Elevation of Trimethylamine-N-Oxide in Chronic Kidney Disease: Contribution of Decreased Glomerular Filtration Rate
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Biochemical Parameters
2.2. Renal Excretion of TMAO and Precursors
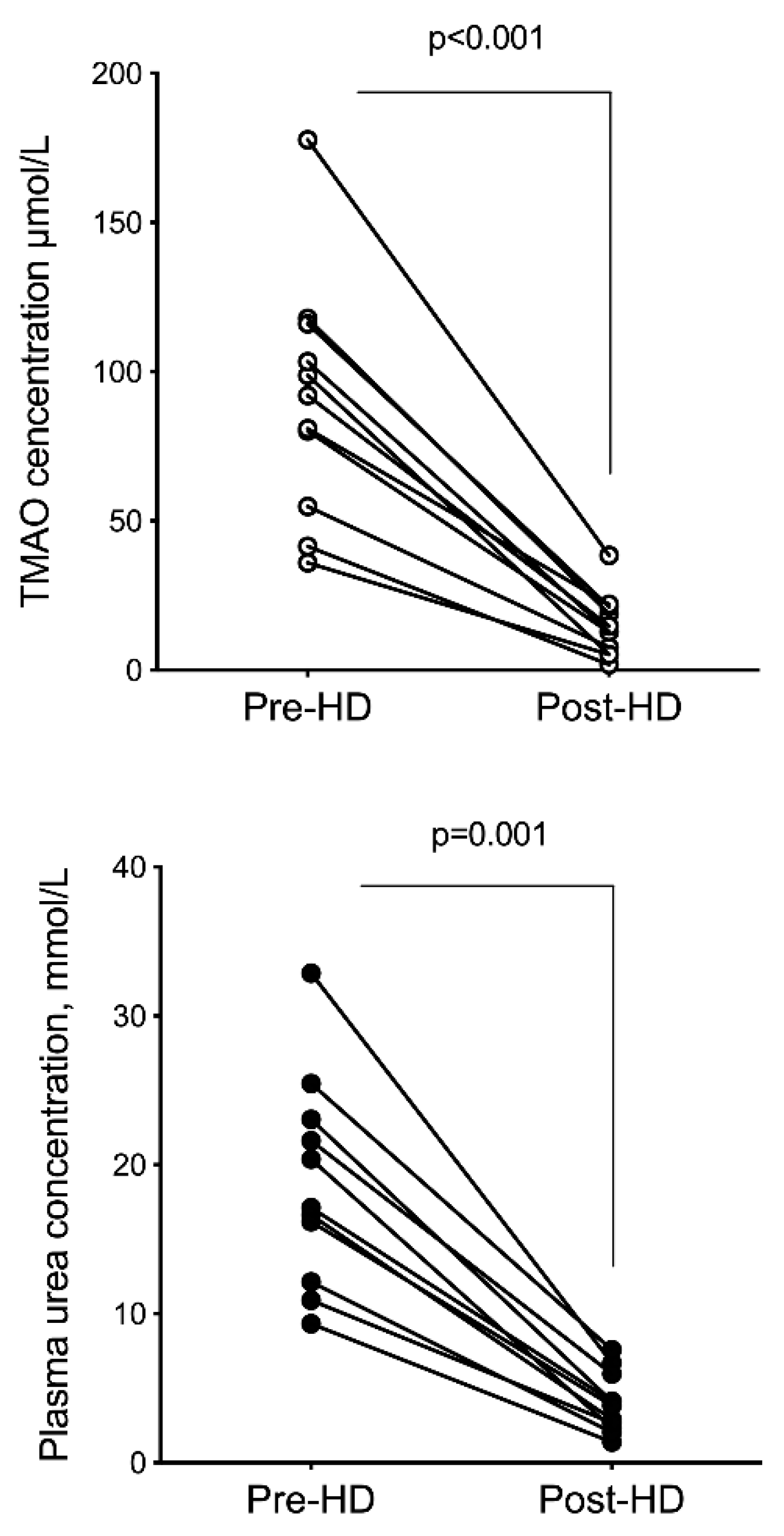
2.3. Hemodialysis Removal
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Subjects
5.2. Anthropometric Data
5.3. Blood Sampling
5.4. Glomerular Filtration Rate Assessment
5.4.1. Measured GFR (mGFR)
5.4.2. Estimated GFR (eGFR)
5.5. Clearance and Fractional Excretion Calculations
5.6. Quantification of Methylamines
5.7. Other Biochemical Measurements
5.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bain, M.A.; Fornasini, G.; Evans, A.M. Trimethylamine: Metabolic, pharmacokinetic and safety aspects. Curr. Drug Metab. 2005, 6, 227–240. [Google Scholar] [CrossRef] [PubMed]
- al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The exogenous origin of trimethylamine in the mouse. Metabolism 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Dietary precursors of trimethylamine in man: A pilot study. Food Chem. Toxicol. Int. J. 1999, 37, 515–520. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.D.; Lee, J.A.; Lee, H.A.; Sadler, P.J.; Wilkie, D.R.; Woodham, R.H. Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: Identification of trimethylamine-N-oxide. Biochim. Biophys. Acta 1991, 1096, 101–107. [Google Scholar] [CrossRef]
- De La Huerga, J.; Popper, H.; Steigmann, F. Urinary excretion of choline and trimethylamines after intravenous administration of choline in liver diseases. J. Lab. Clin. Med. 1951, 38, 904–910. [Google Scholar]
- Treacy, E.P.; Akerman, B.R.; Chow, L.M.; Youil, R.; Bibeau, C.; Lin, J.; Bruce, A.G.; Knight, M.; Danks, D.M.; Cashman, J.R.; et al. Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum. Mol. Genet. 1998, 7, 839–845. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.H.