Deoxynivalenol Affects Proliferation and Expression of Activation-Related Molecules in Major Porcine T-Cell Subsets
Abstract
:1. Introduction
2. Results
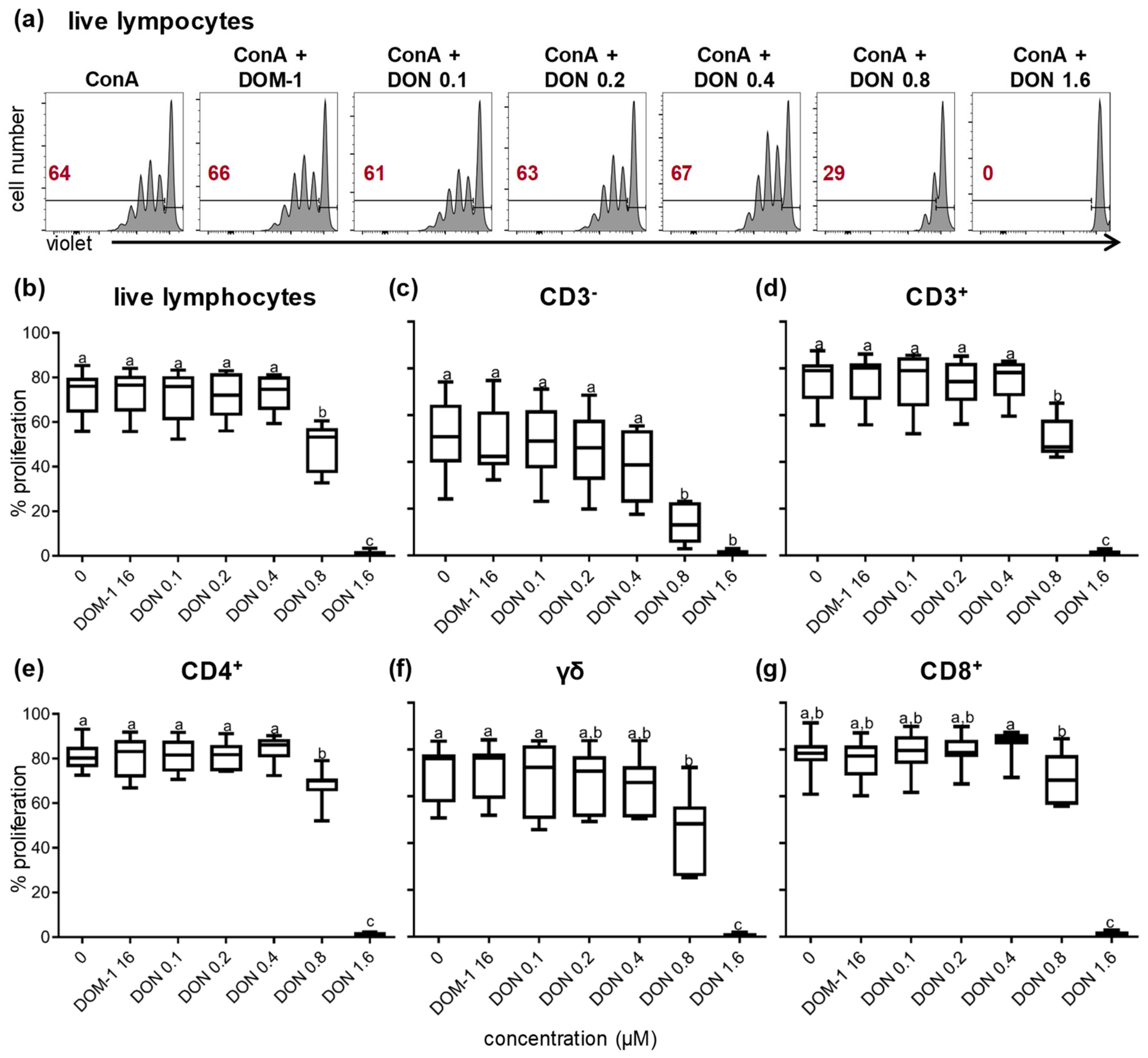
2.1. Cell Viability and Lymphocyte Proliferation
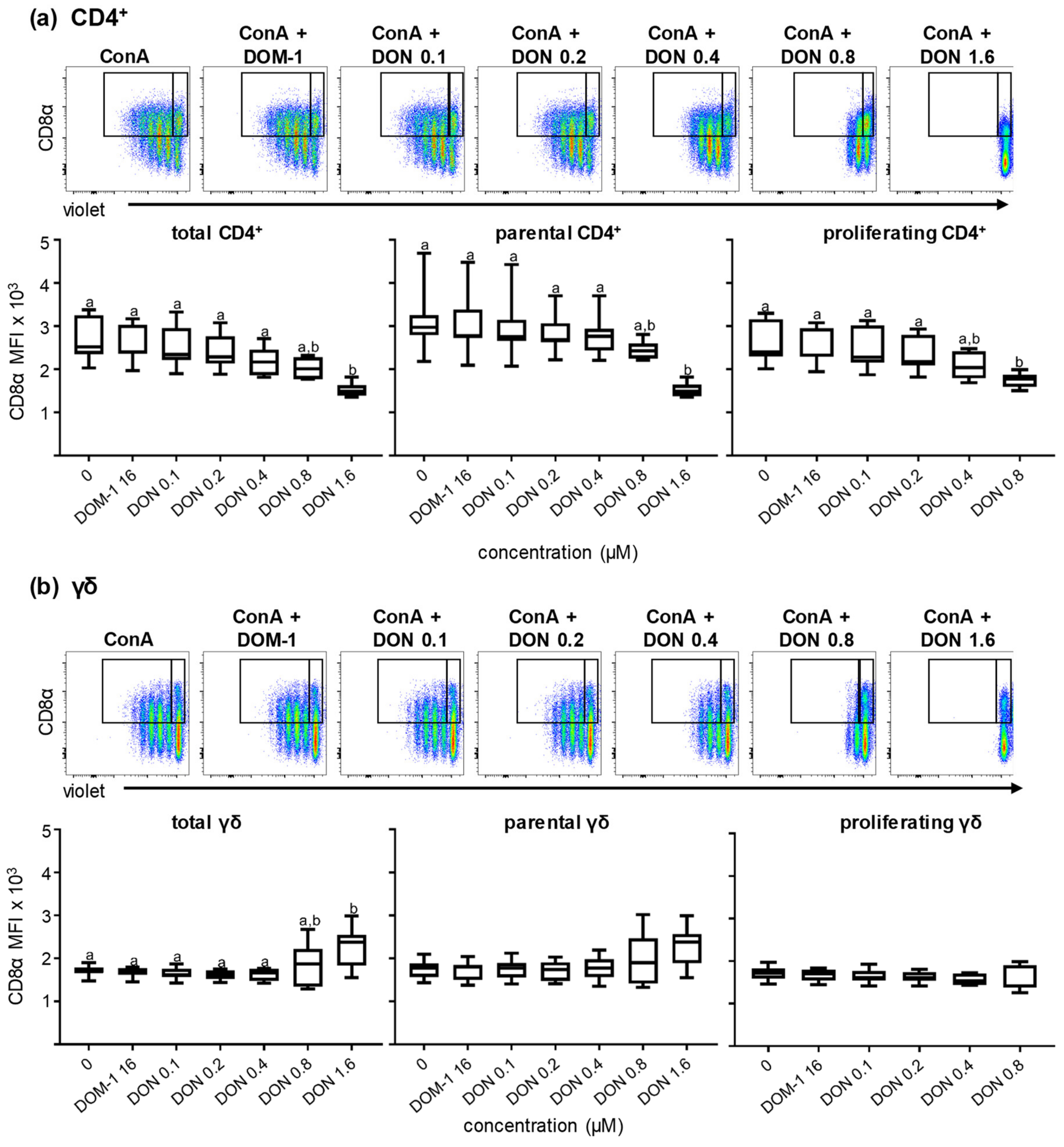
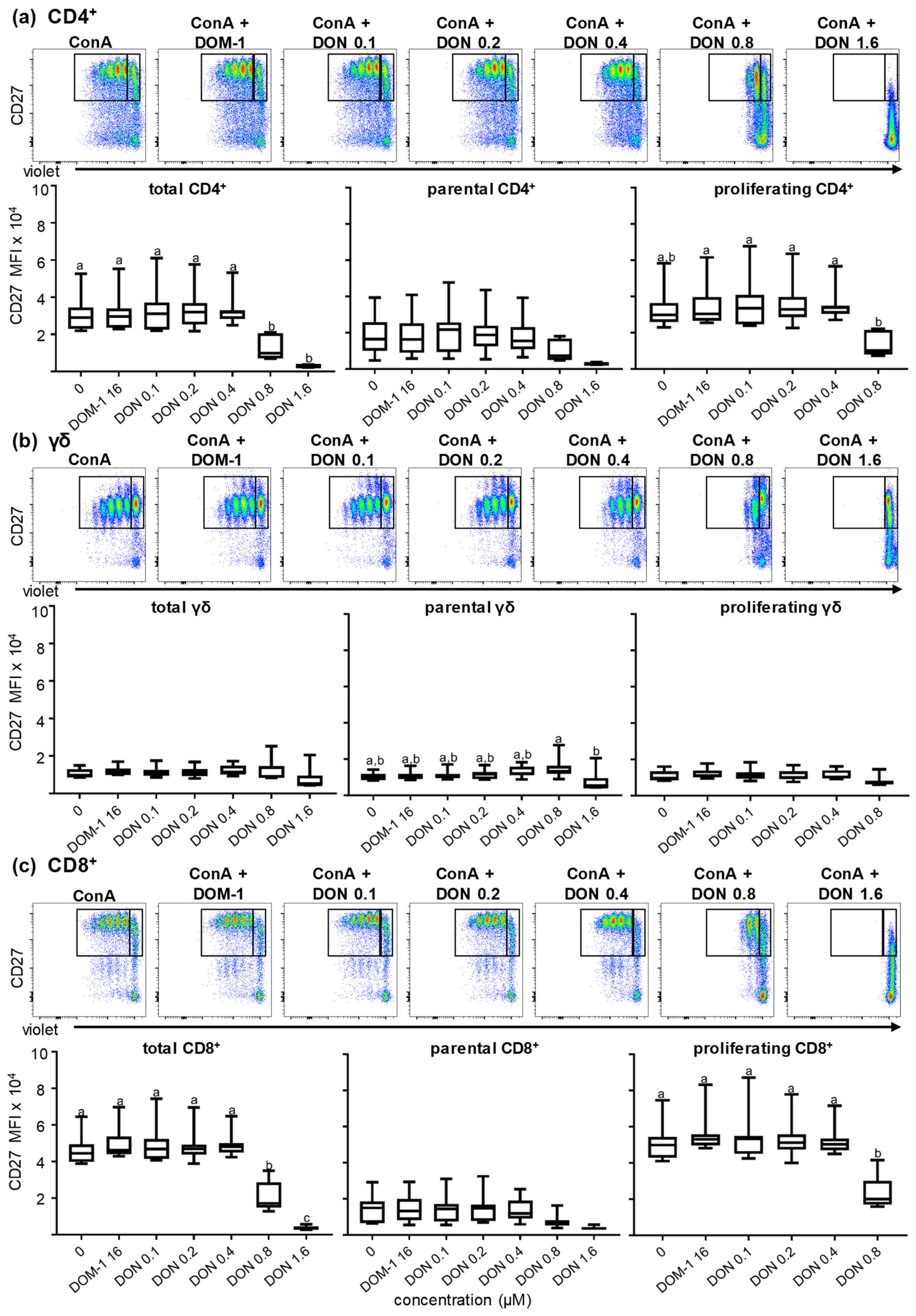
2.2. Effect of DON on the Expression of the Activation Marker CD8α and the Costimulatory Molecules CD27 and CD28 within the Major T-cell Subsets
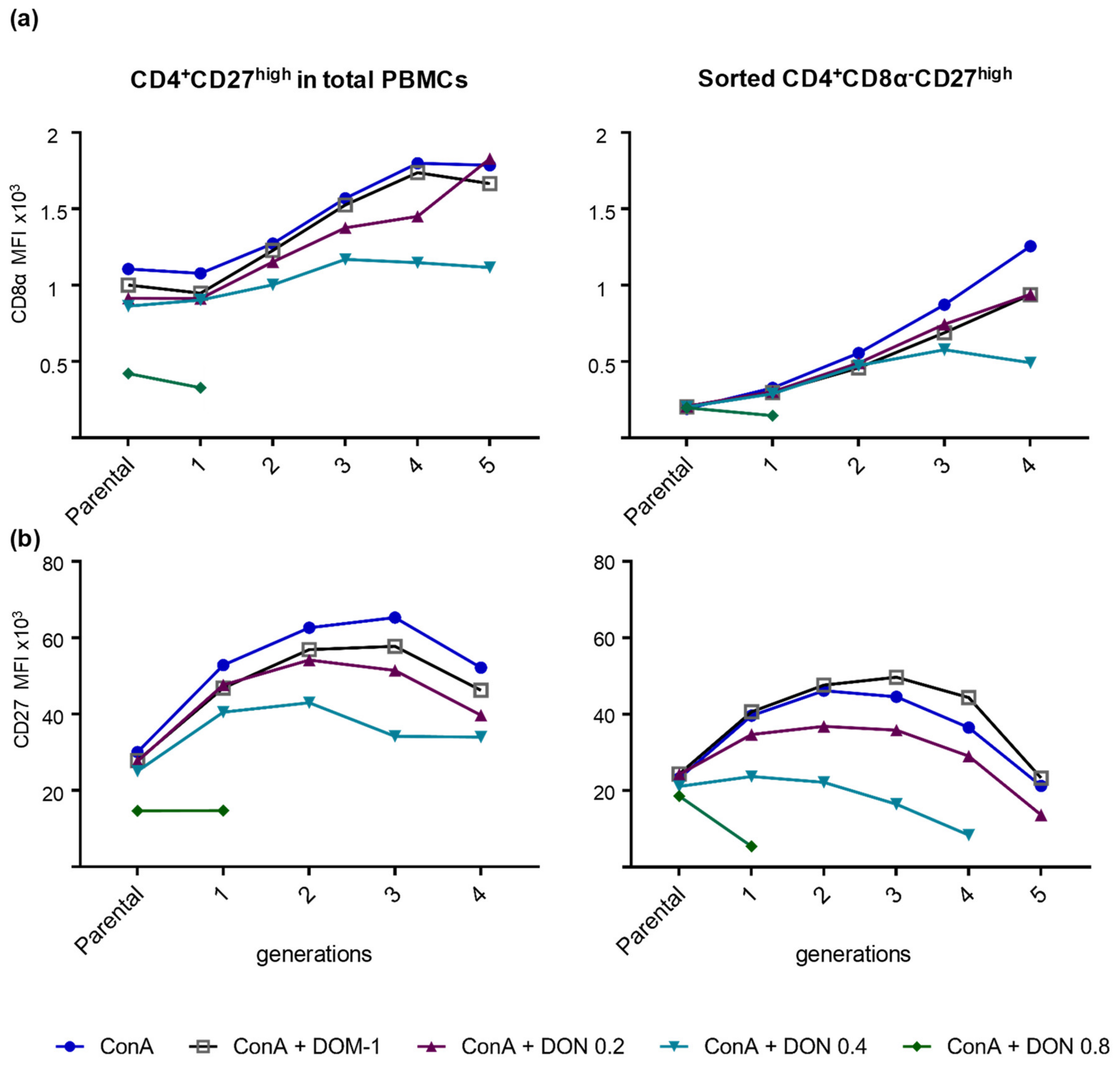
2.2.1. CD8α Expression in the Presence of Different DON Concentrations
2.2.2. CD28 Expression in the Presence of Different DON Concentrations
2.2.3. CD27 Expression in the Presence of Different DON Concentrations
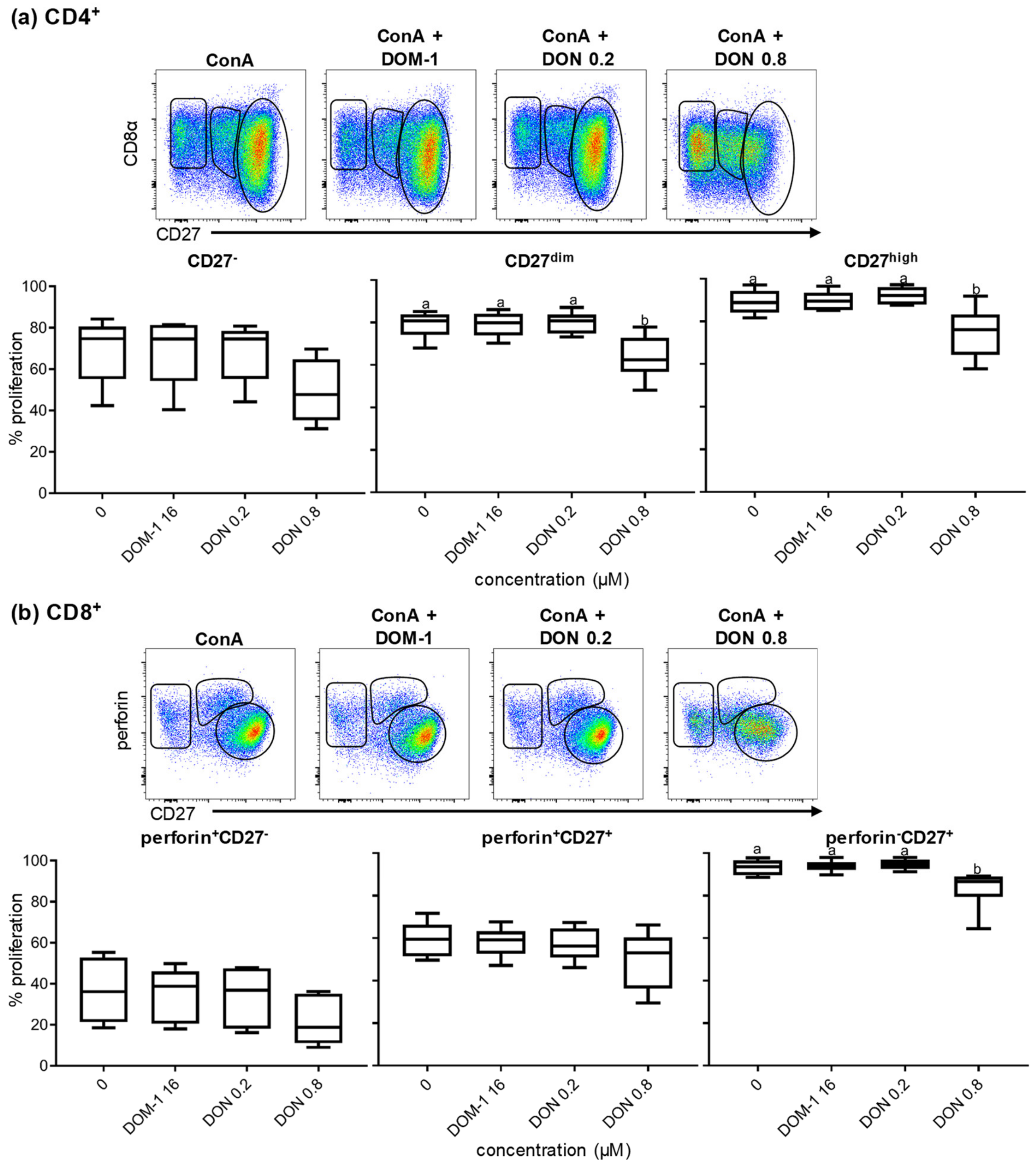
2.3. Proliferation of CD27-Defined CD4+ and CD8+ T-Cell Subsets in the Presence of DON
2.4. Phenotypic Changes in CD8α and CD27 Expression in Proliferating Sorted and Unsorted Naïve CD4+ T Cells in the Presence of DON
3. Discussion
4. Materials and Methods
4.1. Animals and Cell Isolation
4.2. Proliferation Assays
4.3. Flow Cytometry Staining and Antibodies
4.4. Fluorescence-Activated Cell Sorting (FACS) and Antibodies
4.5. Proliferation Assay and In Vitro Cultivation of Sorted T Cells
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pestka, J.J. Deoxynivalenol-induced proinflammatory gene expression: Mechanisms and pathological sequelae. Toxins 2010, 2, 1300–1317. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porcine Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Pinilla, V.; Provost, C.; Gagnon, C.A.; Chorfi, Y. In vivo effect of deoxynivalenol (DON) naturally contaminated feed on porcine reproductive and respiratory syndrome virus (PRRSV) infection. Vet. Microbiol. 