In Vitro Activity of Neem (Azadirachta indica) Oil on Growth and Ochratoxin A Production by Aspergillus carbonarius Isolates
Abstract
1. Introduction
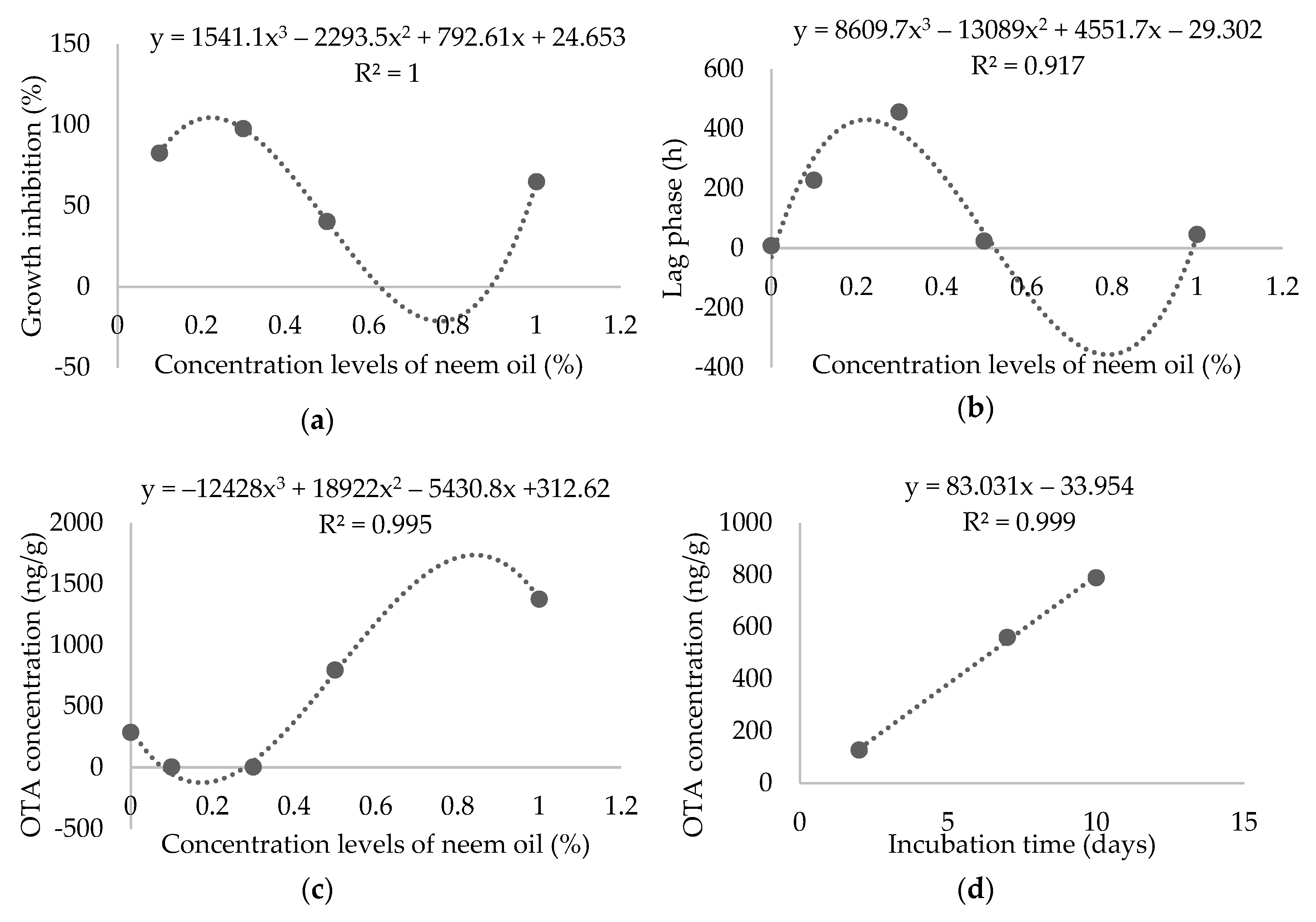
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Strains
5.2. Culture Medium
5.3. Inoculation and Incubation Conditions
5.4. Growth Assessment
5.5. Ochratoxin A Extraction from Culture
5.6. OTA Detection and Quantification
5.7. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Pitt, J.I. Toxigenic fungi and mycotoxins. Br. Med. Bull. 2000, 56, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Gams, W.; Christensen, M.; Onions, A.H.S.; Pitt, J.I.; Samson, R.A. Infrageneric taxa of Aspergillus. In Advances in Penicillium and Aspergillus Systematics; Samson, R.A., Pitt, J.I., Eds.; Plenum: New York, NY, USA, 1985; pp. 55–61. [Google Scholar]
- Varga, J.; Frisvad, J.C.; Kocsubé, S.; Brankovics, B.; Tóth, B.; Sziget, G.; Samson, R.A. New and revisited species in Aspergillus section Nigri. Stud. Mycol. 2011, 69, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus species and mycotoxins: Occurrence and importance in major food commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Cabañes, F.J.; Bragulat, M.R. Black aspergilli and ochratoxin A-producing species in foods. Curr. Opin. Food Sci. 2018, 23, 1–10. [Google Scholar] [CrossRef]
- CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems, n. 139; CAST: Motor City, IA, USA, 2003; p. 199. [Google Scholar]
- Tao, Y.; Xiea, S.; Xua, F.; Liub, A.; Wangb, Y.; Chenb, D.; Pana, Y.; Huangb, L.; Penga, D.; Wanga, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v. 56. 1993. Available online: https://monographs.iarc.fr/iarc-monographs-on-the-evaluation-of-carcinogenic-risks-to-humans-65/ (accessed on 4 April 2018).
- Pfohl-Leszkowicz, A.; Petkova-Bocharova, T.; Chernozemsky, I.N.; Castegnaro, M. Balkan endemic nephropathy and associated urinary tract tumours: A review on aetiological causes and the potential role of mycotoxins. Food Addit. Contam. 2002, 19, 282–302. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.; Ricke, A.S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential Oils as Feed Additives-Future Perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef]