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Gul, A.; Sarnak, M.J. Cardiovascular risk factors in chronic kidney disease. Kidney Int. 2005, 68, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Bain, M.A.; Faull, R.; Fornasini, G.; Milne, R.W.; Evans, A.M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum Trimethylamine-N-Oxide is Elevated in CKD and Correlates with Coronary Atherosclerosis Burden. J. Am. Soc. Nephrol. 2016, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, C.; Hällqvist, J.; Qureshi, A.R.; Barany, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PLoS ONE 2016, 11, e0141738. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.B.; Morse, B.L.; Djurdjev, O.; Tang, M.; Muirhead, N.; Barrett, B.; Holmes, D.T.; Madore, F.; Clase, C.M.; Rigatto, C.; et al. CanPREDDICT Investigators. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016, 89, 1144–1152. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Hai, X.; Landeras, V.; Dobre, M.A.; DeOreo, P.; Meyer, T.W.; Hostetter, T.H. Mechanism of Prominent Trimethylamine Oxide (TMAO) Accumulation in Hemodialysis Patients. PLoS ONE 2015, 10, e0143731. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of Trimethylamine N-Oxide With Nutritional and Inflammatory Biomarkers and Cardiovascular Outcomes in Patients New to Dialysis. J. Ren Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef]
- Levey, A.S.; Perrone, R.D.; Madias, N.E. Serum creatinine and renal function. Annu. Rev. Med. 1988, 39, 465–490. [Google Scholar] [CrossRef]
- Klein, J.D.; Blount, M.A.; Sands, J.M. Urea transport in the kidney. Compr. Physiol. 2011, 1, 699–729. [Google Scholar] [PubMed]
- Kokko, J.P. The role of the collecting duct in urinary concentration. Kidney Int. 1987, 31, 606–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gessner, A.; König, J.; Fromm, M.F. Contribution of multidrug and toxin extrusion protein 1 (MATE1) to renal secretion of trimethylamine-N-oxide (TMAO). Sci. Rep. 2018, 8, 6659. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Mizuno, T.; Mochizuki, T.; Kimura, M.; Matsuki, S.; Irie, S.; Ieiri, I.; Maeda, K.; Kusuhara, H. Involvement of Organic Cation Transporters in the Kinetics of Trimethylamine N-oxide. J. Pharm. Sci. 2017, 106, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Teft, W.A.; Morse, B.L.; Leake, B.F.; Wilson, A.; Mansell, S.E.; Hegele, R.A.; Ho, R.H.; Kim, R.B. Identification and Characterization of Trimethylamine-N-oxide Uptake and Efflux Transporters. Mol. Pharm. 2010, 14, 310–318. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Z.; Cajka, T.; Buffa, J.A.; Nemet, I.; Hurd, A.G.; Gu, X.; Skye, S.M.; Roberts, A.B.; Wu, Y.; et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018, 3, 99096. [Google Scholar] [CrossRef]
- Johnson, C.; Prokopienko, A.J.; West, R.E.; Nolin, T.D.; Stubbs, J.R. Decreased Kidney Function Is Associated with Enhanced Hepatic Flavin Monooxygenase Activity and Increased Circulating Trimethylamine N-Oxide Concentrations in Mice. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 1304–1309. [Google Scholar] [CrossRef]
- Hartiala, J.A.; Tang, W.H.W.; Wang, Z.; Crow, A.L.; Stewart, A.F.R.; Roberts, R.; McPherson, R.; Erdmann, J.; Willenborg, C.; Hazen, S.L.; et al. Genome-wide association study and targeted metabolomics identifies sex-specific association of CPS1 with coronary artery disease. Nat. Commun 2016, 7, 10558. [Google Scholar] [CrossRef]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Martin, S.S.; Blaha, M.J.; Elshazly, M.B.; Toth, P.P.; Kwiterovich, P.O.; Blumenthal, R.S.; Jones, S.R. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013, 310, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Depner, T.A.; Daugirdas, J.T. Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J. Am. Soc. Nephrol. 1996, 7, 780–785. [Google Scholar] [PubMed]
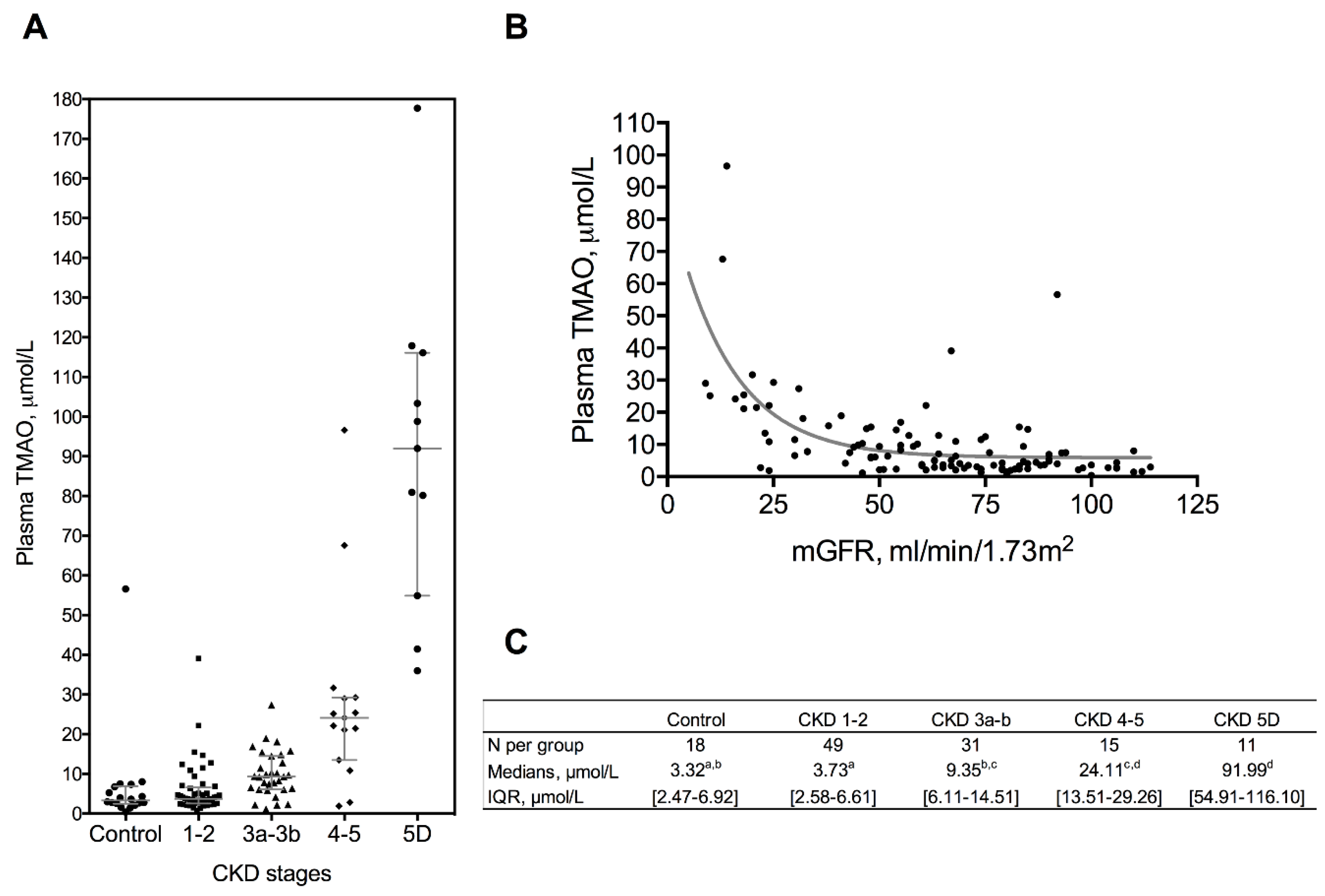
| Variable | Controls | CKD Patients | Hemodialysis | p-Value | ||
|---|---|---|---|---|---|---|
| Stage 1–2 | Stage 3a–3b | Stage 4–5 | ||||
| Sex, male/female | 13/5 | 26/23 | 9/22 | 12/3 | 7/4 | 0.0054 |
| Age, y | 41 [34–47] a | 46 [30–58] a | 64 [45–69] b,c | 50 [33–74] a,c | 62 [48–75] a,c | 0.0014 |
