 2005, 437, 495–496. [Google Scholar] [CrossRef]
- Cremer, S.; Armitage, S.A.O.; Paul, S.-H. Social immunity. Curr. Biol. 2007, 17, PR693–R702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekaris, K.; Moore, R.; Rode, E.; Fry, B. Mad, bad and dangerous to know: The biochemistry, ecology and evolution of slow loris venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saviola, A.J.; Chiszar, D.; Busch, C.; Mackessy, S.P. Molecular basis for prey relocation in viperid snakes. BMC Biol. 2013, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.K.L.; Guenard, B.; Lewis, O.T. Trait-based ecology of terrestrial arthropods. Biol. Rev. 2019, 94, 999–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, D.C.; Jeanne, R.L. Venom source of a sex pheromone in the social wasp Polistes fuscatus (Hymenoptera: Vespidae). J. Chem. Ecol. 1983, 9, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hölldobler, B.; Wilson, E.O. The Ants; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Wigger, E.; Kuhn-Nentwig, L.; Nentwig, W. The venom optimisation hypothesis: A spider injects large venom quantities only into difficult prey types. Toxicon 2002, 40, 749–752. [Google Scholar] [CrossRef]
- Young, B.A.; Lee, C.E.; Daley, K.M. Do snakes meter venom? BioScience 2002, 52, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- Pintor, A.F.; Krockenberger, A.K.; Seymour, J.E. Costs of venom production in the common death adder (Acanthophis antarcticus). Toxicon 2010, 56, 1035–1042. [Google Scholar] [CrossRef]
- Nisani, Z.; Dunbar, S.G.; Hayes, W.K. Cost of venom regeneration in Parabuthus transvaalicus (Arachnida: Buthidae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 509–513. [Google Scholar] [CrossRef]
- Smith, M.T.; Ortega, J.; Beaupre, S.J. Metabolic cost of venom replenishment by Prairie Rattlesnakes (Crotalus viridis viridis). Toxicon 2014, 86, 1–7. [Google Scholar] [CrossRef]
- Enzor, L.A.; Wilborn, R.E.; Bennett, W.A. Toxicity and metabolic costs of the Atlantic stingray (Dasyatis sabina) venom delivery system in relation to its role in life history. J. Exp. Mar. Biol. Ecol. 2011, 409, 235–239. [Google Scholar] [CrossRef]
- Nisani, Z.; Boskovic, D.S.; Dunbar, S.G.; Kelln, W.; Hayes, W.K. Investigating the chemical profile of regenerated scorpion (Parabuthus transvaalicus) venom in relation to metabolic cost and toxicity. Toxicon 2012, 60, 315–323. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D.; Mason, R. Cost of producing venom in three North American pitviper species. Copeia 2006, 2006, 818–825. [Google Scholar] [CrossRef]
- Boevé, J.-L.; Kuhn-Nentwig, L.; Keller, S.; Nentwig, W. Quantity and quality of venom released by a spider (Cupiennius salei, Ctenidae). Toxicon 1995, 33, 1347–1357. [Google Scholar] [CrossRef]
- Cooper, A.M.; Kelln, W.J.; Hayes, W.K. Venom regeneration in the centipede Scolopendra polymorpha: evidence for asynchronous venom component synthesis. Zoology 2014, 117, 398–414. [Google Scholar] [CrossRef]
- Young, B.A.; Zahn, K. Venom flow in rattlesnakes: mechanics and metering. J. Exp. Biol. 2001, 204, 4345–4351. [Google Scholar]
- Rein, J.O. Sting use in two species of Parabuthus scorpions (Buthidae). J. Arachnol. 1993, 21, 60–63. [Google Scholar]
- Kenning, M.; Muller, C.H.G.; Sombke, A. The ultimate legs of Chilopoda (Myriapoda): A review on their morphological disparity and functional variability. PeerJ 2017, 5, e4023. [Google Scholar] [CrossRef] [Green Version]
- Nelsen, D.R.; Kelln, W.; Hayes, W.K. Poke but don’t pinch: Risk assessment and venom metering in the western black widow spider, Latrodectus hesperus. Anim. Behav. 2014, 89, 107–114. [Google Scholar] [CrossRef]