2014, 174, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, H.K.; Shinozuka, J.; Islam, Z.; Pestka, J.J. Satratoxin G interaction with 40S and 60S ribosomal subunits precedes apoptosis in the macrophage. Toxicol. Appl. Pharmacol. 2009, 237, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garreau de Loubresse, N.; Prokhorova, I.; Holtkamp, W.; Rodnina, M.V.; Yusupova, G.; Yusupov, M. Structural basis for the inhibition of the eukaryotic ribosome. Nature 2014, 513, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.K.; Pestka, J.J. Deoxynivalenol induces p38 interaction with the ribosome in monocytes and macrophages. Toxicol. Sci. 2008, 105, 59–66. [Google Scholar] [CrossRef]
- Zhou, H.-R.; Islam, Z.; Pestka, J.J. Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxic trichothecene deoxynivalenol. Toxicol. Sci. 2005, 87, 113–122. [Google Scholar] [CrossRef]
- Zhou, H.-R.; Lau, A.S.; Pestka, J.J. Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol. Sci. 2003, 74, 335–344. [Google Scholar] [CrossRef]
- Taranu, I.; Marina, D.E.; Burlacu, R.; Pinton, P.; Damian, V.; Oswald, I.P. Comparative aspects of in vitro proliferation of human and porcine lymphocytes exposed to mycotoxins. Arch. Anim. Nutr. 2010, 64, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Daenicke, S.; Keese, C.; Goyarts, T.; Döll, S. Effects of deoxynivalenol (DON) and related compounds on bovine peripheral blood mononuclear cells (PBMC) in vitro and in vivo. Mycotoxin Res. 2011, 27, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Novak, B.; Vatzia, E.; Springler, A.; Pierron, A.; Gerner, W.; Reisinger, N.; Hessenberger, S.; Schatzmayr, G.; Mayer, E. Bovine peripheral blood mononuclear cells are more sensitive to deoxynivalenol than those derived from poultry and swine. Toxins 