Analysis of the Use of Cylindrospermopsin and/or Microcystin-Contaminated Water in the Growth, Mineral Content, and Contamination of Spinacia oleracea and Lactuca sativa
Abstract
1. Introduction
2. Results and Discussion
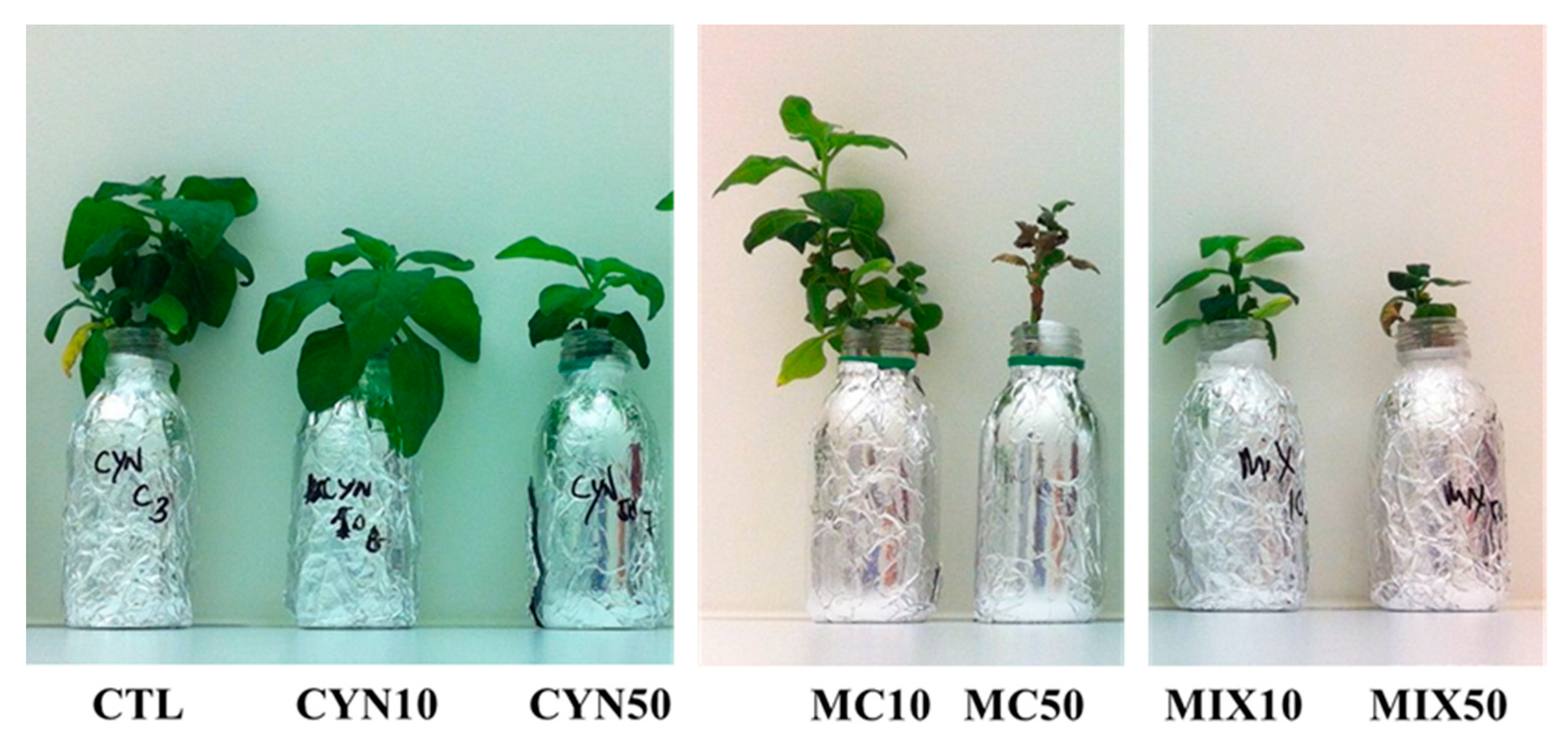
2.1. Effects of CYN, MC and CYN/MC Mixture on Spinach and Lettuce Growth
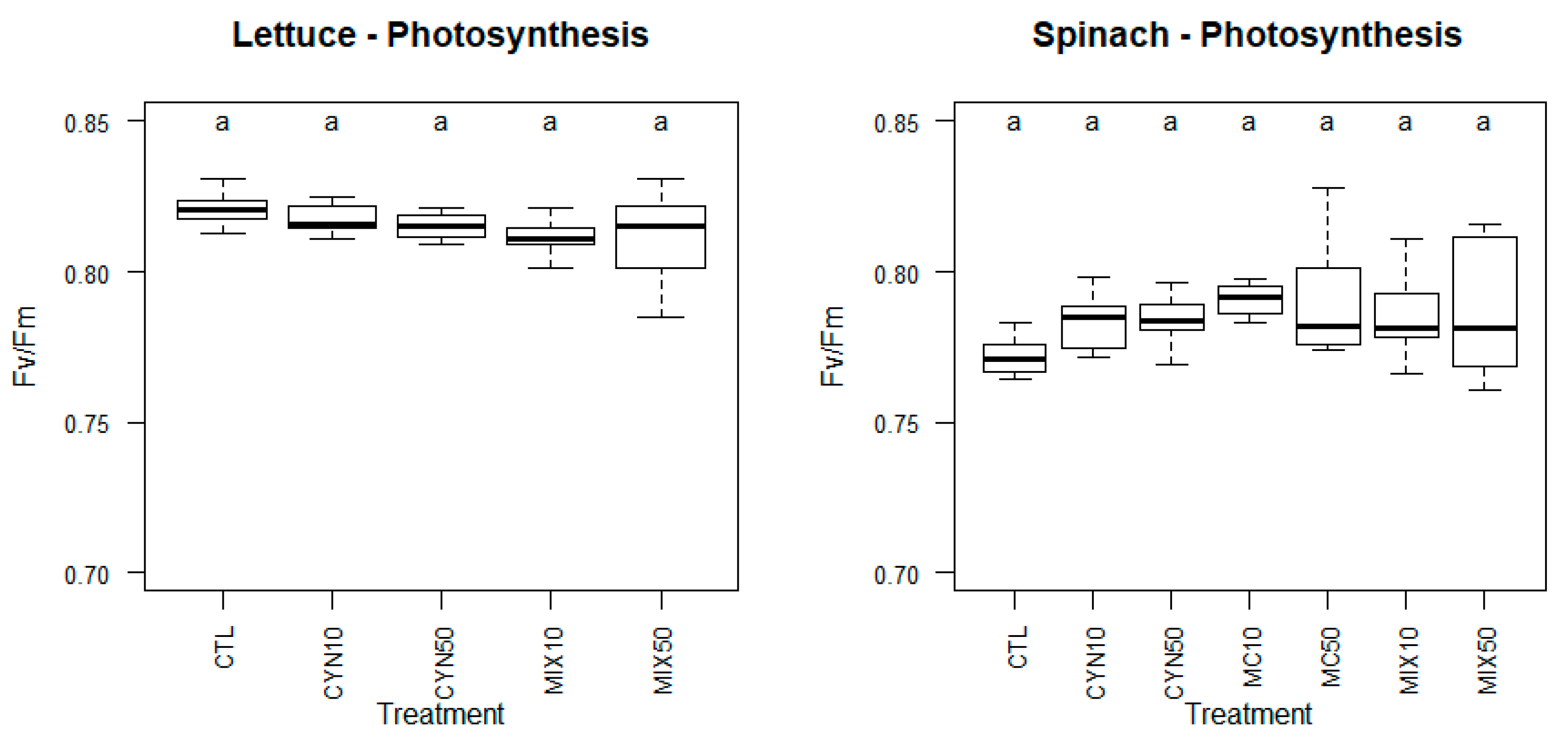
2.2. Effects of CYN, MC and CYN/MC Mixture on Lettuce and Spinach Photosynthetic Capacity
2.3. Effects of CYN, MC and CYN/MC Mixture on Lettuce and Spinach Mineral Content
2.3.1. Macronutrients
2.3.2. Micronutrients
2.4. Accumulation of CYN and MC in Plant Leaves
3. Conclusions
4. Materials and Methods
4.1. Biological Material
4.2. Cyanobacterial Crude Extracts and Quantification of MC and CYN
4.3. Exposure Experiments
4.4. Physiological Parameters: Plant Fresh Weight and Photosynthetic Capacity
4.5. Mineral Content
4.6. MC Extraction and Quantification from Spinach
4.7. CYN Extraction and Quantification from Spinach and Lettuce
4.8. CYN/MC MIX Simultaneous Extraction and Quantification from Spinach and Lettuce
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.N.; Némery, J.; Gratiot, N.; Strady, E.; Tran, V.Q.; Nguyen, A.T.; Aimé, J.; Peyne, A. Nutrient dynamics and eutrophication assessment in the tropical river system of Saigon—Dongnai (southern Vietnam). Sci. Total Environ. 2019, 653, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Catherine, A.; Bernard, C.; Spoof, L.; Bruno, M. Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, 1st ed.; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1, pp. 107–126. [Google Scholar]
- International Agency for Research on Cancer. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. In Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Publications: Lyon, France, 2010; Volume 94, pp. 1–412. [Google Scholar]
- Craig, M.; Luu, H.A.; McCready, T.L.; Williams, D.; Andersen, R.J.; Holmes, C.F.B. Molecular mechanisms underlying the interaction of motuporin and microycystins with type-1 and type-2A protein phosphatases. Biochem. Cell Biol. Biol. Cell. 1996, 74, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, T.; Zhang, Y.-L.; Guo, Z.-L.; Xu, L.-H. Effect of microcystin-LR on protein phosphatase 2A and its function in human amniotic epithelial cells. J. Zhejiang Univ. Sci. B 2011, 12, 951–960. [Google Scholar] [CrossRef]
- Christen, V.; Meili, N.; Fent, K. Microcystin-LR induces endoplasmatic reticulum stress and leads to induction of NFκB, interferon-alpha, and tumor necrosis factor-alpha. Environ. Sci. Technol. 2013, 47, 3378–3385. [Google Scholar] [CrossRef]
