Chemical and Biological Characteristics of Antimicrobial α-Helical Peptides Found in Solitary Wasp Venoms and Their Interactions with Model Membranes
Abstract
1. Introduction
2. Chemical and Biological Characterization
2.1. Mastoparans
2.2. Eumenitins
2.3. Protonectin
2.4. Decoralin
2.5. Anoplin
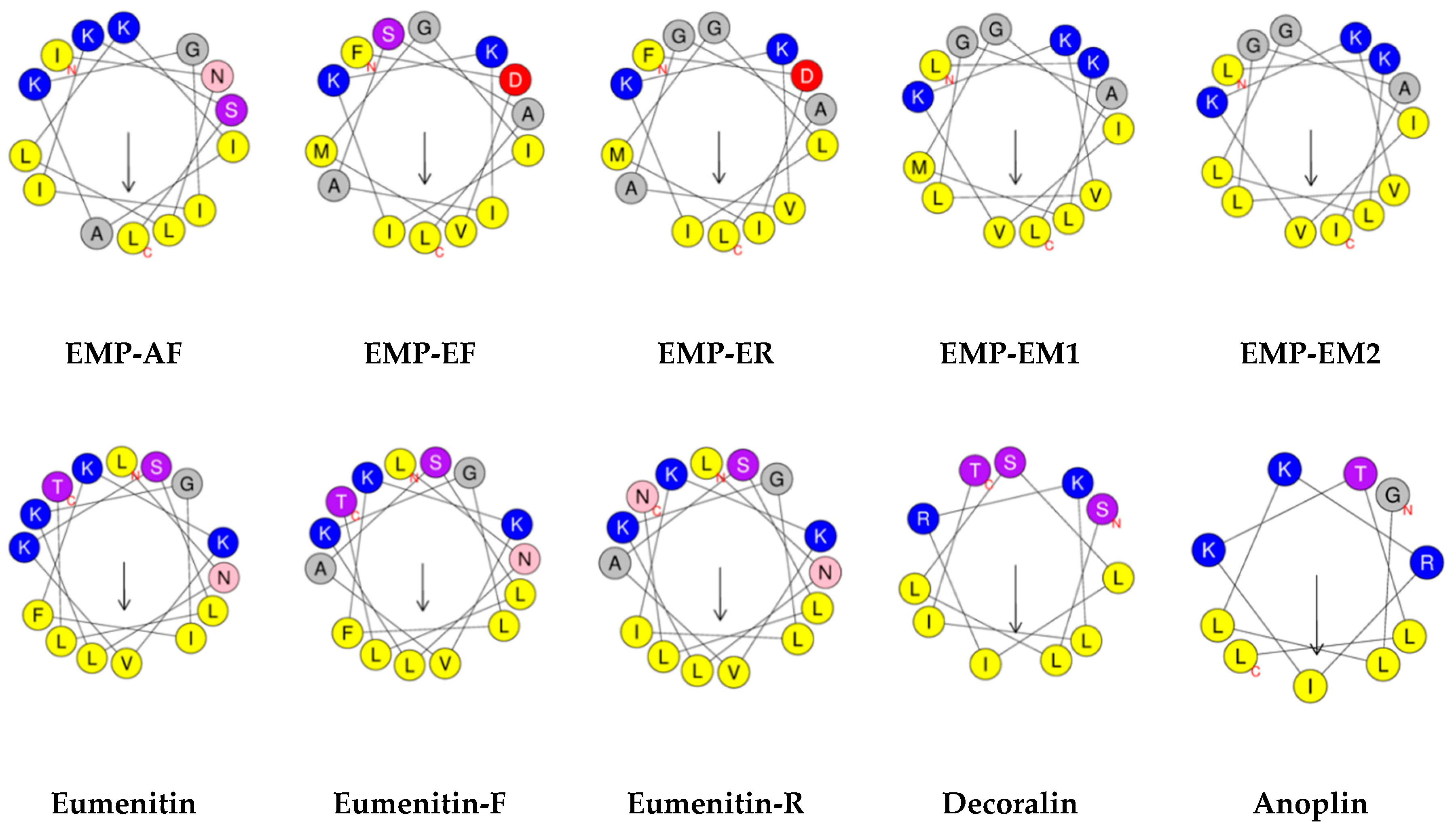
3. Physicochemical Properties and Secondary Structure
3.1. Sequence and Chain Length
3.2. N- and C-Termini
3.3. Charge and Helix Macro-Dipole
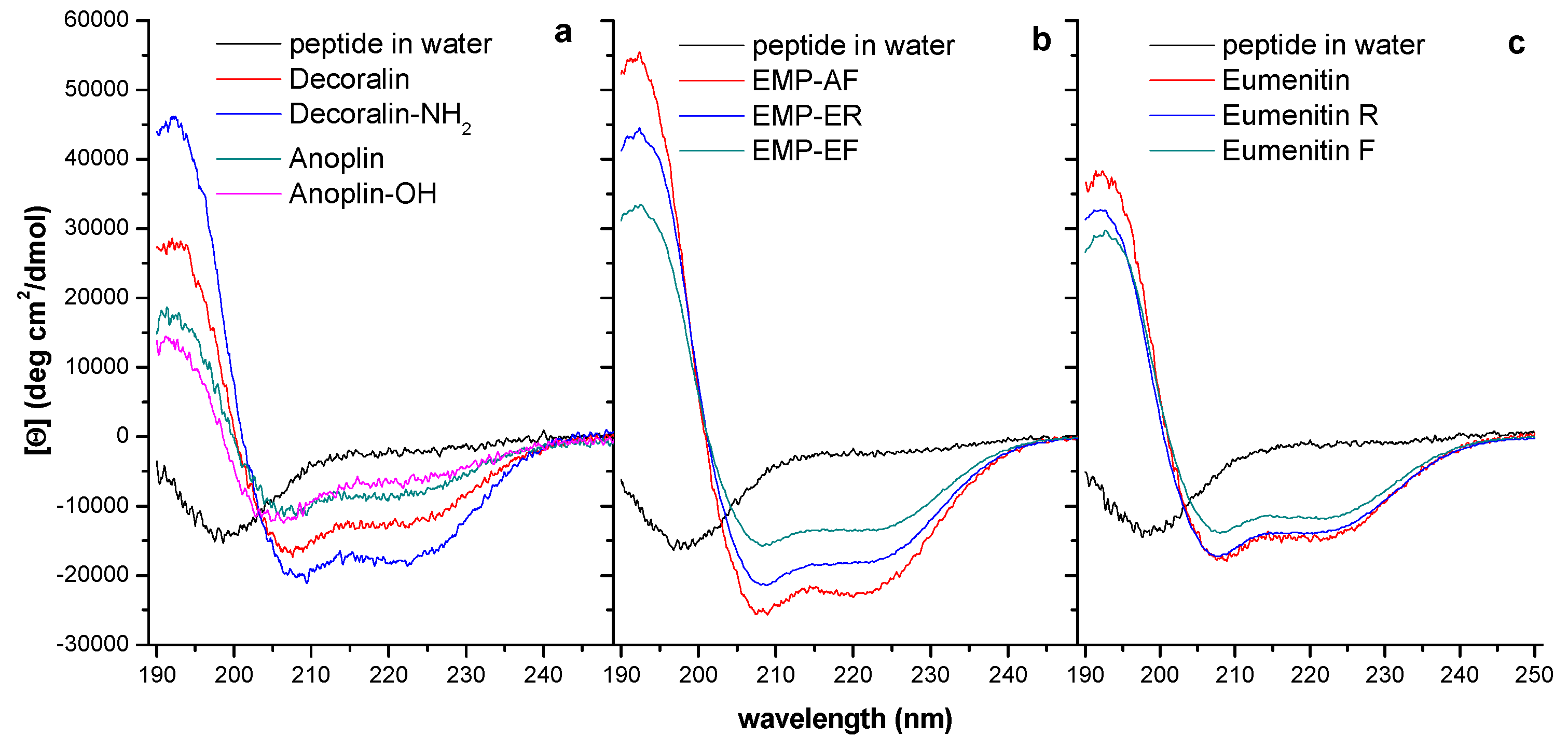
3.4. Helical Propensity and Helicity
3.5. Amphipathicity and Hydrophobic Moment
3.6. Hydrophobicity
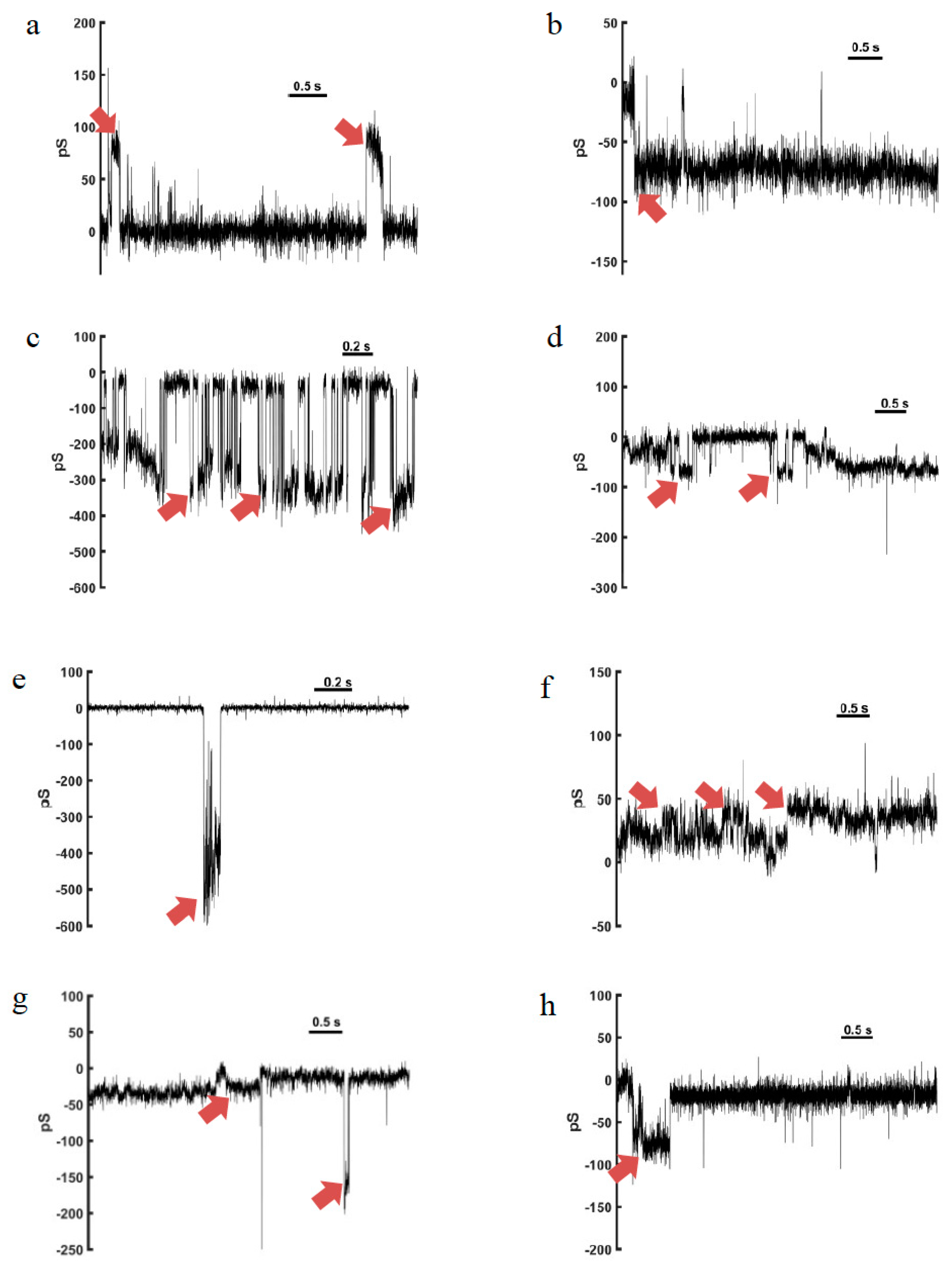
4. Channel-Like Pore-Forming Properties
4.1. Anoplin
4.2. Eumenitin
4.3. Eumenitin-F and Eumenitin-R
4.4. EMP-EF and EMP-ER
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EMP | eumenine mastoparan |
| PMTX | pompilidotoxin |
| HPLC | high performance liquid chromatography |
| CD | circular dichroism |
| TFE | 2,2,2-trifluoroethanol |
| SDS | sodium dodecylsulfate |
| NMR | nuclear magnetic resonance |
| KLA | lysine-leucine-alanine (amino acid sequence) |
| DPhPC | 1,2-diphytanoyl-sn-glycero-3-phosphocholine |
References
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J.; Craik, D.J. The vast structural diversity of antimicrobial peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Narayana, J.L.; Chen, J.-Y. Antimicrobial peptides: Possible anti-infective agents. Peptides 2015, 72, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef] [PubMed]
- Kuhn-Nentwig, L. Antimicrobial and cytolytic peptides of venomous arthropods. Cell. Mol. Life Sci. 2003, 60, 2651–2668. [Google Scholar] [CrossRef] [PubMed]
- Bulet, P.; Stöcklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Insects, arachnids and centipedes venom: A powerful weapon against bacteria. A literature review. Toxicon 2017, 130, 91–103. [Google Scholar] [CrossRef]
- Konno, K.; Hisada, M.; Naoki, H.; Itagaki, Y.; Kawai, N.; Miwa, A.; Yasuhara, T.; Morimoto, Y.; Nakata, Y. Structure and biological activities of eumenine mastoparan-AF (EMP-AF), a novel mast cell degranulating peptide in the venom of the solitary wasp (Anterhynchium flavomarginatum micado). Toxicon 2000, 38, 1505–1515. [Google Scholar] [CrossRef]
- Konno, K.; Hisada, M.; Fontana, R.; Lorenzi, C.C.B.; Naoki, H.; Itagaki, Y.; Miwa, A.; Kawai, N.; Nakata, Y.; Yasuhara, T.; et al. Anoplin, a novel antimicrobial peptide from the venom of the solitary wasp Anoplius samariensis. Biochim. Biophys. Acta 2001, 1550, 70–80. [Google Scholar] [CrossRef]
- Konno, K.; Hisada, M.; Naoki, H.; Itagaki, Y.; Fontana, R.; Rangel, M.; Oliveira, J.S.; Cabrera, M.P.; Ruggiero Neto, J.; Hide, I.; et al. Eumenitin, a novel antimicrobial peptide from the venom of the solitary eumenine wasp Eumenes rubronotatus. Peptides 2006, 27, 2624–2631. [Google Scholar] [CrossRef]