2018, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.; Xiao, Y.; Borst, J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 2003, 198, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.; Gravestein, L.A.; Tesselaar, K.; van Lier, R.A.; Schumacher, T.N.; Borst, J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000, 1, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Hendriks, J.; Xiao, Y. CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 2005, 17, 275–281. [Google Scholar] [CrossRef]
- Van Oosterwijk, M.F.; Juwana, H.; Arens, R.; Tesselaar, K.; van Oers, M.H.J.; Eldering, E.; van Lier, R.A.W. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int. Immunol. 2007, 19, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Peperzak, V.; Keller, A.M.; Borst, J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J. Immunol. 2008, 181, 1071–1082. [Google Scholar] [CrossRef]
- Reutner, K.; Leitner, J.; Essler, S.E.; Witter, K.; Patzl, M.; Steinberger, P.; Saalmüller, A.; Gerner, W. Porcine CD27: identification, expression and functional aspects in lymphocyte subsets in swine. Dev. Comp. Immunol. 2012, 38, 321–331. [Google Scholar] [CrossRef]
- Acuto, O.; Michel, F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol. 2003, 3, 939–951. [Google Scholar] [CrossRef]
- Beyersdorf, N.; Kerkau, T.; Hünig, T. CD28 co-stimulation in T-cell homeostasis: A recent perspective. Immunotargets Ther. 2015, 4, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Boise, L.H.; Minn, A.J.; Noel, P.J.; June, C.H.; Accavitti, M.A.; Lindsten, T.; Thompson, C.B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 1995, 3, 87–98. [Google Scholar] [CrossRef]