- Pawar, V.C.; Thaker, V.S. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 2007, 49, 316–323. [Google Scholar] [CrossRef] [PubMed]
- El-Nagerabi, S.A.F.; Al-Bahry, S.N.; Elshafie, A.E.; AlHilali, S. Effect of Hibiscus sabdariffa extract and Nigella sativa oil on the growth and aflatoxin B1 production of Aspergillus flavus and Aspergillus parasiticus strains. Food Control 2012, 25, 59–63. [Google Scholar] [CrossRef]
- Ferreira, F.D.; Kemmelmeier, C.; Arrotéia, C.C.; Costa, C.L.; Mallmann, C.A.; Janeiro, V.; Ferreira, F.M.; Mossini, S.A.; Silva, E.L.; Machinski, M., Jr. Inhibitory effect of the essential oil of Curcuma longa L. and curcumin on aflatoxin production by Aspergillus flavus Link. Food Chem. 2013, 136, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Passone, M.A.; Girardi, N.S.; Etcheverry, M. Antifungal and antiaflatoxigenic activity by vapor contact of three essential oils, and effects of environmental factors on their efficacy. LWT Food Sci. Technol. 2013, 53, 434–444. [Google Scholar] [CrossRef]
- Manso, S.; Pezo, D.; Gómez-Lus, R.; Nerín, C. Diminution of aflatoxin B1 production caused by an active packaging containing cinnamon essential oil. Food Control 2014, 45, 101–108. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B. Neem (Azadirachta indica): An Indian traditional panacea with modern molecular basis. Phytomedicine 2017, 34, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Quelemes, P.V.; Perfeito, M.L.G.; Guimarães, M.A.; dos Santos, R.C.; Lima, D.F.; Nascimento, C.; Silva, M.P.N.; Soares, M.J.S.; Ropke, C.D.; Eaton, P.; et al. Effect of neem (Azadirachta indica A. Juss) leaf extract on resistant Staphylococcus aureus biofilm formation and Schistosoma mansoni worms. J. Ethnopharmacol. 2015, 175, 287–294. [Google Scholar] [CrossRef]
- Suresh, G.; Narasimhan, N.S.; Masilamani, S.; Partho, R.D.; Gopalakrishnan, G. Antifungal Fractions and Compounds from Uncrushed Green Leaves of Azadirachta indica. Phytoparasitica 1997, 25, 33–39. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Afshan, F.; Ghiasuddin, S.F.; Naqvi, S.N.H.; Tariq, R.M. New insect-growth-regulator meliacin butenolides from the leaves of Azadirachta indica A. Juss. J. Chem. Soc. Perkin Trans. 1999, 16, 2367–2370. [Google Scholar] [CrossRef]