- Shine, R.; Schwaner, T. Prey constriction by venomous snakes: A review, and new data on Australian species. Copeia 1985, 1985, 1067–1071. [Google Scholar] [CrossRef]
- Nisani, Z.; Hayes, W.K. Defensive stinging by Parabuthus transvaalicus scorpions: risk assessment and venom metering. Anim. Behav. 2011, 81, 627–633. [Google Scholar] [CrossRef]
- Silveira, P.V.; Nishioka, S.d.A. Venomous snake bite without clinical envenoming (’dry-bite’). A neglected problem in Brazil. Trop. Geogr. Med. 1995, 47, 83–85. [Google Scholar]
- Dugon, M.M.; Arthur, W. Prey orientation and the role of venom availability in the predatory behaviour of the centipede Scolopendra subspinipes mutilans (Arthropoda: Chilopoda). J. Insect Physiol. 2012, 58, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.K. Factors associated with the mass of venom expended by prairie rattlesnakes (Crotalus v. virdis) feeding on mice. Toxicon 1992, 30, 449–460. [Google Scholar] [CrossRef]
- Evans, E.R.J.; Northfield, T.D.; Daly, N.L.; Wilson, D.T. Venom costs and optimization in scorpions. Front. Ecol. Evol. 2019, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Malli, H.; Kuhn-Nentwig, L.; Imboden, H.; Nentwig, W. Effects of size, mortility and paralysation time of prey on the quantity of venom injected by the hunting spider Cupiennius salei. J. Exp. Biol. 1999, 202, 2083–2089. [Google Scholar] [PubMed]
- Hayes, W.K. Venom metering by juvenile prairie rattlesnakes, Crotalus v. viridis: effects of prey size and experience. Anim. Behav. 1995, 50, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Hayes, W.K.; Lavin-Murcio, P.; Kardong, K.V. Northern pacific rattlesnakes (Crotalus viridis oreganus) meter venom when feeding on prey of different sizes. Copeia 1995, 1995, 337–343. [Google Scholar] [CrossRef]
- Wullschleger, B.; Nentwig, W. Influence of venom availability on a spider’s prey-choice behaviour. Funct. Ecol. 2002, 16, 802–807. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.-H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.A.; Mayhew, M.L.; Jin, J.; Herzig, V.; Undheim, E.A.B.; Sombke, A.; Fry, B.G.; Meritt, D.J.; King, G.F. The assassin bug Pristhesancus plagipennis produces two distinct venoms in separate gland lumens. Nat. Commun. 2018, 9, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inceoglu, B.; Lango, J.; Jing, J.; Chen, L.; Doymaz, F.; Pessah, I.N.; Hammock, B.D. One scorpion, two venoms: prevenom of Parabuthus transvaalicus acts as an alternative type of venom with distinct mechanism of action. Proc. Natl. Acad. Sci. USA 2003, 100, 922–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenstern, D.; Hamilton, B.; Korbie, D.; Clauser, K.R.; Haas, B.J.; Bowlay, G.; Jones, A.; Venter, D.J.; King, G.F. Biochemical venom modulation in spiders is achieved via compartmentalized toxin production and storage. Sneak Peek Curr. Biol. 2019. [Google Scholar] [CrossRef]
- Lira, A.F.A.; Santos, A.B.; Silva, N.A.; Martins, R.D. Threat level influences the use of venom in a scorpion species, Tityus stigmurus (Scorpiones, Buthidae). Acta Ethol. 2017, 20, 291–295. [Google Scholar] [CrossRef]
- Gangur, A.N.; Smout, M.; Liddell, M.J.; Seymour, J.E.; Wilson, D.; Northfield, T.D. Changes in predator exposure, but not in diet, induce phenotypic plasticity in scorpion venom. Proc. Biol. Sci. 2017, 284, 20171364. [Google Scholar] [CrossRef] [Green Version]
- Haridass, E.T.; Ananthakrishnan, T.N. Functional morphology of pylorus and rectal glands in Reduviidae (Insecta — Heteroptera). Proc. Indian Acad. Sci. 1981, 90, 483–493. [Google Scholar] [CrossRef]