- Burr, J.S.; Savage, N.D.; Messah, G.E.; Kimzey, S.L.; Shaw, A.S.; Arch, R.H.; Green, J.M. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J. Immunol. 2001, 166, 5331–5335. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, K.; Wu, L.; Garza, K.M.; La Rose, J.; Khoo, W.; Odermatt, B.; Mak, T.W.; Ohashi, P.S.; Rottapel, R. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2001, 2, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Saalmüller, A.; Werner, T.; Fachinger, V. T-helper cells from naive to committed. Vet. Immunol. Immunopathol. 2002, 87, 137–145. [Google Scholar] [CrossRef]
- Reutner, K.; Leitner, J.; Müllebner, A.; Ladinig, A.; Essler, S.E.; Duvigneau, J.C.; Ritzmann, M.; Steinberger, P.; Saalmüller, A.; Gerner, W. CD27 expression discriminates porcine T helper cells with functionally distinct properties. Vet. Res. 2013, 44, 18. [Google Scholar] [CrossRef] [PubMed]
- Talker, S.C.; Käser, T.; Reutner, K.; Sedlak, C.; Mair, K.H.; Koinig, H.; Graage, R.; Viehmann, M.; Klingler, E.; Ladinig, A.; et al. Phenotypic maturation of porcine NK- and T-cell subsets. Dev. Comp. Immunol. 2013, 40, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, I.M.; Talker, S.C.; Käser, T.; Stadler, M.; Reiter, L.; Ladinig, A.; Milburn, J.V.; Hammer, S.E.; Mair, K.H.; Saalmüller, A.; et al. Expression of T-Bet, Eomesodermin, and GATA-3 Correlates With Distinct Phenotypes and Functional Properties in Porcine γδ T Cells. Front. Immunol. 2019, 10, 396. [Google Scholar] [CrossRef]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.-P.F.L.; Schatzmayr, G.; He, J.W.; Zhou, T.; Moll, W.-D.; et al. Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci. Rep. 2016, 6, 29105. [Google Scholar] [CrossRef] [Green Version]
- Springler, A.; Hessenberger, S.; Reisinger, N.; Kern, C.; Nagl, V.; Schatzmayr, G.; Mayer, E. Deoxynivalenol and its metabolite deepoxy-deoxynivalenol: Multi-parameter analysis for the evaluation of cytotoxicity and cellular effects. Mycotoxin Res. 2017, 33, 25–37. [Google Scholar] [CrossRef]