- Singh, S.; Asthana, R.K. Assessment of microcystin concentration in carp and catfish: A case study from Lakshmikund Pond, Varanasi, India. Bull. Environ. Contam. Toxicol. 2014, 92, 687–692. [Google Scholar] [CrossRef]
- Gkelis, S.; Zaoutsos, N. Cyanotoxin occurrence and potentially toxin producing cyanobacteria in freshwaters of Greece: A multi-disciplinary approach. Toxicon 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Trainer, V.L.; Hardy, F.J. Integrative monitoring of marine and freshwater harmful algae in Washington State for public health protection. Toxins 2015, 7, 1206–1234. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res. Rev. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Štraser, A.; Filipič, M.; Žegura, B. Genotoxic effects of the cyanobacterial hepatotoxin cylindrospermopsin in the HepG2 cell line. Arch. Toxicol. 2011, 85, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Prieto, A.I.; Maisanaba, S.; Gutiérrez-Praena, D.; Mellado-García, P.; Jos, Á.; Cameán, A.M. Mutagenic and genotoxic potential of pure Cylindrospermopsin by a battery of in vitro tests. Food Chem. Toxicol. 2018, 121, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, S.; Cameán, A.M.; Jos, A. In vitro toxicological assessment of cylindrospermopsin: A review. Toxins 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 2003, 18, 243–251. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Guzmán-Guillén, R.; Ortega, A.P.; Llana-Ruíz-Cabello, M.; Campos, A.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. New Method for Simultaneous Determination of Microcystins and Cylindrospermopsin in Vegetable Matrices by SPE-UPLC-MS/MS. Toxins 2018, 10, 406. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Chia, M.A.; do Carmo Bittencourt-Oliveira, M. Potential human health risk assessment of cylindrospermopsin accumulation and depuration in lettuce and arugula. Harmful Algae 2017, 68, 217–223. [Google Scholar] [CrossRef]
- Bihn, E.A.; Smart, C.D.; Hoepting, C.A.; Worobo, R.W. Use of Surface Water in the Production of Fresh Fruits and Vegetables: A Survey of Fresh Produce Growers and Their Water Management Practices. Food Prot. Trends 2013, 33, 307–314. [Google Scholar]
- Chen, J.; Song, L.; Dai, J.; Gan, N.; Liu, Z. Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 2004, 43, 393–400. [Google Scholar] [CrossRef]
- Pereira, S.; Saker, M.L.; Vale, M.; Vasconcelos, V.M. Comparison of sensitivity of grasses (Lolium perenne L. and Festuca rubra L.) and lettuce (Lactuca sativa L.) exposed to water contaminated with microcystins. Bull. Environ. Contam. Toxicol. 2009, 83, 81–84. [Google Scholar] [CrossRef]