- Konno, K.; Rangel, M.; Oliveira, J.S.; Cabrera, M.P.S.; Fontana, R.; Hirata, I.Y.; Nakata, Y.; Mori, K.; Kawano, M.; Fuchino, H.; et al. Decoralin, a novel linear cationic α-helical peptide from the venom of the solitary eumenine wasp Oreumenes decoratus. Peptides 2007, 28, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; Cabrera, M.P.S.; Kazuma, K.; Ando, K.; Wang, X.; Kato, M.; Nihei, K.; Hirata, I.Y.; Cross, T.; Garcia, A.N.; et al. Chemical and biological characterization of four new antimicrobial and α-helical peptides from the venoms of two solitary eumenine wasps. Toxicon 2011, 57, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Rangel, M.; de Oliveira, J.S.; Fontana, R.; Kawano, M.; Fuchino, H.; Hide, I.; Yasuhara, T.; Nakata, Y. New mastoparan peptides in the venom of the solitary eumenine wasp Eumenes micado. Toxins 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.P.S.; Souza, B.M.; Fontana, R.; Konno, K.; Palma, M.S.; de Azevedo, W.F., Jr.; Ruggiero Neto, J. Conformation and lytic activity of eumenine mastoparan: A new antimicrobial peptide from wasp venom. J. Pept. Res. 2004, 64, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.P.S.; Arcisio-Miranda, M.; Costa, S.T.B.; Konno, K.; Ruggiero, J.R.; Procopio, J.; Ruggiero Neto, J. Study of the mechanism of action of Anoplin, a helical antimicrobial decapeptide with ion channel-like activity, and the role of the amidated C-terminus. J. Pept. Sci. 2008, 14, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Arcisio-Miranda, M.; Cabrera, M.P.S.; Konno, K.; Rangel, M.; Procopio, J. Effects of the cationic antimicrobial peptide Eumenitin from the venom of solitary wasp Eumenes rubronotatus in planar lipid bilayers: Surface charge and pore formation activity. Toxicon 2008, 51, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Kazuma, K.; Nihei, K. Peptide toxins in solitary wasp venoms. Toxins 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Piek, T. Neurotoxic kinins from wasp and ant venoms. Toxicon 1991, 29, 139–149. [Google Scholar] [CrossRef]
- Nakanishi, K.; Goodnow, R.; Konno, K.; Niwa, M.; Bukownik, R.; Kalimopoulos, T.A.; Usherwood, P.N.R.; Eldefrawi, A.T.; Eldefrawi, M.E. Philanthotoxin-433 (PhTX-433), a non-competitive glutamate receptor inhibitor. Pure Appl. Chem. 1990, 62, 1223–1230. [Google Scholar] [CrossRef]
- Konno, K.; Kawai, N. Pompilidotoxins: Novel peptide neurotoxins blocking sodium channel inactivation from solitary wasp venom. Curr. Med. Chem. Cent. Nerv. Syst. Agents 2004, 4, 139–146. [Google Scholar] [CrossRef]
- Hirai, Y.; Yasuhara, T.; Yoshida, H.; Nakajima, T.; Fujino, M.; Kitada, C. A new mast cell degranulating peptide “mastoparan” in the venom of Vespula lewisii. Chem. Pharm. Bull. 1979, 27, 1942–1944. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Shinada, T.; Ohfune, Y.; Hisada, M.; Yasuda, A.; Naoki, H.; Nakajima, T. Novel biologically active peptides from the venom of Polistes rothneyi Iwatai. Biol. Pharm. Bull. 2006, 29, 2493–2497. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T. Pharmacological biochemistry of vespid venoms. In Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioural Aspects; Piek, T., Ed.; Academic Press: London, UK, 1986; pp. 309–327. ISBN 0-12-554771-4. [Google Scholar]