- Van der Nat, J.M.; van der Sluis, W.G.; Hart, L.A.; Dijk, H.V.; de Silva, K.T.D.; Labadie, R.P. Activity-Guided Isolation and Identification of Azadirachta indica Bark Extract Constituents which Specifically Inhibit Chemiluminescence Production by Activated Human Polymorphonuclear Leukocytes. Planta Med. 1991, 57, 65–68. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Suresh, G.; Gopalakrishnan, G.; Banumathy, B.; Masilamani, S. Identification of Antifungal Compounds from the Seed Oil of Azadirachta indica. Phytoparasitica 1998, 26, 109–116. [Google Scholar] [CrossRef]
- Roychoudhury, R. Chapter 18—Neem Products. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 545–562. [Google Scholar] [CrossRef]
- Brahmachari, G. Neem–An Omnipotent Plant: A Retrospection. ChemBioChem 2004, 5, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Del Serrone, P.; Failla, S.; Nicoletti, M. Natural control of bacteria affecting meat quality by a neem (Azadirachta indica A. Juss) cake extract. Nat. Prod. Res. 2015, 29, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Al Akeel, R.; Mateen, A.; Janardhan, K.; Gupta, V.C. Analysis of anti-bacterial and anti oxidative activity of Azadirachta indica bark using various solvents extracts. Saudi J. Biol. Sci. 2017, 24, 11–14. [Google Scholar] [CrossRef]
- Al Saiqali, M.; Tangutur, A.D.; Banoth, C.; Bhukya, B. Antimicrobial and anticancer potential of low molecular weight polypeptides extracted and characterized from leaves of Azadirachta indica. Int. J. Biol. Macromol. 2018, 114, 906–921. [Google Scholar] [CrossRef]
- Parida, M.M.; Upadhyay, C.; Pandya, G.; Jana, A.M. Inhibitory potential of neem (Azadirachta indica Juss) leaves on Dengue virus type-2 replication. J. Ethnopharmacol. 2002, 79, 273–278. [Google Scholar] [CrossRef]
- Chaube, S.K.; Shrivastav, T.G.; Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.K. Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. SpringerPlus 2014, 3, 464. [Google Scholar] [CrossRef]
- European Commission. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.ViewReview&id=721 (accessed on 13 August 2019).