- Miles, P.W. Studies on the salivary physiology of plant bugs: The chemistry of formation of the sheath material. Insect Physiol. 1963, 10, 147–160. [Google Scholar] [CrossRef]
- Edwards, J.S. The action and composition of the saliva ofan assassin bug Platymeris rhadamanthus Gaerst. (Hemiptera, Reduviidae). J. Exp. Biol. 1961, 38, 61–77. [Google Scholar]
- Morrison, M.N. Gel electrophoresis studies with references to functional morphology of the salivary glands of Acanthaspis pedestris Stal. (Insecta: Heteroptera: Reduviidae). Proc. Indian Acad. Sci. 1989, 98, 167–173. [Google Scholar] [CrossRef]
- Zhong, H.Y.; Wei, C.; Zhang, Y.L. Gross morphology and ultrastructure of salivary glands of the mute cicada Karenia caelatata Distant (Hemiptera: Cicadoidea). Micron 2013, 45, 83–91. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.H.; Alewood, P.F.; Lewis, R.J. Intraspecific variations in Conus geographus defence-evoked venom and estimation of the human lethal dose. Toxicon 2014, 91, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, J.E.; Bertke, E.M. Ultrastructure of the venom gland of the scorpion, Centruroides sculpturatus (Ewing). J. Morphol. 1972, 137, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Kerchove, C.M.; Carneiro, S.M.; Markus, R.P.; Yamanouye, N. Stimulation of the α-adrenoceptor triggers the venom production cycle in the venom gland of Bothrops jararaca. J. Exp. Biol. 2004, 207, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanouye, N.; Britto, L.R.; Carneiro, S.M.; Markus, R.P. Control of venom production and secretion by sympathetic outflow in the snake Bothrops jararaca. J. Exp. Biol. 1997, 200, 2547–2556. [Google Scholar]
- Taub, A.M. Ophidian cephalic glands. J. Morphol. 1966, 118, 529–541. [Google Scholar] [CrossRef]
- Järlfors, U.; Smith, D.S.; Russell, F.E. Nerve endings in the venom gland of the spider Latrodectus mactans. Toxicon 1969, 7, 263–264. [Google Scholar] [CrossRef]
- Undheim, E.A.; Hamilton, B.R.; Kurniawan, N.D.; Bowlay, G.; Cribb, B.W.; Merritt, D.J.; Fry, B.G.; King, G.F.; Venter, D.J. Production and packaging of a biological arsenal: evolution of centipede venoms under morphological constraint. Proc. Natl. Acad. Sci. USA 2015, 112, 4026–4031. [Google Scholar] [CrossRef] [Green Version]
- Young, B.A.; Herzog, F.; Friedel, P.; Rammensee, S.; Bausch, A.; van Hemmen, J.L. Tears of venom: hydrodynamics of reptilian envenomation. Phys. Rev. Lett. 2011, 106, 198103. [Google Scholar] [CrossRef]
- Richter, S.; Helm, C.; Meunier, F.A.; Hering, L.; Campbell, L.I.; Drukewitz, S.H.; Undheim, E.A.B.; Jenner, R.A.; Schiavo, G.; Bleidorn, C. Comparative analyses of glycerotoxin expression unveil a novel structural organization of the bloodworm venom system. BMC Evol. Biol. 2017, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.D.; Mueller, A.; Clayton, D.; Starobova, H.; Hamilton, B.R.; Payne, R.J.; Vetter, I.; King, G.F.; Undheim, E.A.B. A comprehensive portrait of the venom of the giant red bull ant, Myrmecia gulosa, reveals a hyperdiverse hymenopteran toxin gene family. Sci. Adv. 2018, 4, eaau4640. [Google Scholar] [CrossRef] [Green Version]