| BMI, kg/m2 | 24.1 [22.7–25.7] | 24.2 [20.6–26.0] | 24.5 [21.6–27.2] | 25.3 [21.0–30.9] | 22.4 [21.1–27.5] | 0.6427 |
| Systolic BP, mmHg | 127.6 ± 21.0 | 122.8 ± 17.3 | 134.2 ± 21.9 | 128.5 ± 22.3 | 115.5 ± 19.1 | 0.1538 |
| Diastolic BP, mmHg | 82.8 ± 13.5 | 77.8 ± 13.6 | 79.2 ± 13.3 | 79.7 ± 17.6 | 59.0 ± 2.8 | 0.2261 |
| Creatinine, µmol/L | 67.9 ± 14.6 a | 87.8 ± 18.0 a,b | 119.6 ± 36.6 b | 268.8 ± 113.2 c | 689.0 ± 149.8 d | <0.0001 |
| mGFR, mL/min. 1.73 m2 | 100 [93–107] a | 74 [66–83] b | 48 [41–54] c | 20 [14–24] c | NA | <0.0001 |
| Urea, mmol/L | 4.4 [3.6–6.2] a | 5.9 [5.0–7.9] a,b | 9.3 [7.0–11.7] b,c | 21.5 [11.1–30.1] c | 16.1 [13.0–24.2] c | <0.0001 |
| UA/C, mg/mmol | 0.8 [0.5–1.2] a | 1.2 [0.6–3.5] a,b | 2.5 [0.6–49.7] b,c | 38.3 [1.0–61.9] c | NA | 0.0001 |
| Uric acid, mmol/L | 252 [185–310] a | 275 [222–348] a | 317 [279–356] a,b | 555 [465–624] b | ND | 0.0008 |
| Protein intake, g/kg/day | 1.03 ± 0.34 | 0.96 ± 0.35 | 0.86 ± 0.23 | 0.81 ± 0.14 | 1.26 ± 0.47 | 0.3814 |
| Bicarbonates, mmol/L | 25.0 [24.0–27.0] a | 25.0 [23.8–27.0] a | 24.5 [23.3–26.0] a,b | 20.5 [18.0–32.8] a,b | 22.0 [20.0–24.0] b | 0.0122 |
| Proteins, g/L | 74 [71–78] a | 74 [71–78] a | 72 [66–74] a,b | 72 [66–76] a,b | 66 [65–71] b | 0.0015 |
| Triglycerides, mmol/L | 0.84 [0.66–1.11] a,c | 1.00 [0.77–1.40] a,b | 1.17 [1.00–1.54] b | 1.55 [0.81–1.95] b | 1.24 [1.04–1.53] b,c | 0.0021 |
| Total cholesterol, mmol/L | 4.82 [3.80–5.81] a,b | 5.22 [4.39–5.81] a | 4.84 [3.85–5.58] a,b | 3.99 [3.57–4.50] b | 3.26 [2.79–4.00] b | 0.0004 |
| HDL-cholesterol, mmol/L | 1.03 [0.85–1.47] | 1.16 [1.02–1.43] | 1.11 [0.98–1.29] | 0.95 [0.82–1.27] | 1.14 [0.94–1.21] | 0.1569 |
| LDL-cholesterol, mmol/L | 3.42 [2.36–4.06] a | 3.45 [2.73–3.97] a | 3.00 [2.10–3.49] a,b | 2.45 [1.71–2.81] b | 1.82 [1.22–2.16] b | <0.0001 |
| Lipid Lowering treatments, % | 0.0 | 2.4 | 30.0 | 40.0 | 81.8 | <0.0001 |
| Variable | rs | 95% CI | |
|---|---|---|---|
| Age, y | 0.30 | 0.12 to 0.47 | ** |
| BMI, kg/m2 | 0.17 | −0.02 to 0.35 | ns |
| UA/C, mg/mmol | 0.24 | 0.06 to 0.42 | ** |