- Gowda, N.K.S.; Malathi, V.; Suganthi, R.U. Effect of some chemical and herbal compounds on growth of Aspergillus parasiticus and aflatoxin production. Anim. Feed Sci. Tech. 2004, 116, 281–291. [Google Scholar] [CrossRef]
- Zeringue, H.J.; Bhatnagar, D. Effects of neem leaf volatiles on submerged cultures of aflatoxigenic Aspergillus parasiticus. Appl. Environ. Microbiol. 1994, 60, 3543–3547. [Google Scholar]
- Razzaghi-Abyaneh, M.; Allameh, A.; Tiraihi, T.; Shams-Ghahfarokhi, M.; Ghorbanian, M. Morphological alterations in toxigenic Aspergillus parasiticus exposed to neem (Azadirachta indica) leaf and seed aqueous extracts. Mycopathologia 2005, 159, 565–570. [Google Scholar] [CrossRef]
- Sitara, U.; Niaz, I.; Naseem, J. Antifungal effect of essential oils on in vitro growth of pathogenic fungi. Pak. J. Bot. 2008, 40, 409–414. [Google Scholar]
- Bhatnagar, D.; McCormick, S.P. The Inhibitory Effect of Neem (Azadirachta indica) Leaf Extracts on Aflatoxin Synthesis in Aspergillus parasiticus. J. Am. Oil Chem. Soc. 1988, 65, 1166–1168. [Google Scholar] [CrossRef]
- Zeringue, H.J.; Bhatnagar, D. Inhibition of Aflatoxin Production in Aspergillus flavus Infected Cotton Bolls After Treatment with Neem (Azadirachta indica) Leaf Extracts. J. Am. Oil Chem. Soc. 1990, 67, 215–216. [Google Scholar] [CrossRef]
- Zeringue, H.J.; Shih, B.Y.; Bhatnagar, D. Effects of clarified neem oil on growth and aflatoxin B formation in submerged and plate cultures of aflatoxigenic Aspergillus spp. Phytoparasitica 2001, 29, 1–4. [Google Scholar] [CrossRef]
- Garcia, R.P.; Garcia, M.I. Laboratory evaluation of neem derivatives against Aspergillus growth and aflatoxin formation. Philipp. Agric. Sci. 1990, 73, 333–342. [Google Scholar]
- Bok, J.W.; Keller, N.P. LaeA, a Regulator of Secondary Metabolism in Aspergillus spp. Eukaryot. Cell 2004, 3, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Lind, A.L.; Smith, T.D.; Saterlee, T.; Calvo, A.M.; Rokas, A. Regulation of Secondary Metabolism by the Velvet Complex is Temperature-Responsive in Aspergillus. Genes Genomes Genet. 2016, 6, 4023–4033. [Google Scholar]
- Roze, L.V.; Chanda, A.; Linz, J.E. Compartmentalization and molecular traffic in secondary metabolism: A new understanding of established cellular processes. Fungal Genet. Biol. 2011, 48, 35–48. [Google Scholar] [CrossRef]