- Ziegman, R.; Undheim, E.A.B.; Baillie, G.; Jones, A.; Alewood, P.F. Investigation of the estuarine stonefish (Synanceia horrida) venom composition. J. Proteomics 2019, 201, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Grund, L.Z.; Lima, C. Thalassophryne nattereri fish venom: from the envenoming to the understanding of the immune system. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2014, 20, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegman, R.; Alewood, P. Bioactive components in fish venoms. Toxins 2015, 7, 1497–1531. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Raghuraman, S.; Schmidt, E.W.; Safavi-Hemami, H. Linking neuroethology to the chemical biology of natural products: interactions between cone snails and their fish prey, a case study. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2017, 203, 717–735. [Google Scholar] [CrossRef]
- Ponte, G.; Modica, M.V. Salivary glands in predatory mollusks: Evolutionary considerations. Front. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Göransson, U.; Jacobsson, E.; Strand, M.; Andersson, H.S. The toxins of nemertean worms. Toxins 2019, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Moser, W.E.; Desser, S.S. Morphological, histochemical, and ultrastructural characterization of the salivary glands and proboscises of three species of glossiphoniid leeches (Hirudinea: Rhynchobdellida). J. Morphol. 1995, 225, 1–18. [Google Scholar] [CrossRef]
- Marshall, C.G.; Lent, C.M. Excitability and secretory activity in the salivary gland cells of jawed leeches (Hirudinea: Gnathobdellida). J. Exp. Biol. 1988, 137, 89–105. [Google Scholar]
- Drukewitz, S.H.; Fuhrmann, N.; Undheim, E.A.B.; Blanke, A.; Giribaldi, J.; Mary, R.; Laconde, G.; Dutertre, S.; von Reumont, B.M. A dipteran’s novel sucker punch: Evolution of arthropod atypical venom with a neurotoxic component in robber flies (Asilidae, Diptera). Toxins 2018, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Beutel, R.G.; Friedrich, F.; Aspöck, U. The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta). Zool. J. Linn. Soc. 2010, 158, 533–562. [Google Scholar] [CrossRef]
- Villas-Boas, I.M.; Bonfá, G.; Tambourgi, D.V. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon 2018, 153, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.D.; Banajee, K.H.; Foil, L.D.; Macaluso, K.R. Transmission mechanisms of an emerging insect-borne rickettsial pathogen. Parasit. Vectors 2016, 9, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Reumont, B.M.; Blanke, A.; Richter, S.; Alvarez, F.; Bleidorn, C.; Jenner, R.A. The first venomous crustacean revealed by transcriptomics and functional morphology: remipede venom glands express a unique toxin cocktail dominated by enzymes and a neurotoxin. Mol. Biol. Evol. 2014, 31, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murienne, J.; Harvey, M.S.; Giribet, G. First molecular phylogeny of the major clades of Pseudoscorpiones (Arthropoda: Chelicerata). Mol. Phylogenet. Evol. 2008, 49, 170–184. [Google Scholar] [CrossRef]
- Ebert, T.A. Adaptive aspects of phenotypic plasticity in echinoderms. Oceanologica Acta 1996, 19, 347–355. [Google Scholar]
- Coppard, S.E.; Kroh, A.; Smith, A.B. The evolution of pedicellariae in echinoids: An arms race against pests and parasites. Acta Zool. 2010, 93, 125–148. [Google Scholar] [CrossRef]
- Peiren, N.; Vanrobaeys, F.; de Graaf, D.C.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim. Biophys. Acta Proteins Proteom. 2005, 1752, 1–5. [Google Scholar] [CrossRef]
- Escoubas, P.; Sollod, B.; King, G.F. Venom landscapes: mining the complexity of spider venoms via a combined cDNA and mass spectrometric approach. Toxicon 2006, 47, 650–663. [Google Scholar] [CrossRef]
- Lavergne, V.; Harliwong, I.; Jones, A.; Miller, D.; Taft, R.J.; Alewood, P.F. Optimized deep-targeted proteotranscriptomic profiling reveals unexplored Conus toxin diversity and novel cysteine frameworks. Proc. Natl. Acad. Sci. USA 2015, 112, E3782–E3791. [Google Scholar] [CrossRef] [Green Version]