- Gehringer, M.M.; Kewada, V.; Coates, N.; Downing, T.G. The use of Lepidium sativum in a plant bioassay system for the detection of microcystin-LR. Toxicon 2003, 41, 871–876. [Google Scholar] [CrossRef]
- El Khalloufi, F.; El Ghazali, I.; Saqrane, S.; Oufdou, K.; Vasconcelos, V.; Oudra, B. Phytotoxic effects of a natural bloom extract containing microcystins on Lycopersicon esculentum. Ecotoxicol. Environ. Saf. 2012, 79, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S.; Hofmann, J.; Hübner, B. Effects on growth and physiological parameters in wheat (Triticum aestivum L.) grown in soil and irrigated with cyanobacterial toxin contaminated water. Environ. Toxicol. Chem. 2007, 26, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S.; Aulhorn, M.; Grimm, B. Influence of a cyanobacterial crude extract containing microcystin-LR on the physiology and antioxidative defence systems of different spinach variants. New Phytol. 2007, 175, 482–489. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Jung, K.; Lundvall, L.; Neumann, S.; Peuthert, A. Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environ. Toxicol. Chem. 2006, 25, 2381–2387. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Barakate, A.; Codd, G.A. Inhibition of plant protein synthesis by the cyanobacterial hepatotoxin, cylindrospermopsin. FEMS Microbiol. Lett. 2004, 235, 125–129. [Google Scholar] [CrossRef]
- Máthé, C.; Vasas, G. Microcystin-LR and cylindrospermopsin induced alterations in chromatin organization of plant cells. Mar. Drugs 2013, 11, 3689–3717. [Google Scholar] [CrossRef]
- Freitas, M.; Azevedo, J.; Pinto, E.; Neves, J.; Campos, A.; Vasconcelos, V. Effects of microcystin-LR, cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture on growth, oxidative stress and mineral content in lettuce plants (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2015, 116, 59–67. [Google Scholar] [CrossRef]
- Prieto, A.; Campos, A.; Cameán, A.; Vasconcelos, V. Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol. Environ. Saf. 2011, 74, 1973–1980. [Google Scholar] [CrossRef]
- Vasas, G.; Gáspár, A.; Surányi, G.; Batta, G.; Gyémánt, G.; M-Hamvas, M.; Máthé, C.; Grigorszky, I.; Molnár, E.; Borbély, G. Capillary electrophoretic assay and purification of cylindrospermopsin, a cyanobacterial toxin from Aphanizomenon ovalisporum, by plant test (Blue-Green Sinapis Test). Anal. Biochem. 2002, 302, 95–103. [Google Scholar] [CrossRef]