- Mayer, E.; Novak, B.; Springler, A.; Schwartz-Zimmermann, H.E.; Nagl, V.; Reisinger, N.; Hessenberger, S.; Schatzmayr, G. Effects of deoxynivalenol (DON) and its microbial biotransformation product deepoxy-deoxynivalenol (DOM-1) on a trout, pig, mouse, and human cell line. Mycotoxin Res. 2017, 33, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimczok, D.; Döll, S.; Rau, H.; Goyarts, T.; Wundrack, N.; Naumann, M.; Dänicke, S.; Rothkötter, H.-J. The Fusarium toxin deoxynivalenol disrupts phenotype and function of monocyte-derived dendritic cells in vivo and in vitro. Immunobiology 2007, 212, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Razafimanjato, H.; Benzaria, A.; Taïeb, N.; Guo, X.-J.; Vidal, N.; Di Scala, C.; Varini, K.; Maresca, M. The ribotoxin deoxynivalenol affects the viability and functions of glial cells. Glia 2011, 59, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Charoenpornsook, K.; Fitzpatrick, J.L.; Smith, J.E. The effects of four mycotoxins on the mitogen stimulated proliferation of bovine peripheral blood mononuclear cells in vitro. Mycopathologia 1998, 143, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Goyarts, T.; Dänicke, S.; Tiemann, U.; Rothkötter, H.-J. Effect of the Fusarium toxin deoxynivalenol (DON) on IgA, IgM and IgG concentrations and proliferation of porcine blood lymphocytes. Toxicol. In Vitro 2006, 20, 858–867. [Google Scholar] [CrossRef]
- Appay, V.; Dunbar, P.R.; Callan, M.; Klenerman, P.; Gillespie, G.M.A.; Papagno, L.; Ogg, G.S.; King, A.; Lechner, F.; Spina, C.A.; et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002, 8, 379–385. [Google Scholar] [CrossRef]
- Fritsch, R.D.; Shen, X.; Sims, G.P.; Hathcock, K.S.; Hodes, R.J.; Lipsky, P.E. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J. Immunol. 2005, 175, 6489–6497. [Google Scholar] [CrossRef]