- Sunagar, K.; Moran, Y. The rise and fall of an evolutionary innovation: contrasting strategies of venom evolution in ancient and young animals. PLoS Genet. 2015, 11, e1005596. [Google Scholar] [CrossRef]
- Pekár, S.; Bočánek, O.; Michálek, O.; Petráková, L.; Haddad, C.R.; Šedo, O.; Zdráhal, Z. Venom gland size and venom complexity—essential trophic adaptations of venomous predators: A case study using spiders. Mol. Ecol. 2018, 27, 4257–4269. [Google Scholar] [CrossRef] [PubMed]
- Phuong, M.A.; Mahardika, G.N.; Alfaro, M.E. Dietary breadth is positively correlated with venom complexity in cone snails. BMC Genomics 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weese, D.A.; Duda, T.F., Jr. Effects of predator-prey interactions on predator traits: Differentiation of diets and venoms of a marine snail. Toxins 2019, 11, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahari, S.; Bickford, D.; Fry, B.G.; Kini, R.M. Expression pattern of three-finger toxin and phospholipase A2 genes in the venom glands of two sea snakes, Lapemis curtus and Acalyptophis peronii: comparison of evolution of these toxins in land snakes, sea kraits and sea snakes. BMC Evol. Biol. 2007, 7, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, T.N.W.; Koludarov, I.; Ali, S.A.; Dobson, J.; Zdenek, C.N.; Dashevsky, D.; Op den Brouw, B.; Masci, P.P.; Nouwens, A.; Josh, P.; et al. Rapid radiations and the race to redundancy: An investigation of the evolution of australian elapid snake venoms. Toxins 2016, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Jenner, R.A.; Von Reumont, B.M.; Campbell, L.I.; Undheim, E.A.B. Parallel evolution of complex centipede venoms revealed by comparative proteotranscriptomic analyses. Mol. Biol. Evol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, M.J.; Rokyta, D.R. Venom-gland transcriptomics and venom proteomics of the giant Florida blue centipede, Scolopendra viridis. Toxicon 2018, 152, 121–136. [Google Scholar] [CrossRef]
- Prashanth, J.R.; Dutertre, S.; Lewis, R.J. Pharmacology of predatory and defensive venom peptides in cone snails. Mol. Biosyst. 2017, 13, 2453–2465. [Google Scholar] [CrossRef]
- Touchard, A.; Aili, S.R.; Fox, E.G.P.; Escoubas, P.; Orivel, J.; Nicholson, G.M.; Dejean, A. The biochemical toxin arsenal from ant venoms. Toxins 2016, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Undheim, E.A.B.; Jones, A.; Clauser, K.R.; Holland, J.W.; Pineda, S.S.; King, G.F.; Fry, B.G. Clawing through evolution: Toxin diversification and convergence in the ancient lineage Chilopoda (centipedes). Mol. Biol. Evol. 2014, 31, 2124–2148. [Google Scholar] [CrossRef] [Green Version]
- Li-Smerin, Y.; Swartz, K.J. Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels. Proc. Natl. Acad. Sci. USA 1998, 95, 8585–8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, R.E.; Warren, V.A.; Kraus, R.L.; Hwang, J.C.; Liu, C.J.; Dai, G.; Brochu, R.M.; Kohler, M.G.; Gao, Y.D.; Garsky, V.M.; et al. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry 2002, 41, 14734–14747. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, E.; Cassulini, R.R.; Silva, D.F.; Clement, H.; Schiavon, E.; Zamudio, F.Z.; Odell, G.; Arcangeli, A.; Clare, J.J.; Alagon, A.; et al. Target promiscuity and heterogeneous effects of tarantula venom peptides affecting Na+ and K+ ion channels. J. Biol. Chem. 2010, 285, 4130–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosmans, F.; Swartz, K.J. Targeting voltage sensors in sodium channels with spider toxins. Trends Pharmacol. Sci. 2010, 31, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingerd, J.S.; Mozar, C.A.; Ussing, C.A.; Murali, S.S.; Chin, Y.K.; Cristofori-Armstrong, B.; Durek, T.; Gilchrist, J.; Vaughan, C.W.; Bosmans, F.; et al. The tarantula toxin beta/delta-TRTX-Pre1a highlights the importance of the S1-S2 voltage-sensor region for sodium channel subtype selectivity. Sci. Rep. 2017, 7, 974. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Kim, P.I.; Lee, S.K.; Lee, C.W.; Eu, Y.J.; Lee, D.G.; Earm, Y.E.; Kim, J.I. Lipid membrane interaction and antimicrobial activity of GsMTx-4, an inhibitor of mechanosensitive channel. Biochem. Biophys. Res. Commun. 2006, 340, 633–638. [Google Scholar] [CrossRef]
- Diochot, S.; Baron, A.; Rash, L.D.; Deval, E.; Escoubas, P.; Scarzello, S.; Salinas, M.; Lazdunski, M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004, 23, 1516–1525. [Google Scholar] [CrossRef]
- Lee, J.Y.P.; Saez, N.J.; Cristofori-Armstrong, B.; Anangi, R.; King, G.F.; Smith, M.T.; Rash, L.D. Inhibition of acid-sensing ion channels by diminazene and APETx2 evoke partial and highly variable antihyperalgesia in a rat model of inflammatory pain. Br. J. Pharmacol. 2018, 175, 2204–2218. [Google Scholar] [CrossRef]
- Peigneur, S.; Beress, L.; Moller, C.; Mari, F.; Forssmann, W.G.; Tytgat, J. A natural point mutation changes both target selectivity and mechanism of action of sea anemone toxins. FASEB J. 2012, 26, 5141–5151. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.E.; Cristofori-Armstrong, B.; Anangi, R.; Rosengren, K.J.; Lau, C.H.; Mobli, M.; Brust, A.; Alewood, P.F.; King, G.F.; Rash, L.D. Understanding the molecular basis of toxin promiscuity: The analgesic sea anemone peptide APETx2 interacts with acid-sensing ion channel 3 and hERG channels via overlapping pharmacophores. J. Med. Chem. 2014, 57, 9195–9203. [Google Scholar] [CrossRef]
- Jin, A.H.; Cristofori-Armstrong, B.; Rash, L.D.; Roman-Gonzalez, S.A.; Espinosa, R.A.; Lewis, R.J.; Alewood, P.F.; Vetter, I. Novel conorfamides from Conus austini venom modulate both nicotinic acetylcholine receptors and acid-sensing ion channels. Biochem. Pharmacol. 2019, 164, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Pekar, S.; Liznarova, E.; Bocanek, O.; Zdrahal, Z. Venom of prey-specialized spiders is more toxic to their preferred prey: A result of prey-specific toxins. J. Anim. Ecol. 2018, 87, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
| Function | Example of Venomous Animal | References |
|---|---|---|
| Intraspecific competition | Platypus, sea anemones, slow loris | [51,52,53] |
| Food storage | Moles, shrews, parasitoid wasps | [54,55] |
| (Pre-)Digestion | Sea anemones, assassin bugs, centipedes, remipedes, vipers | [56,57,58,59,60] |
| Offspring care | Sea anemones, cubozoan jellyfish, parasitoid wasps, saw flies | [55,61,62,63] |
| Mating | Scorpions | [27] |
| Habitat creation | Ants | [64] |
| Antimicrobial ointment | Ants, wasps | [65] |
| Ectoparasite deterrent | Slow loris | [66] |
| Antivenom | Tawny crazy ant (Nylanderia fulva) | [28] |
| Prey homing device | Rattlesnakes | [67] |
| Intraspecific communication | Ants, wasps | [68,69,70] |
| Animal group | General Venom System Morphology | Type of Modulation |
|---|---|---|
| Coleoid cephalopods | Two pairs of potential venom glands, injected through muscular salivary papilla [120]. | Quantitative regulation, direct qualitative modulation. |
| Tonnoid, muricid, and colubrariid snails | One or two lobes in venom glands that open through common duct into buccal mass [120]. | Quantitative regulation, potentially direct qualitative modulation. |