- Beyer, D.; Surányi, G.; Vasas, G.; Roszik, J.; Erdodi, F.; M-Hamvas, M.; Bácsi, I.; Bátori, R.; Serfozo, Z.; Szigeti, Z.M.; et al. Cylindrospermopsin induces alterations of root histology and microtubule organization in common reed (Phragmites australis) plantlets cultured in vitro. Toxicon 2009, 54, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Mathe, C.; Garda, T.; Beyer, D.; Vasas, G. Cellular Effects of Cylindrospermopsin (Cyanobacterial Alkaloid Toxin) and its Potential Medical Consequences. Curr. Med. Chem. 2017, 24, 91–109. [Google Scholar] [CrossRef] [PubMed]
- M-Hamvas, M.; Ajtay, K.; Beyer, D.; Jámbrik, K.; Vasas, G.; Surányi, G.; Máthé, C. Cylindrospermopsin induces biochemical changes leading to programmed cell death in plants. Apoptosis 2017, 22, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.; Azevedo, J.; Freitas, M.; Pinto, E.; Almeida, A.; Vasconcelos, V.; Campos, A. Analysis of the use of microcystin-contaminated water in the growth and nutritional quality of the root-vegetable, Daucus carota. Environ. Sci. Pollut. Res. 2017, 24, 752–764. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Campos, A.; Machado, J.; Freitas, M.; Azevedo, J.; Pinto, E.; Almeida, A.; Cameán, A.M.; Vasconcelos, V. Effects of Chrysosporum (Aphanizomenon) ovalisporum extracts containing cylindrospermopsin on growth, photosynthetic capacity, and mineral content of carrots (Daucus carota). Ecotoxicology 2017, 26, 22–31. [Google Scholar] [CrossRef]
- Pereira, A.L.; Azevedo, J.; Vasconcelos, V. Assessment of uptake and phytotoxicity of cyanobacterial extracts containing microcystins or cylindrospermopsin on parsley (Petroselinum crispum L.) and coriander (Coriandrum sativum L.). Environ. Sci. Pollut. Res. 2017, 24, 1999–2009. [Google Scholar] [CrossRef]
- Freitas, M.; Campos, A.; Azevedo, J.; Barreiro, A.; Planchon, S.; Renaut, J.; Vasconcelos, V. Lettuce (Lactuca sativa L.) leaf-proteome profiles after exposure to cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture: A concentration-dependent response. Phytochemistry 2015, 110, 91–103. [Google Scholar] [CrossRef]
- El Khalloufi, F.; Oufdou, K.; Bertrand, M.; Lahrouni, M.; Oudra, B.; Ortet, P.; Barakat, M.; Heulin, T.; Achouak, W. Microbiote shift in the Medicago sativa rhizosphere in response to cyanotoxins extract exposure. Sci. Total Environ. 2016, 539, 135–142. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; El Khalloufi, F.; Benidire, L.; Albert, S.; Göttfert, M.; Caviedes, M.A.; Rodriguez-Llorente, I.D.; Oudra, B.; Pajuelo, E. Microcystin-tolerant Rhizobium protects plants and improves nitrogen assimilation in Vicia faba irrigated with microcystin-containing waters. Environ. Sci. Pollut. Res. 2016, 23, 10037–10049. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; El Khalloufi, F.; Baz, M.; Lafuente, A.; Dary, M.; Pajuelo, E.; Oudra, B. Physiological and biochemical defense reactions of Vicia faba L.-Rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ. Sci. Pollut. Res. 2013, 20, 5405–5415. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2017, 13, 998E. [Google Scholar] [CrossRef]