- Appay, V.; van Lier, R.A.W.; Sallusto, F.; Roederer, M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 2008, 73, 975–983. [Google Scholar] [CrossRef]
- Wan, L.Y.M.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef]
- Yang, W.; Yu, M.; Fu, J.; Bao, W.; Wang, D.; Hao, L.; Yao, P.; Nüssler, A.K.; Yan, H.; Liu, L. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food Chem. Toxicol. 2014, 64, 383–396. [Google Scholar] [CrossRef]
- Croft, M. Co-stimulatory members of the TNFR family: Keys to effective T-cell immunity? Nat. Rev. Immunol. 2003, 3, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Nolte, M.A.; van Olffen, R.W.; van Gisbergen, K.P.J.M.; van Lier, R.A.W. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive evaluation of cytotoxicity and cellular effects. Mycotoxin Res. 2017, 33, 25–37. [Google Scholar] [CrossRef]
- Appleman, L.J.; Berezovskaya, A.; Grass, I.; Boussiotis, V.A. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J. Immunol. 2000, 164, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Saalmüller, A.; Pauly, T.; Höhlich, B.J.; Pfaff, E. Characterization of porcine T lymphocytes and their immune response against viral antigens. J. Biotechnol. 1999, 73, 223–233. [Google Scholar] [CrossRef]
- Hamann, D.; Baars, P.A.; Rep, M.H.; Hooibrink, B.; Kerkhof-Garde, S.R.; Klein, M.R.; van Lier, R.A. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997, 186, 1407–1418. [Google Scholar] [CrossRef]
- Denyer, M.S.; Wileman, T.E.; Stirling, C.M.A.; Zuber, B.; Takamatsu, H.-H. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet. Immunol. Immunopathol. 2006, 110, 279–292. [Google Scholar] [CrossRef]