- Arroteia, C.C.; Kemmelmeier, C.; Junior, M.M. Effect of aqueous and oily extracts of Neem [Azadirachta indica A. Juss (Meliaceae)] on patulin production in apples contaminated with Penicillium expansum. Ciência Rural. 2007, 37, 1518–1523. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Allameh, A.; Razzaghi-abyane, M.; Shams, M.; Rezaee, M.B.; Jaimand, K. Effects of neem leaf extract on production of aflatoxins and activities of fatty acid synthetase, isocitrate dehydrogenase and glutathione S-transferase in Aspergillus parasiticus. Mycopathologia 2002, 154, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Zaika, L.L.; Buchanan, R.L. Review of compounds affecting the biosynthesis or bioregulation of aflatoxins. J. Food Prot. 1987, 50, 691–708. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, C.; Violante, M.; Combina, M.; Palacio, G.; Dalcero, A. Mycoflora and ochratoxin-producing strains of Aspergillus section Nigri in wine grapes in Argentina. Lett. Appl. Microbiol. 2004, 39, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Bragulat, M.R.; Abarca, M.L.; Cabañes, F.J. An easy screening method for fungi producing ochratoxin A in pure culture. Int. J. Food Microbiol. 2001, 71, 139–144. [Google Scholar] [CrossRef]
- Scudamore, K.A.; MacDonald, S.J. A collaborative study of an HPLC method for determination of ochratoxin A in wheat using immunoaffinity column clean-up. Food Addit. Contam. 1998, 15, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.P.; Keough, M.J. (Eds.) Experimental Design Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; pp. 1–537. [Google Scholar]
| Strains | Concentration Levels of Neem Oil (%) | |||
|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | 1.0 | |
| FRR5690 | 73.7 ± 8.5 | 96.3 ± 0.3 | 45.1 ± 1.7 | 58.5 ± 1.7 |
| A2034 | 91.1 ± 1.1 | 98.8 ± 0 | 34.5 ± 2.8 | 70.8 ± 3.1 |
| RCG1 | 97.8 ± 2.2 | 100 ± 0 | 47.3 ± 9.3 | 62.2 ± 4.0 |
| RCG2 | 87.1 ± 0.6 | 98.9 ± 0 | 36.4 ± 4.4 | 65.6 ± 4.9 |
| RCG3 | 96.3 ± 2.3 | 98.2 ± 0.6 | 40.9 ± 1.1 | 61.5 ± 3.6 |
| RCG4 | 49.2 ± 4.5 | 93.7 ± 1.3 | 37.1 ± 1.7 | 69.5 ± 2.9 |
| Means ± SD | 82.5 ± 17.7 b | 97.6 ± 2.2 a | 40.2 ± 6.1 d | 64.7 ± 5.4 c |
| Source | df | Type III SS | MS | F | p-Value |
|---|---|---|---|---|---|
| (C) | 3 | 32,923.07 | 10,974.36 | 118.79 | <0.0001* |
| Covariate: (ST) | 1 | 340.49 | 340.49 | 3.69 | 0.0591 |
| Error | 67 | 6189.78 | 92.38 | ||
| Contrast | df | Contrast SS | MS | F | p-value |
| (C)-linear | 1 | 11,077.80 | 11,077.80 | 119.91 | <0.0001* |
| (C)-quadratic | 1 | 392.93 | 392.93 | 4.25 | 0.0431* |
| (C)-cubic | 1 | 21,452.34 | 21,452.34 | 232.21 | <0.0001* |
| Parameter | Estimate | SE | p-value | ||
| (C)-linear | −110.94 | 10.13 | <0.0001* | ||
| (C)-quadratic | 9.34 | 4.53 | 0.0431* | ||
| (C)-cubic | 154.39 | 10.13 | <0.0001* |
| Strains | Concentration Levels of Neem Oil (%) | ||||
|---|---|---|---|---|---|
| 0 (Control) | 0.1 | 0.3 | 0.5 | 1.0 | |
| FRR5690 | 7.4 ± 3.8 | 101.7 ± 27.0 | ≥540 | 29.6 ± 1.3 | 52.5 ± 2.6 |
| A2034 | 5.6 ± 1.7 | 53.2 ± 8.2 | ≥540 | 16.6 ± 4.2 | 40.7 ± 4.5 |