- El Khalloufi, F.; Oufdou, K.; Lahrouni, M.; El Ghazali, I.; Saqrane, S.; Vasconcelos, V.; Oudra, B. Allelopatic effects of cyanobacteria extracts containing microcystins on Medicago sativa-Rhizobia symbiosis. Ecotoxicol. Environ. Saf. 2011, 74, 431–438. [Google Scholar] [CrossRef] [PubMed]
- McElhiney, J.; Lawton, L.A.; Leifert, C. Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 2001, 39, 1411–1420. [Google Scholar] [CrossRef]
- Saqrane, S.; Ouahid, Y.; El Ghazali, I.; Oudra, B.; Bouarab, L.; del Campo, F.F. Physiological changes in Triticum durum, Zea mays, Pisum sativum and Lens esculenta cultivars, caused by irrigation with water contaminated with microcystins: A laboratory experimental approach. Toxicon 2009, 53, 786–796. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; Faghire, M.; Peix, A.; El Khalloufi, F.; Vasconcelos, V.; Oudra, B. Cyanobacterial extracts containing microcystins affect the growth, nodulation process and nitrogen uptake of faba bean (Vicia faba L., Fabaceae). Ecotoxicology 2012, 21, 681–687. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.Q.; Hu, L.B.; Shi, Z.Q. Microcystin-LR-induced phytotoxicity in rice crown root is associated with the cross-talk between auxin and nitric oxide. Chemosphere 2013, 93, 283–293. [Google Scholar] [CrossRef]
- Lee, S.; Jiang, X.; Manubolu, M.; Riedl, K.; Ludsin, S.A.; Martin, J.F.; Lee, J. Fresh produce and their soils accumulate cyanotoxins from irrigation water: Implications for public health and food security. Food Res. Int. 2017, 102, 234–245. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, X.; Liu, H.; Liang, C. Effect of irrigation with microcystins-contaminated water on growth and fruit quality of Cucumis sativus L. and the health risk. Agric. Water Manag. 2018, 204, 91–99. [Google Scholar] [CrossRef]
- Cao, Q.; Steinman, A.D.; Wan, X.; Xie, L. Bioaccumulation of microcystin congeners in soil-plant system and human health risk assessment: A field study from Lake Taihu region of China. Environ. Pollut. 2018, 240, 44–50. [Google Scholar] [CrossRef]
- Liang, C.; Wang, W.; Wang, Y. Effect of irrigation with microcystins-contaminated water on growth, yield and grain quality of rice (Oryza sativa). Environ. Earth Sci. 2016, 75, 505. [Google Scholar] [CrossRef]