| Uric Acid, µM | 0.31 | 0.10 to 0.50 | ** |
| Bicarbonates, mM | −0.22 | −0.43 to 0.01 | * |
| Protein intake, g/kg/day | 0.06 | −0.21 to 0.31 | ns |
| Triglycerides | 0.17 | −0.02 to 0.35 | ns |
| HDL-cholesterol, mmol/L | −0.10 | −0.28 to 0.09 | ns |
| LDL-cholesterol, mmol/L | −0.16 | −0.34 to 0.03 | ns |
| TMA, µmol/L | −0.14 | −0.32 to 0.06 | ns |
| Choline, µmol/L | 0.18 | −0.01 to 0.36 | ns |
| Betain, µmol/L | −0.05 | −0.23 to 0.15 | ns |
| Carnitine, µmol/L | 0.20 | 0.01 to 0.38 | * |
| Analyte | Controls | CKD Patients | Hemodialysis | p-Value | ||
|---|---|---|---|---|---|---|
| Stage 1–2 | Stage 3a–3b | Stage 4–5 | ||||
| N | 18 | 49 | 31 | 15 | 11 | |
| Choline, µmol/L | 1.10 ± 0.22 a | 1.03 ± 0.21 a | 1.11 ± 0.27 a | 1.31 ± 0.28 a | 3.32 ± 1.02 b | <0.0001 |
| Betain, µmol/L | 40.15 [29.98–56.25] | 29.90 [31.33–38.50] | 32.20 [22.40–39.20] | 33.40 [21.30–37.40] | 40.13 [24.98–58.43] | 0.0516 |
| Carnitine, µmol/L | 52.83 ± 18.55 a | 49.71 ± 13.07 a | 57.58 ± 16.91 a | 79.44 ± 31.62 b | 21.11 ± 7.73 c | <0.0001 |
| TMA, µmol/L | 0.28 [0.26–0.32] a | 0.27 [0.25–0.31] a | 0.21 [0.18–0.28] b | 0.23 [0.21–0.28] a,b | ND | <0.0001 |
| Group | Parameter | mGFR, mL/min/1.73 m2 | FR Na % | pTMAO µmol/L | Cl TMAO mL/min/1.73 m2 | FE TMAO % | pCreat µmol/L | Cl Creatinine mL/min/1.73m2 | FE Creat % | pUrea mmol/L | Cl Urea mL/min/1.73m2 | FE Urea % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | median | 98 | 99.4 | 2.4 | 109 | 103 | 72 | 125 | 127 | 4.8 | 53 | 51 |
| n = 5 | IQR | [91–105 | [98.8–99.6] | [2.1–30.7] | [50–145] | [55–144] | [68–82] | [104–148] | [114–141] | [3.7–5.5] | [39–77] | [42–76] |
| CKD stages 1–2 | median | 73 | 99.4 | 3.5 | 71 | 106 | 94 | 94 | 133 | 5.5 | 38 | 56 |
| n = 19 | IQR | [67–79] | [99.0–99.7] | [2.4–4.9] | [57–89] | [86–118] | [84–109] | [81–116] | [120–146] | [3.8–6.4] | [31–52] | [46–66] |
| CKD stages 3–5 | median | 51 | 99.1 | 9.2 | 55 | 108 | 123 | 73 | 153 | 8.3 | 30 | 61 |
| n = 8 | IQR | [46–55] | [97.9–99.5] | [5.4–14.0] | [43–67] | [98–130] | [96–151] | [61–84] | [132–161] | [6.3–10.7] | [18–44] | [51–82] |