- Novak, B.; Rainer, V.; Sulyok, M.; Haltrich, D.; Schatzmayr, G.; Mayer, E. Twenty-eight fungal secondary metabolites detected in pig feed samples: Their occurrence, relevance and cytotoxic effects in vitro. Toxins 2019, 11, 537. [Google Scholar] [CrossRef]
- Saalmüller, A.; Jonjic, S.; Bühring, H.J.; Reddehase, M.J.; Koszinowski, U.H. Monoclonal antibodies reactive with swine lymphocytes. II. Detection of an antigen on resting T cells down-regulated after activation. J. Immunol. 1987, 138, 1852–1857. [Google Scholar]
- Leitner, J.; Reutner, K.; Essler, S.E.; Popow, I.; Gerner, W.; Steinberger, P.; Saalmüller, A. Porcine SWC1 is CD52—Final determination by the use of a retroviral cDNA expression library. Vet. Immunol. Immunopathol. 2012, 146, 27–34. [Google Scholar] [CrossRef]
- Mair, K.H.; Stadler, M.; Talker, S.C.; Forberg, H.; Storset, A.K.; Müllebner, A.; Duvigneau, J.C.; Hammer, S.E.; Saalmüller, A.; Gerner, W. Porcine CD3(+)NKp46(+) lymphocytes have NK-cell characteristics and are present in increased frequencies in the lungs of influenza-infected animals. Front. Immunol. 2016, 7, 263. [Google Scholar] [CrossRef]


| Antigen | Clone | Isotype | Fluorochrome | Labeling Strategy | Source of Primary Ab |
|---|---|---|---|---|---|
| Analysis of co-Stimulatory Molecules | |||||
| CD3 | BB23-8E6-8C8 | IgG2a | PerCP-Cy5.5 | Directly conjugated | BD Biosciences |
| CD4 | 74-12-4 | IgG2b | FITC | Directly conjugated | BD Biosciences |
| CD8α | 11/295/33 | IgG2a | Alexa647 | Directly conjugated | In house |
| TCR-γδ | PPT16 | IgG2b | BV605 | Biotin-Streptavidin 3 | In house |
| CD28 1 | 3D11 2 | IgG1 | PE | Secondary antibody 4 | In house 2 |
| CD27 1 | b30c7 | IgG1 | PE | Secondary antibody 4 | In house |
| Analysis of Perforin, CD27 and CD8α Expression | |||||
| CD27 | b30c7 | IgG1 | Alexa647 | Secondary antibody 5 | In house |