- Corbel, S.; Bouaïcha, N.; Nélieu, S.; Mougin, C. Soil irrigation with water and toxic cyanobacterial microcystins accelerates tomato development. Environ. Chem. Lett. 2015, 13, 447–452. [Google Scholar] [CrossRef]
- Terao, K.; Ohmori, S.; Igarashi, K.; Ohtani, I.; Watanabe, M.F.; Harada, K.I.; Ito, E.; Watanabe, M. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezakia natans. Toxicon 1994, 32, 833–843. [Google Scholar] [CrossRef]
- Runnegar, M.; Berndt, N.; Kaplowitz, N. Microcystin uptake and inhibition of protein phosphatases: Effects of chemoprotectants and self-inhibition in relation to known hepatic transporters. Toxicol. Appl. Pharmacol. 1995, 134, 264–272. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Dawson, R.M. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef]
- Bolhar-Nordenkampf, H.R.; Hofer, M.; Lechner, E.G. Analysis of light-induced reduction of the photochemical capacity in field-grown plants. Evidence for photoinhibition? Photosynth. Res. 1991, 27, 31–39. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Campos, A.; Azevedo, J.; Neves, J.; Freitas, M.; Guzmán-Guillén, R.; Cameán, A.M.; Renaut, J.; Vasconcelos, V. Exposure of Lycopersicon Esculentum to microcystin-LR: Effects in the leaf proteome and toxin translocation from water to leaves and fruits. Toxins 2014, 6, 1837–1854. [Google Scholar] [CrossRef]
- do Carmo Bittencourt-Oliveira, M.; Cordeiro-Araújo, M.K.; Chia, M.A.; de Toledo Arruda-Neto, J.D.; de Oliveira, Ê.T.; dos Santos, F. Lettuce irrigated with contaminated water: Photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol. Environ. Saf. 2016, 128, 83–90. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Cary, NC, USA, 2003; ISBN 0878938230. [Google Scholar]
- Runnegar, M.T.; Xie, C.; Snider, B.B.; Wallace, G.A.; Weinreb, S.M.; Kuhlenkamp, J. In vitro hepatotoxicity of the cyanobacterial alkaloid cyclindrospermopsin and related synthetic analogues. Toxicol. Sci. 2002, 67, 81–87. [Google Scholar] [CrossRef]
- Prieto, A.I.; Guzmán-Guillén, R.; Díez-Quijada, L.; Campos, A.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. Validation of a method for cylindrospermopsin determination in vegetables: Application to real samples such as lettuce (Lactuca sativa L.). Toxins 2018, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Rivasseau, C.; Martins, S.; Hennion, M.C. Determination of some physicochemical parameters of microcystins (cyanobacterial toxins) and trace level analysis in environmental samples using liquid chromatography. J. Chromatogr. A 1998, 799, 155–169. [Google Scholar] [CrossRef]