- Sforça, M.L.; Oyama, S., Jr.; Canduri, F.; Lorenzi, C.C.B.; Pertinez, T.A.; Konno, K.; Souza, B.M.; Palma, M.S.; Ruggiero Neto, J.; de Azevedo, W.F., Jr.; et al. How C-terminal carboxyamidation alters the mast cell degranulating activity of peptides from the venom of the eumenine solitary wasp. Biochemistry 2004, 43, 5608–5617. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Shinada, T.; Ohfune, Y.; Hisada, M.; Yasuda, A.; Naoki, H.; Nakajima, T. Novel mastoparan and protonectin analogs isolated from a solitary wasp, Orancistrocerus drewseni drewseni. Amino Acids 2009, 37, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, S.H. Isolation and molecular cloning of venom peptides from Orancistrocerus drewseni (Hymenoptera: Eumenidae). Toxicon 2010, 55, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, S.H. Differential gene expression profiles in the venom gland/sac of Eumenes pomiformis (Hymenoptera: Eumenidae). Toxicon 2010, 55, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Guerra, M.E.; Fadel, V.; Maltarollo, V.G.; Baldissera, G.; Honorio, K.M.; Ruggiero, J.R.; dos Santos Cabrera, M.P. MD simulations and multivariate studies for modeling the antileishmanial activity of peptides. Chem. Biol. Drug Des. 2017, 90, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Hisada, M.; Itagaki, Y.; Naoki, H.; Kawai, N.; Miwa, A.; Yasuhara, T.; Takayama, H. Isolation and structure of pompilidotoxins (PMTXs), novel neurotoxins in solitary wasp venoms. Biochem. Biophys. Res. Commun. 1998, 250, 612–616. [Google Scholar] [CrossRef]
- Ifrah, D.; Doisy, X.; Ryge, T.S.; Hansen, P.R. Structure-activity relationship study of anoplin. J. Pept. Sci. 2005, 11, 113–121. [Google Scholar] [CrossRef]
- Won, A.; Khan, M.; Gustin, S.; Akpawu, A.; Seebun, D.; Avis, T.J.; Leung, B.O.; Hitchcock, A.P.; Ianoul, A. Investigating the effects of L- to D-amino acid substitution and deamidation on the activity and membrane interactions of antimicrobial peptide anoplin. Biochim. Biophys. Acta 2011, 1808, 1592–1600. [Google Scholar] [CrossRef]
- Won, A.; Pripotnev, S.; Ruscito, A.; Ianoul, A. Effect of point mutations on the secondary structure and membrane interaction of antimicrobial peptide anoplin. J. Phys. Chem. B 2011, 115, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Munk, J.K.; Uggerhøj, L.E.; Poulsen, T.J.; Frimodt-Møller, N.; Wimmer, R.; Nyberg, N.T.; Hansen, P.R. Synthetic analogs of anoplin show improved antimicrobial activities. J. Pept. Sci. 2013, 19, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Slootweg, J.C.; van Schaik, T.B.; van Ufford, H.C.Q.; Breukink, E.; Liskamp, R.M.J.; Rijkers, D.T.S. Improving the biological activity of the antimicrobial peptide anoplin by membrane anchoring through a lipophilic amino acid derivative. Bioorgan. Med. Chem. Lett. 2013, 23, 3749–3752. [Google Scholar] [CrossRef] [PubMed]