| CD4 | 74-12-4 | IgG2b | FITC | Secondary antibody 6 | In house |
| CD8α 1 | 11/295/33 | IgG2α | PE-Cy7 | Secondary antibody 7 | In house |
| CD8β 1 | PG164a | IgG2a | PE-Cy7 | Secondary antibody 7 | In house |
| Perforin | δ-G9 | IgG2b | PerCP-eFluor710 | Directly conjugated | eBioscience |
| Antigen | Clone | Isotype | Fluorochrome | Labeling Strategy | Source of Primary Ab |
|---|---|---|---|---|---|
| Staining for Cell Sorting | |||||
| CD4 | 74-12-4 | IgG2b | FITC | Directly conjugated | BD Biosciences |
| CD27 | b30c7 | IgG1 | PE | Secondary antibody 1 | In house |
| CD8α | 11/295/33 | IgG2a | Alexa647 | Secondary antibody 2 | In house |
| CD172a | 77-22-15A | IgG2b | eFluor450 | Biotin-Streptavidin 3 | In house |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vatzia, E.; Pierron, A.; Saalmüller, A.; Mayer, E.; Gerner, W. Deoxynivalenol Affects Proliferation and Expression of Activation-Related Molecules in Major Porcine T-Cell Subsets. Toxins 2019, 11, 644. https://doi.org/10.3390/toxins11110644
Vatzia E, Pierron A, Saalmüller A, Mayer E, Gerner W. Deoxynivalenol Affects Proliferation and Expression of Activation-Related Molecules in Major Porcine T-Cell Subsets. Toxins. 2019; 11(11):644. https://doi.org/10.3390/toxins11110644
Chicago/Turabian StyleVatzia, Eleni, Alix Pierron, Armin Saalmüller, Elisabeth Mayer, and Wilhelm Gerner. 2019. "Deoxynivalenol Affects Proliferation and Expression of Activation-Related Molecules in Major Porcine T-Cell Subsets" Toxins 11, no. 11: 644. https://doi.org/10.3390/toxins11110644
APA StyleVatzia, E., Pierron, A., Saalmüller, A., Mayer, E., & Gerner, W. (2019). Deoxynivalenol Affects Proliferation and Expression of Activation-Related Molecules in Major Porcine T-Cell Subsets. Toxins, 11(11), 644. https://doi.org/10.3390/toxins11110644