| Nemertea | Proboscis with venom secreting cells, but no direct injection apparatus [121]. | Potential qualitative modulation by spatially heterogeneous toxin storage along proboscis. |
| Glycerid polychaetes | Toxin-producing “lappets” secreting venom into large muscular and glandular venom reservoir, which is presumably also involved in venom expulsion [114]. | Quantitative regulation. |
| Leeches | Secretory cells dispersed along the buccal cavity in jawed leeches (Arhynchobdellida); presence of two paired salivary glands in jawless leeches (Glossiphoniidae) [122,123]. | Quantitative regulation and direct qualitative modulation in Glossiphoniidae; only quantitative regulation in Arhynchobdellida. |
| Robber flies (Asiliidae) | Two pairs of venom glands secreting venom to a separate venom pump [124]. | Quantitative regulation, direct qualitative modulation. |
| Larval neuropterans | Paired venom gland opening directly into the venom delivering canal of the jaws [125]. | Quantitative regulation. |
| Aculeate hymenoptera | Filamentous glands, venom stored in large venom reservoir. Additional Dufour’s gland [115]. | Quantitative regulation, possibly direct qualitative modulation if Dufour’s gland involved. |
| *Lepidopteran caterpillars | Various variations on venom gland-associated spines [126]. | None. |
| Fleas | Single pair of salivary/venom glands [127]. | Quantitative regulation. |
| Centipedes | Composite venom glands consisting of numerous “secretory units” that empty into a chitinous duct (“calyx”). In most giant centipedes (Scolopendromorpha), the calyx is greatly extended, with secretory units organized perpendicular to length of the gland. Heterogeneous toxin production [112]. | Quantitative regulation in all, direct qualitative modulation in giant centipedes. |
| Remipedes | Venom glands secrete into large venom reservoir immediately proximal to venom delivery structure [128]. | Quantitative regulation. |
| Spiders | Paired muscular venom glands with branch-like ductules leading to a common duct. Spitting spiders (Scytodidae) with extra lobe. | Indirect qualitative modulation; direct qualitative modulation in spitting spiders. |
| Iocheiratan pseudoscorpions | Venom glands in pedipalpal fingers, either in both, or in either, with separate outlets [129]. | Quantitative regulation, potential direct qualitative modulation in species with venom glands in both pedipalpal fingers. |
| *Echinoderms | Venomous spines, venomous pedicellaria [130,131]. | None. Potential spatial heterogeneity of toxins with different functions. |
| *Fish, except lampreys, fang blennies, and jaw eels | Venomous spines connected to or covered in venom-producing glands/tissue. | None. |
| *Frogs, salamanders | Spines or ribs piercing venom glands. | None. |
| Colubroid snakes | Venom glands with branch-like ductules leading to a short duct connected to front or rear fangs. | Quantitative regulation, indirect qualitative modulation. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins 2019, 11, 666. https://doi.org/10.3390/toxins11110666
Schendel V, Rash LD, Jenner RA, Undheim EAB. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins. 2019; 11(11):666. https://doi.org/10.3390/toxins11110666
Chicago/Turabian StyleSchendel, Vanessa, Lachlan D. Rash, Ronald A. Jenner, and Eivind A. B. Undheim. 2019. "The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution" Toxins 11, no. 11: 666. https://doi.org/10.3390/toxins11110666
APA StyleSchendel, V., Rash, L. D., Jenner, R. A., & Undheim, E. A. B. (2019). The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins, 11(11), 666. https://doi.org/10.3390/toxins11110666