- Jindrichova, B.; Burketova, L.; Novotna, Z. Novel properties of antimicrobial peptide anoplin. Biochem. Biophys. Res. Commun. 2014, 444, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Zheng, X.; Yang, X.; Ma, P.; Cai, Y.; Zhang, B.; Chen, Y. Design of novel analogues of short antimicrobial peptide anoplin with improved antimicrobial activity. J. Pept. Sci. 2014, 20, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Munk, J.K.; Ritz, C.; Fliedner, F.P.; Frimodt-Møller, N.; Hansen, P.R. Novel method to identify the optimal antimicrobial peptide in a combination matrix, using anoplin as an example. Antimicrob. Agents Chemother. 2014, 58, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Uggerhøj, L.E.; Poulsen, T.J.; Munk, J.K.; Fredborg, M.; Sondergaard, T.E.; Frimodt-Møller, N.; Hansen, P.R.; Wimmer, R. Rational design of alpha-helical antimicrobial peptides: do’s and don’ts. ChemBioChem 2015, 16, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Aschi, M.; Luzi, C.; Fiorillo, A.; Bozzi, A. Folding propensity of anoplin: A molecular dynamics study of the native peptide and four mutated isoforms. Biopolymers 2015, 103, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Sørensen, K.K.; HjáLlmarsdóttir, M.A.; Sigurjónsson, Ó.E.; Jensen, K.J.; Másson, M.; Thygesen, M.B. Antimicrobial peptide shows enhanced activity and reduced toxicity upon grafting to chitosan polymers. Chem. Commun. 2015, 51, 11611–11614. [Google Scholar] [CrossRef]
- Daben, M.; Libardo, J.; Nagella, S.; Lugo, A.; Pierce, S.; Angeles-Boza, A.M. Copper-binding tripeptide motif increases potency of the antimicrobial peptide Anoplin via Reactive Oxygen Species generation. Biochem. Biophys. Res. Commun. 2015, 456, 446–451. [Google Scholar] [CrossRef]
- Chionis, K.; Krikorian, D.; Koukkou, A.I.; Sakarellos-Daitsiotis, M.; Panou-Pomonis, E. Synthesis and biological activity of lipophilic analogs of the cationic antimicrobial active peptides anoplin. J. Pept. Sci. 2016, 22, 731–736. [Google Scholar] [CrossRef]
- Salas, R.L.; Garcia, J.K.D.L.; Miranda, A.C.R.; Rivera, W.L.; Nellas, R.B.; Sabido, P.M.G. Effects of truncation of the peptide chain on the secondary structure and bioactivities of palmitoylated anoplin. Peptides 2018, 104, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Ji, Y.; Shin, J.-S.; Lee, S.; Lee, S.H. Venom peptides from solitary hunting wasps induce feeding disorder in lepidopteran larvae. Peptides 2011, 32, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic α-helical antimicrobial peptides—A systematic study of the effects of structural and physical properties on biological activity. Eur. J. Biochem. 2001, 268, 5589–5600. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, J.M.; Qian, H.; York, E.J.; Stewart, J.M.; Baldwin, R.L. Parameters of helix-coil transition theory for alanine-based peptides of varying chain lengths in water. Biopolymers 1991, 31, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Rivas, L. Animal antimicrobial peptides: An overview. Biopolymers 1998, 47, 415–433. [Google Scholar] [CrossRef]
- Schulz, G.E.; Schirmer, R.H. Principles of Protein Structure; Springer: New York, NY, USA, 1978; pp. 10–16. [Google Scholar]
- Sitaram, N.; Nagaraj, R. Interaction of antimicrobial peptides with biological and model membranes: Structural and charge requirements for activity. Biochim. Biophys. Acta 1999, 1462, 29–54. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugishita, K.; Harada, M.; Fujii, N.; Miyajima, K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1997, 1327, 119–130. [Google Scholar] [CrossRef]