- Valério, E.; Vasconcelos, V.; Campos, A. New Insights on the Mode of Action of Microcystins in Animal Cells—A Review. Mini-Reviews Med. Chem. 2016, 16, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Crush, J.R.; Briggs, L.R.; Sprosen, J.M.; Nichols, S.N. Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ. Toxicol. 2008, 23, 246–252. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al Shehri, A.M. Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Peuthert, A.; Chakrabarti, S.; Pflugmacher, S. Uptake of microcystins-LR and -LF (cyanobacterial toxins) in seedlings of several important agricultural plant species and the correlation with cellular damage (lipid peroxidation). Environ. Toxicol. 2007, 22, 436–442. [Google Scholar] [CrossRef]
- Kittler, K.; Schreiner, M.; Krumbein, A.; Manzei, S.; Koch, M.; Rohn, S.; Maul, R. Uptake of the cyanobacterial toxin cylindrospermopsin in Brassica vegetables. Food Chem. 2012, 133, 875–879. [Google Scholar] [CrossRef]
- Campos, A.; Araújo, P.; Pinheiro, C.; Azevedo, J.; Osório, H.; Vasconcelos, V. Effects on growth, antioxidant enzyme activity and levels of extracellular proteins in the green alga Chlorella vulgaris exposed to crude cyanobacterial extracts and pure microcystin and cylindrospermopsin. Ecotoxicol. Environ. Saf. 2013, 94, 45–53. [Google Scholar] [CrossRef]
- Jensen, M.H.; Malter, A.J. Protected Agriculture: A Global Review; World Bank Tech. Pap.; The World Bank: Washington, DC, USA, 1995; Volume 253, p. 157. [Google Scholar]
- Pinheiro, C.; Azevedo, J.; Campos, A.; Loureiro, S.; Vasconcelos, V. Absence of negative allelopathic effects of cylindrospermopsin and microcystin-LR on selected marine and freshwater phytoplankton species. Hydrobiologia 2013, 705, 27–42. [Google Scholar] [CrossRef]
- Welker, M.; Bickel, H.; Fastner, J. HPLC-PDA detection of cylindrospermopsin—Opportunities and limits. Water Res. 2002, 36, 4659–4663. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Prieto, A.I.; Moreno, I.; Soria, M.E.; Cameán, A.M. Effects of thermal treatments during cooking, microwave oven and boiling, on the unconjugated microcystin concentration in muscle of fish (Oreochromis niloticus). Food Chem. Toxicol. 2011, 49, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Egozcue, J.J.; Barceló-Vidal, C.; Martín-Ferńandez, J.A.; Jarauta-Bragulat, E.; Díaz-Barrero, J.L.; Mateu-Figueras, G. Elements of Simplicial Linear Algebra and Geometry. In Compositional Data Analysis: Theory and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 139–157. [Google Scholar]