| RCG1 | 13.1 ± 0.8 | 531.7 ± 14.4 | ≥540 | 31.9 ± 6.2 | 40.5 ± 6.7 |
| RCG2 | 8.4 ± 2.0 | 87.6 ± 4.1 | ≥540 | 26.5 ± 2.0 | 45.8 ± 3.3 |
| RCG3 | 2.9 ± 2.6 | ≥540 | ≥540 | 18.0 ± 3.7 | 41.3 ± 2.8 |
| RCG4 | 6.5 ± 0.5 | 154.0 ± 15.4 | 36.0 ± 6.9 | 15.7 ± 3.6 | 47.9 ± 0.6 |
| Means ± SD | 7.5 ± 3.7 c | 226.6 ± 207.5 b | 456.2 ± 193.3 a | 23.2 ± 7.4 c | 44.9 ± 5.6 c |
| Source | df | Type III SS | MS | F | p-Value |
|---|---|---|---|---|---|
| (C) | 4 | 2,640,845.40 | 660,211.35 | 42.09 | <0.0001* |
| Covariate: (ST) | 1 | 23,815.89 | 23,815.89 | 1.52 | 0.2213 |
| Error | 83 | 1,301,845.28 | 15,684.88 | ||
| Contrast | df | Contrast SS | MS | F | p-value |
| (C)-linear | 1 | 29,536.64 | 29,536.64 | 1.88 | 0.1737 |
| (C)-quadratic | 1 | 1,431,427.76 | 1,431,427.76 | 91.26 | <0.0001* |
| (C)-cubic | 1 | 347,184.26 | 347,184.26 | 22.13 | <0.0001* |
| Parameter | Estimate | SE | p-value | ||
| (C)-linear | −128.48 | 93.62 | 0.1737 | ||
| (C)-quadratic | −1057.38 | 110.68 | <0.0001* | ||
| (C)-cubic | 444.36 | 94.45 | <0.0001* |
| Strains | Incubation Time (Days) | OTA Concentration (ng/g) | ||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.3 | 0.5 | 1.0 | ||
| FRR5690 | 2 | 62.5 ± 4.7 | Nd | Nd | 368.8 ± 34.3 | Nd |
| 7 | 267.9 ± 46.5 | Nd | Nd | 637.7 ± 35.4 | 2817.2 ± 219.9 | |
| 10 | 334.3 ± 7.4 | Nd | 28.2 ± 3.2 | 986.3 ± 86.1 | 2954.7 ± 54.4 | |
| A2034 | 2 | 117.9 ± 2.1 | Nd | Nd | 468.2 ± 12.1 | 110.5 ± 4.0 |
| 7 | 225.5 ± 10.0 | 13.8 ± 0.0 | Nd | 624.4 ± 18.4 | 454.5 ± 4.5 | |
| 10 | 325.7 ± 20.3 | 22.2 ± 2.2 | Nd | 741.3 ± 21.6 | 720.2 ± 1.3 | |
| RCG1 | 2 | 201.6 ± 2.2 | Nd | NG | 232.2 ± 25.9 | 168.0 ± 12.2 |
| 7 | 278.8 ± 10.8 | Nd | NG | 358.0 ± 8.0 | 268.3 ± 0.6 | |
| 10 | 396.3 ± 7.8 | Nd | NG | 432.9 ± 2.0 | 258.5 ± 26.5 | |
| 2 | 102.6 ± 6.4 | Nd | Nd | 512.7 ± 30.7 | 160.2 ± 11.0 | |
| RCG2 | 7 | 573.2 ± 19.2 | Nd | Nd | 2281.2 ± 79.2 | 1808.3 ± 50.4 |
| 10 | 758.6 ± 58.6 | Nd | Nd | 1785.0 ± 10.2 | 6164.4 ± 329.4 | |
| 2 | 227.8 ± 49.8 | Nd | Nd | 232.5 ± 56.6 | 280.1 ± 81.4 | |
| RCG3 | 7 | 384.3 ± 25.8 | Nd | Nd | 2232.6 ± 273.4 | 1904.2 ± 155.8 |
| 10 | 528.6 ± 33.0 | Nd | Nd | 1403.5 ± 300.7 | 3054.8 ± 199.9 | |
| 2 | 86.4 ± 7.5 | Nd | Nd | 542.9 ± 41.1 | Nd | |
| RCG4 | 7 | 160.7 ± 6.1 | Nd | Nd | 183.5 ± 5.5 | 1507.4 ± 9.4 |
| 10 | 203.8 ± 6.8 | Nd | Nd | 278.5 ± 16.5 | 2103.7 ± 102.9 | |
| 2 | 122.3 ± 68.8 cC | Nd dC | 0.8 ± 0.4 dC | 392.9 ± 132.8 bC | 120.1 ± 105.1 aC | |
| Means ± SD | 7 | 309.6 ± 142.2 cB | 3.13 ± 4.9 dB | 0.8 ± 0.4 dB | 1025.2 ± 869.7bB | 1460.0 ± 905.3 aB |
| 10 | 424.6 ± 184.7 cA | 4.5 ± 8.2 dA | 5.4 ± 10.6 dA | 965.7 ± 597.7 bA | 2542.7 ± 1987.7 aA | |