- Dathe, M.; Wieprecht, T.; Nikolenko, H.; Handel, L.; Maloy, W.L.; MacDonald, D.L.; Beyermann, M.; Bienert, M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997, 403, 208–212. [Google Scholar] [CrossRef]
- Dathe, M.; Nikolenko, H.; Meyer, J.; Beyermann, M.; Bienert, M. Optimization of the antimicrobial activity of Magainin peptides by modification of charge. FEBS Lett. 2001, 501, 146–150. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Magainins as paradigm for the mode of action of pore forming polypeptides Biochim. Biophys. Acta 1998, 1376, 391–400. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lim, Y.F.; Russo, E.; Schneider, P.; Bolliger, L.; Edenharter, A.; Altmann, K.H.; Halin, C.; Hiss, J.A.; Schneider, G. Multidimensional design of anticancer peptides. Angew. Chem. Int. Ed. 2015, 54, 10370–10374. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.T.; Pedron, C.N.; Araujo, I.; Silva, P.I., Jr.; Silva, F.D.; Oliveira, V.X. Decoralin analogs with increased resistance to degradation and lower hemolytic activity. ChemistrySelect 2017, 2, 18–23. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Pedron, C.N.; Silva Lima, J.A.; Silva, P.I., Jr.; Silva, F.D.; Oliveira, V.X. Antimicrobial activity of leucine-substituted decoralin analogs with lower hemolytic activity. J. Pept. Sci. 2017, 23, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.T.; Andrade, G.T.; Sato, R.H.; Pedron, C.N.; Manieri, T.M.; Cerchiaro, G.; Ribeiro, A.O.; Fuente-Nunez, C.; Oliveira, V.X., Jr. Natural and redesigned wasp venom peptides with selective antitumoral activity. Beilstein J. Org. Chem. 2018, 14, 1693–1703. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Silva, F.D.; Pedron, C.N.; Capurro, M.L.; Fuente-Nunez, C.; Oliveira, V.X., Jr. Peptide design enables reengineering of an inactive wasp venom peptide into synthetic antiplasmodial agents. ChemistrySelect 2018, 3, 5859–5863. [Google Scholar] [CrossRef]
- Hol, W.G.; van Duijnen, P.T.; Berendsen, H.J.C. The α-helix dipole and the properties of proteins. Nature 1978, 273, 443–446. [Google Scholar] [CrossRef]
- Joshi, H.V.; Meier, M.S. The effect of a peptide helix macrodipole on the pKa of an Asp side chain carboxylate. J. Am. Chem. Soc. 1996, 118, 12038–12044. [Google Scholar] [CrossRef]
- Rohl, C.A.; Baldwin, R.L. Deciphering rules of helix stability in peptides. Methods Enzymol. 1998, 295, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Fasman, G.D. Conformational parameters for amino acids in helical, β-sheet, and random coil regions calculated from proteins. Biochemistry 1974, 13, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Gans, P.J.; Lyu, P.C.; Manning, M.C.; Woody, R.W. The helix-coil transition in heterogeneous peptides with specific side-chain interactions: Theory and comparison with CD spectral data. Biopolymers 1991, 31, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, K.T.; DeGrado, W.F. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science 1990, 250, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Marqusee, S.; Robbins, H.V.; Baldwin, R.L. Unusually stable helix formation in short alanine-based peptides. Proc. Natl. Acad. Sci. USA 1989, 86, 5286–5290. [Google Scholar] [CrossRef] [PubMed]