| p-value | <0.0001 | 0.401 | 0.134 | 0.048 | 0.899 | 0.001 | 0.002 | 0.121 | 0.005 | 0.051 | 0.721 | |
| Analyte | Pre-Dialysis | Post-Dialysis | FR | p |
|---|---|---|---|---|
| TMAO, µmol/L | 90.84 ± 40.11 | 14.65 ± 10.39 | 84.91 ± 6.49 | <0.0001 |
| Choline, µmol/L | 3.32 ± 1.02 | 1.76 ± 0.64 | 46.72 ± 14.30 | <0.0001 |
| Betaine, µmol/L | 42.68 ± 17.34 | 16.34 ± 7.62 | 61.26 ± 10.53 | <0.0001 |
| Carnitine, µmol/L | 21.11 ± 7.73 | 4.45 ± 1.50 | 77.89 ± 7.29 | <0.0001 |
| Urea, mmol/L | 18.7 ± 6.93 | 3.97 ± 2.01 | 79.21 ± 5.66 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelletier, C.C.; Croyal, M.; Ene, L.; Aguesse, A.; Billon-Crossouard, S.; Krempf, M.; Lemoine, S.; Guebre-Egziabher, F.; Juillard, L.; Soulage, C.O. Elevation of Trimethylamine-N-Oxide in Chronic Kidney Disease: Contribution of Decreased Glomerular Filtration Rate. Toxins 2019, 11, 635. https://doi.org/10.3390/toxins11110635
Pelletier CC, Croyal M, Ene L, Aguesse A, Billon-Crossouard S, Krempf M, Lemoine S, Guebre-Egziabher F, Juillard L, Soulage CO. Elevation of Trimethylamine-N-Oxide in Chronic Kidney Disease: Contribution of Decreased Glomerular Filtration Rate. Toxins. 2019; 11(11):635. https://doi.org/10.3390/toxins11110635
Chicago/Turabian StylePelletier, Caroline C., Mikael Croyal, Lavinia Ene, Audrey Aguesse, Stephanie Billon-Crossouard, Michel Krempf, Sandrine Lemoine, Fitsum Guebre-Egziabher, Laurent Juillard, and Christophe O. Soulage. 2019. "Elevation of Trimethylamine-N-Oxide in Chronic Kidney Disease: Contribution of Decreased Glomerular Filtration Rate" Toxins 11, no. 11: 635. https://doi.org/10.3390/toxins11110635
APA StylePelletier, C. C., Croyal, M., Ene, L., Aguesse, A., Billon-Crossouard, S., Krempf, M., Lemoine, S., Guebre-Egziabher, F., Juillard, L., & Soulage, C. O. (2019). Elevation of Trimethylamine-N-Oxide in Chronic Kidney Disease: Contribution of Decreased Glomerular Filtration Rate. Toxins, 11(11), 635. https://doi.org/10.3390/toxins11110635