- Parent, S.É.; Parent, L.E.; Egozcue, J.J.; Rozane, D.E.; Hernandes, A.; Lapointe, L.; Hébert-Gentile, V.; Naess, K.; Marchand, S.; Lafond, J.; et al. The plant ionome revisited by the nutrient balance concept. Front. Plant Sci. 2013, 4, 39. [Google Scholar] [CrossRef] [PubMed]

| Levels of MC and CYN in Tissues of Lettuce and Spinach | CYN10 | CYN50 | MC10 | MC50 | MIX10 | MIX50 | MIX10 | MIX50 | |
|---|---|---|---|---|---|---|---|---|---|
| CYN | CYN | MC | MC | MC | MC | CYN | CYN | ||
| (µg/kg fw) | (µg/kg fw) | (µg/kg fw) | (µg/kg fw) | (µg/kg fw) | (µg/kg fw) | (µg/kg fw) | (µg/kg fw) | ||
| Lettuce | leaves | 2.4 ± 0.89 | 9.4 ± 2.38 | nd | nd | < LOD | < LOD | 10.00 ± 2.40 | 41.92 ± 6.37 |
| roots | nd | nd | nd | nd | 0.22 ± 0.08 | 1.10 ± 0.25 | 34.23 ± 11.58 | 110.00 ± 33.29 | |
| Spinach | leaves | 9.52 ± 3.56 | 36.97 ± 10.25 | < LOD | < LOD | < LOD | < LOD | 12.57 ± 4.22 | 119.69 ± 43.93 |
| roots | nd | nd | nd | nd | 0.53 ± 0.32 | 1.31 ± 0.14 | 39.16 ± 31.30 | 24.00 ± 3.76 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llana-Ruiz-Cabello, M.; Jos, A.; Cameán, A.; Oliveira, F.; Barreiro, A.; Machado, J.; Azevedo, J.; Pinto, E.; Almeida, A.; Campos, A.; et al. Analysis of the Use of Cylindrospermopsin and/or Microcystin-Contaminated Water in the Growth, Mineral Content, and Contamination of Spinacia oleracea and Lactuca sativa. Toxins 2019, 11, 624. https://doi.org/10.3390/toxins11110624
Llana-Ruiz-Cabello M, Jos A, Cameán A, Oliveira F, Barreiro A, Machado J, Azevedo J, Pinto E, Almeida A, Campos A, et al. Analysis of the Use of Cylindrospermopsin and/or Microcystin-Contaminated Water in the Growth, Mineral Content, and Contamination of Spinacia oleracea and Lactuca sativa. Toxins. 2019; 11(11):624. https://doi.org/10.3390/toxins11110624
Chicago/Turabian StyleLlana-Ruiz-Cabello, Maria, Angeles Jos, Ana Cameán, Flavio Oliveira, Aldo Barreiro, Joana Machado, Joana Azevedo, Edgar Pinto, Agostinho Almeida, Alexandre Campos, and et al. 2019. "Analysis of the Use of Cylindrospermopsin and/or Microcystin-Contaminated Water in the Growth, Mineral Content, and Contamination of Spinacia oleracea and Lactuca sativa" Toxins 11, no. 11: 624. https://doi.org/10.3390/toxins11110624
APA StyleLlana-Ruiz-Cabello, M., Jos, A., Cameán, A., Oliveira, F., Barreiro, A., Machado, J., Azevedo, J., Pinto, E., Almeida, A., Campos, A., Vasconcelos, V., & Freitas, M. (2019). Analysis of the Use of Cylindrospermopsin and/or Microcystin-Contaminated Water in the Growth, Mineral Content, and Contamination of Spinacia oleracea and Lactuca sativa. Toxins, 11(11), 624. https://doi.org/10.3390/toxins11110624