- Blondelle, S.E.; Forood, B.; Houghten, R.A.; Pérez-Payá, E. Secondary structure induction in aqueous vs membrane-like environments. Biopolymers 1997, 42, 489–498. [Google Scholar] [CrossRef]
- Waterhous, D.V.; Johnson, W.C., Jr. Importance of environment in determining secondary structure in proteins. Biochemistry 1994, 33, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Johnson, C., Jr. Environment affects amino acid preference for secondary structure. Proc. Natl. Acad. Sci. USA 1992, 89, 4462–4465. [Google Scholar] [CrossRef]
- Deber, C.M.; Li, S.C. Peptides in membranes: Helicity and hydrophobicity. Biopolymers 1995, 37, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Blondelle, S.E.; Houghten, R.A. Design of model amphipathic peptides having potent antimicrobial activities. Biochemistry 1992, 31, 12688–12694. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1999, 1462, 71–87. [Google Scholar] [CrossRef]
- Dathe, M.; Schümann, M.; Wieprecht, T.; Winkler, A.; Beyermann, M.; Krause, E.; Matsuzaki, K.; Murase, O.; Bienert, M. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry 1996, 35, 12612–12622. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Schwarz, E.; Komaromy, M.; Wall, R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 1984, 179, 125–142. [Google Scholar] [CrossRef]
- Dathe, M.; Meyer, J.; Beyermann, M.; Maul, B.; Hoischen, C.; Bienert, M. General aspects of peptide selectivity towards lipid bilayers and cell membranes studied by variation of the structural parameters of amphipathic helical model peptides. Biochim. Biophys. Acta 2002, 1558, 171–186. [Google Scholar] [CrossRef]
- Pathak, N.; Salas-Auvert, R.; Ruche, G.; Janna, M.H.; McCarthy, D.; Harrison, R.G. Comparison of the effects of hydrophobicity, amphiphilicity, and α-helicity on the activities of antimicrobial Peptides. Proteins Struct. Funct. Bioinf. 1995, 22, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.; Chandler, D.; Weeks, J.D. Hydrophobicity at small and large length scales. J. Phys. Chem. B 1999, 103, 4570–4577. [Google Scholar] [CrossRef]
- Wieprecht, T.; Dathe, M.; Beyermann, M.; Krause, E.; Maloy, W.L.; MacDonald, D.L.; Bienert, M. Peptide hydrophobicity controls the activity and selectivity of Magainin 2 amide in interaction with membranes. Biochemistry 1997, 36, 6124–6132. [Google Scholar] [CrossRef]
- Leite, N.B.; da Costa, L.C.; Dos Santos Alvares, D.; Dos Santos Cabrera, M.P.; De Souza, B.M.; Palma, M.S.; Ruggiero Neto, J. The effect of acidic residues and amphipathicity on the lytic activities of mastoparan peptides studied by fluorescence and CD spectroscopy. Amino Acids 2011, 40, 91–100. [Google Scholar] [CrossRef]
- Neville, F.; Cahuzac, M.; Konovalov, O.; Ishitsuka, Y.; Lee, K.Y.C.; Kuzmenko, I.; Kale, G.M.; Gidalevitz, D. Lipid head group discrimination by antimicrobial peptide LL-37: Insight into mechanism of action. Biophys. J. 2006, 90, 1275–1287. [Google Scholar] [CrossRef]
- Leontiadou, H.; Mark, A.E.; Marrink, S.J. Antimicrobial peptides an action. J. Am. Chem. Soc. 2006, 128, 12156–12161. [Google Scholar] [CrossRef]