| Source | df | Type III SS | MS | F | p-Value |
|---|---|---|---|---|---|
| (C) | 4 | 75,146,241.75 | 18,786,560.44 | 47.27 | <0.0001* |
| (T) | 2 | 20,290,698.84 | 10,145,349.42 | 25.52 | <0.0001* |
| Covariate: (ST) | 1 | 569,135.62 | 569,135.62 | 1.43 | 0.2326 |
| (C)* (T) | 8 | 37,954,380.17 | 4,744,297.52 | 11.94 | <0.0001* |
| Error | 254 | 100,956,999.01 | |||
| Contrast | df | Contrast SS | MS | F | p-value |
| (C)-linear | 1 | 47,609,459.27 | 47,609,459.27 | 119.78 | <0.0001* |
| (C)-quadratic | 1 | 24,443,018.97 | 24,443,018.97 | 61.50 | <0.0001* |
| (C)-cubic | 1 | 1,320,779.85 | 1,320,779.85 | 3.32 | 0.0695 |
| Parameter | Estimate | SE | p-value | ||
| (C)-linear | 2969.27 | 271.30 | <0.0001* | ||
| (C)-quadratic | 2517.36 | 321.01 | <0.0001* | ||
| (C)-cubic | −494.56 | 271.30 | 0.0695 | ||
| Contrast | df | Contrast SS | MS | F | p-value |
| (T)-linear | 1 | 19,669,576.14 | 19,669,576.14 | 49.49 | <0.0001* |
| (T)-quadratic | 1 | 621,122.70 | 621,122.70 | 1.56 | 0.2124 |
| Parameter | Estimate | SE | p-value | ||
| (T)-linear | 661.14 | 93.98 | <0.0001* | ||
| (T)-quadratic | −203.49 | 162.78 | 0.2124 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.P.; Astoreca, A.L.; Oliveira, Á.A.d.; Salvato, L.A.; Biscoto, G.L.; Keller, L.A.M.; Rosa, C.A.d.R.; Cavaglieri, L.R.; Azevedo, M.I.d.; Keller, K.M. In Vitro Activity of Neem (Azadirachta indica) Oil on Growth and Ochratoxin A Production by Aspergillus carbonarius Isolates. Toxins 2019, 11, 579. https://doi.org/10.3390/toxins11100579
Rodrigues MP, Astoreca AL, Oliveira ÁAd, Salvato LA, Biscoto GL, Keller LAM, Rosa CAdR, Cavaglieri LR, Azevedo MId, Keller KM. In Vitro Activity of Neem (Azadirachta indica) Oil on Growth and Ochratoxin A Production by Aspergillus carbonarius Isolates. Toxins. 2019; 11(10):579. https://doi.org/10.3390/toxins11100579
Chicago/Turabian StyleRodrigues, Mariana Paiva, Andrea Luciana Astoreca, Águida Aparecida de Oliveira, Lauranne Alves Salvato, Gabriela Lago Biscoto, Luiz Antonio Moura Keller, Carlos Alberto da Rocha Rosa, Lilia Renée Cavaglieri, Maria Isabel de Azevedo, and Kelly Moura Keller. 2019. "In Vitro Activity of Neem (Azadirachta indica) Oil on Growth and Ochratoxin A Production by Aspergillus carbonarius Isolates" Toxins 11, no. 10: 579. https://doi.org/10.3390/toxins11100579
APA StyleRodrigues, M. P., Astoreca, A. L., Oliveira, Á. A. d., Salvato, L. A., Biscoto, G. L., Keller, L. A. M., Rosa, C. A. d. R., Cavaglieri, L. R., Azevedo, M. I. d., & Keller, K. M. (2019). In Vitro Activity of Neem (Azadirachta indica) Oil on Growth and Ochratoxin A Production by Aspergillus carbonarius Isolates. Toxins, 11(10), 579. https://doi.org/10.3390/toxins11100579