- Rzepiela, A.J.; Sengupta, D.; Goga, N.; Marrink, S.J. Membrane poration by antimicrobial peptides combining atomistic and coarse-grained descriptions. Faraday Discuss. 2010, 144, 431–443. [Google Scholar] [CrossRef]
- Giladevitz, D.; Ishitzuka, Y.; Murean, A.S.; Konovalov, O.; Waring, A.L.; Lehrer, R.I.; Lee, K.Y.C. Interaction of antimicrobial peptide protegrin with biomembranes. Proc. Natl. Acad. Sci. USA 2003, 100, 6302–6307. [Google Scholar] [CrossRef]
- Andreev, K.; Martynowycz, M.W.; Huang, M.L.; Kuzmenko, I.; Bu, W.; Kirshenbaum, K.; Gidalevitz, D. Hydrophobic interactions modulate antimicrobial peptoid selectivity toward anionic lipid membranes. Biochim. Biophys. Acta 2018, 1860, 1414–1423. [Google Scholar] [CrossRef]
- Rangel, M.; Konno, K.; Brunaldi, K.; Procopio, J.; Freitas, J.C. Neurotoxic activity induced by a haemolytic substance in the extract of the marine sponge Geodia corticostylifera. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 207–215. [Google Scholar] [CrossRef]
- Boland, M.P.; Separovic, F. Membrane interactions of antimicrobial peptides from Australian tree frogs. Biochim. Biophys. Acta 2006, 1758, 1178–1183. [Google Scholar] [CrossRef][Green Version]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Dos Santos Cabrera, M.P.; Arcisio-Miranda, M.; da Costa, L.C.; De Souza, B.M.; Broggio Costa, S.T.; Palma, M.S.; Ruggiero Neto, J.; Procopio, J. Interactions of mast cell degranulating peptides with model membranes: A comparative biophysical study. Arch. Biochem Biophys. 2009, 486, 1–11. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry 1996, 35, 11361–11368. [Google Scholar] [CrossRef]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef]
- Strandberg, K.; Westerberg, S. Composition of phospholipids and phospholipid fatty acids in rat mast cells. Mol. Cell. Biochem. 1976, 11, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Fink, J.; Merrifield, R.B.; Mauzerall, D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc. Natl. Acad. Sci. USA 1988, 85, 5072–5076. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Lohner, K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
| Peptide | Sequence | Peptide | Sequence |
|---|---|---|---|
| EMP-AF | INLLKIAKGIIKSL-NH2 | Eumenitin | LNLKGIFKKVKSLLT |
| EMP-EF | FDVMGIIKKIASAL-NH2 | Eumenitin-F | LNLKGLFKKVASLLT |
| EMP-ER | FDIMGLIKKVAGAL-NH2 | Eumenitin-R | LNLKGLIKKVASLLN |
| EMP-EM1 | LKLMGIVKKVLGAL-NH2 | EpVP1 | INLKGLIKKVASLLT |
| EMP-EM2 | LKLLGIVKKVLGAI-NH2 | Decoralin | SLLSLIRKLIT |
| EpVP2a | FDLLGLVKKVASAL-NH2 | OdVP3 | KDLHTVVSAILQAL-NH2 |
| EpVP2b | FDLLGLVKSVVSAL-NH2 | EMP-OD (OdVP1) | GRILSFIKGLAEHL-NH2 |
| Anoplin | GLLKRIKTLL-NH2 | Orancis-Protonectin (OdVP2) | ILGIITSLLKSL-NH2 |
| Peptides | Antimicrobial Activity (MIC, μM) | Hemolysis (EC50, μM) | Degranulation (EC50, μM) | References | ||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | ||||
| EMP-AF | 3 | 33 | - | 50 | 30 | [8,14] |
| EMP-EF | 30 | 30 | 7.5 | 181 | 60 | [12] |
| EMP-ER | 30 | 30 | 7.5 | 200 | 70 | [12] |
| EMP-EM1 | 7 | 7 | >60 | na | 70 | [13] |
| EMP-EM2 | 3 | 3 | >60 | na | 60 | [13] |
| Eumenitin | 6 | 6 | - | na | 70 | [10] |
| Eumenitin-F | >60 | 30 | 7.5 | 353 | na | [12] |
| Eumenitin-R | 60 | 30 | 7.5 | 530 | na | [12] |
| Decoralin | 40 | 80 | 40 | na | na | [11] |
| Anoplin | 4 | 43 | - | na | 30 | [9] |
| Peptides | N | Q | C-Term | <H> | µ | fH | References | ||
|---|---|---|---|---|---|---|---|---|---|
| TFE | SDS | PC | |||||||
| EMP-AF | 14 | +4 | amide | 0.051 | 0.342 | 0.55 | 0.72 | 0.16 | [14] |
| EMP-EF | 14 | +2 | amide | 0.115 | 0.279 | 0.41 | 0.44 | nd | [12] |
| EMP-ER | 14 | +2 | amide | 0.131 | 0.251 | 0.53 | 0.59 | nd | [12] |
| EMP-EM1 | 14 | +4 | amide | 0.104 | 0.258 | 0.31 | 0.33 | 0.11 | [13] |
| EMP-EM2 | 14 | +4 | amide | 0.138 | 0.278 | 0.37 | 0.41 | < 0.03 | [13] |
| Eumenitin | 15 | +3 | carboxyl | 0.002 | 0.265 | 0.43 | 0.50 | 0.07/0.11 | [10] |
| Eumenitin-F | 15 | +3 | carboxyl | - | 0.256 | 0.34 | 0.44 | nd | [12] |
| Eumenitin-R | 15 | +3 | carboxyl | - | 0.281 | 0.43 | 0.48 | nd | [12] |
| Decoralin | 11 | +3 | carboxyl | 0.028 | 0.393 | 0.38 | 0.50 | rc | [11] |
| Anoplin | 10 | +4 | amide | - | 0.366 | 0.24 | 0.26 | rc | [9] |
| Peptides | Lipid | Vhold (mV) | Conductance (pS) | SEM | References |
|---|---|---|---|---|---|
| Eumenitin | asolectin | −50 | 118.0/160.0 | 3.67/7.07 | [16] |
| asolectin | +50 | 118.0/160.0 | 3.67/7.07 | [16] | |
| DPhPC | +50 | 61.13 | 7.57 | [16] | |
| DPhPC-cholesterol | −100 | None | - | [16] | |
| DPhPC-cholesterol | +100 | None | - | [16] | |
| Eumenitin-R | asolectin | −100 | 82.5 | 17.1 | [12] |
| asolectin | +100 | 118.8 | 44 | [12] | |
| Eumenitin-F | asolectin | −100 | 298.6 | 51 | [12] |
| asolectin | +100 | 187.1 | 67.7 | [12] | |
| EMP-ER | asolectin | −100 | 68.2 | 4 | [12] |
| asolectin | +100 | 61.4 | 3.7 | [12] | |
| EMP-EF | asolectin | −100 | 33.6 | 8.9 | [12] |
| asolectin | +100 | 32.2 | 6.9 | [12] | |
| Anoplin | asolectin | +100 | 50.7 | 3.8 | [15] |
| asolectin | +130 | 58.4 | 2.7 | [15] | |
| asolectin | +150 | 66.8 | 6.7 | [15] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Cabrera, M.P.; Rangel, M.; Ruggiero Neto, J.; Konno, K. Chemical and Biological Characteristics of Antimicrobial α-Helical Peptides Found in Solitary Wasp Venoms and Their Interactions with Model Membranes. Toxins 2019, 11, 559. https://doi.org/10.3390/toxins11100559
dos Santos Cabrera MP, Rangel M, Ruggiero Neto J, Konno K. Chemical and Biological Characteristics of Antimicrobial α-Helical Peptides Found in Solitary Wasp Venoms and Their Interactions with Model Membranes. Toxins. 2019; 11(10):559. https://doi.org/10.3390/toxins11100559
Chicago/Turabian Styledos Santos Cabrera, Marcia Perez, Marisa Rangel, João Ruggiero Neto, and Katsuhiro Konno. 2019. "Chemical and Biological Characteristics of Antimicrobial α-Helical Peptides Found in Solitary Wasp Venoms and Their Interactions with Model Membranes" Toxins 11, no. 10: 559. https://doi.org/10.3390/toxins11100559
APA Styledos Santos Cabrera, M. P., Rangel, M., Ruggiero Neto, J., & Konno, K. (2019). Chemical and Biological Characteristics of Antimicrobial α-Helical Peptides Found in Solitary Wasp Venoms and Their Interactions with Model Membranes. Toxins, 11(10), 559. https://doi.org/10.3390/toxins11100559





