Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms
Abstract
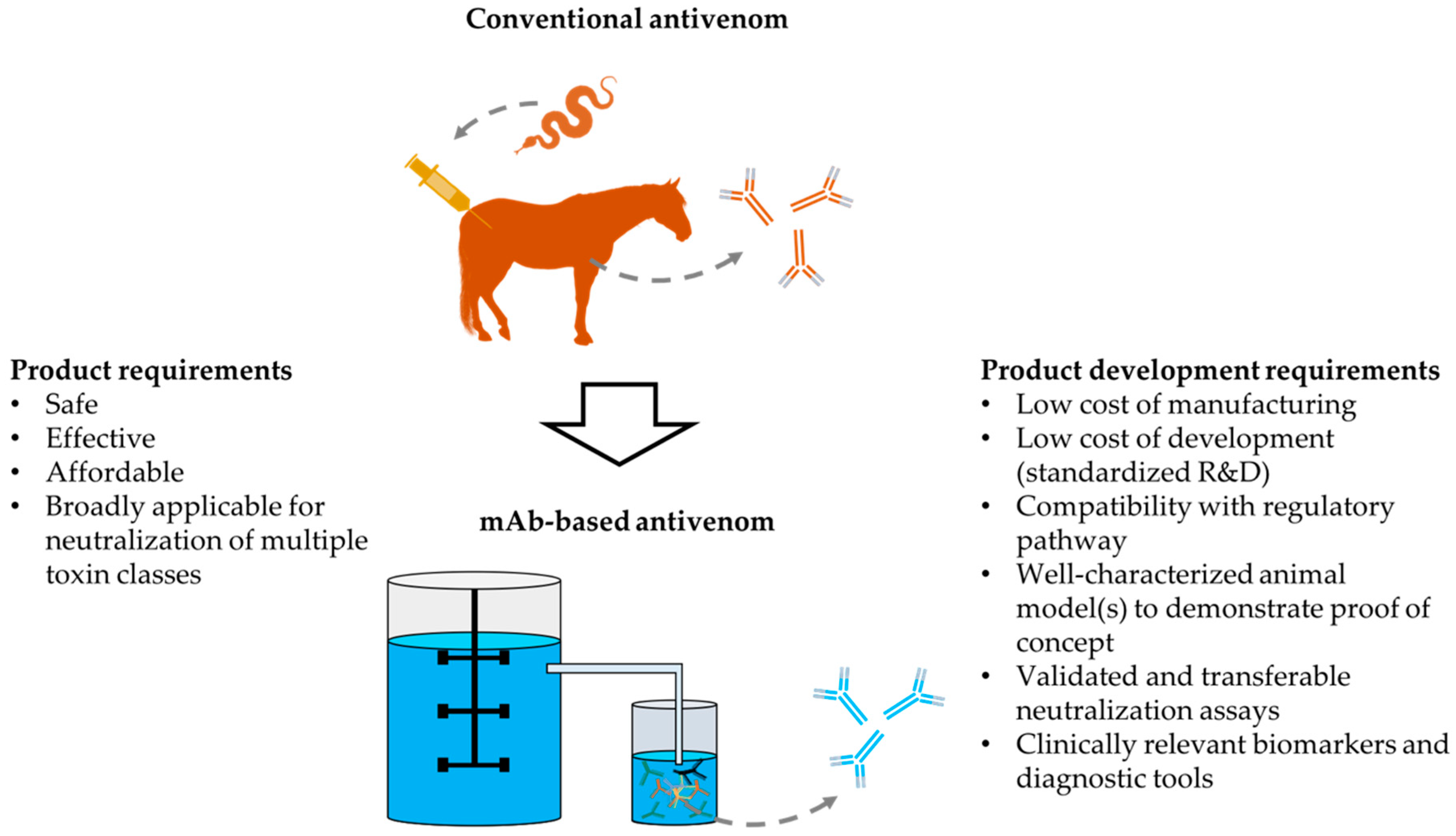
1. Introduction
2. Considerations for Novel Antivenoms and their Markets
3. Development and Manufacturing Aspects
4. Clinical and Regulatory Aspects
5. Conclusions and Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Williams, D.; Fan, H.W.; Warrell, D.A. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon 2010, 56, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Calmette, A. L’immunisation artificielle des animaux contre le venin des serpents, et la thérapeutic expérimentale des morsures venimeuses. Comptes Rendus de la Société de Biologie 1894, 46, 120–124. [Google Scholar]
- Laustsen, A.H.; Engmark, M.; Milbo, C.; Johannesen, J.; Lomonte, B.; Gutiérrez, J.M.; Lohse, B. From Fangs to Pharmacology: The Future of Snakebite Envenoming Therapy. Curr. Pharm. Des. 2016, 22, 5270–5293. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Cook, D.A.; Renjifo, C.; Casewell, N.R.; Currier, R.B.; Wagstaff, S.C. Research strategies to improve snakebite treatment: Challenges and progress. J. Proteom. 2011, 74, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Wieland, T. Managing risks in drug discovery: Reproducibility of published findings. Naunyn-Schmiedebergs Arch. Pharmacol. 2016, 389, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.I. Consequences of Neglect: Analysis of the Sub-Saharan African Snake Antivenom Market and the Global Context. PLoS Negl. Trop. Dis. 2012, 6, e1670. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Meeting on Monoclonal Antibodies against Rabies and Evaluation of Mechanisms to Improve Access to Other Blood-Derived Immunoglobulins; World Health Organization: Silver Spring, MD, USA, 2017. [Google Scholar]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Recombinant snakebite antivenoms: A cost-competitive solution to a neglected tropical disease? PLoS Negl. Trop. Dis. 2017, 11, e0005361. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Johansen, K.H.; Engmark, M.; Andersen, M.R. Snakebites: Costing recombinant antivenoms. Nature 2016, 538, 41. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Meyers, A.J.; McLean, M.D.; Arbabi-Ghahroudi, M.; MacKenzie, R.; Hall, J.C. In Vivo Neutralization of α-Cobratoxin with High-Affinity Llama Single-Domain Antibodies (VHHs) and a VHH-Fc Antibody. PLoS ONE 2013, 8, e69495. [Google Scholar] [CrossRef] [PubMed]
- Julve Parreño, J.M.; Huet, E.; Fernández-Del-Carmen, A.; Segura, A.; Venturi, M.; Gandía, A.; Pan, W.-S.; Albaladejo, I.; Forment, J.; Pla, D.; et al. A synthetic biology approach for consistent production of plant-made recombinant polyclonal antibodies against snake venom toxins. Plant. Biotechnol. J. 2017, 16, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.M.A.; Oliveira, T.S.; Silveira, C.R.F.; Caporrino, M.C.; Rodriguez, D.; Moura-da-Silva, A.M.; Ramos, O.H.P.; Rucavado, A.; Gutiérrez, J.M.; Magalhães, G.S.; et al. A neutralizing recombinant single chain antibody, scFv, against BaP1, A P-I hemorrhagic metalloproteinase from Bothrops asper snake venom. Toxicon 2014, 87, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; John, T.R.; Kaiser, I.I. Specificity and binding affinity of an anti-crotoxin combinatorial antibody selected from a phage-displayed library. Biochem. Pharmacol. 1995, 50, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Gutiérrez, J.; Ramírez, M.; Díaz, C. Neutralization of myotoxic phospholipases A2 from the venom of the snake Bothrops asper by monoclonal antibodies. Toxicon 1992, 30, 239–245. [Google Scholar] [CrossRef]
- Boulain, J.C.; Ménez, A.; Couderc, J.; Faure, G.; Liacopoulos, P.; Fromageot, P. Neutralizing monoclonal antibody specific for Naja nigricollis toxin alpha: Preparation, characterization, and localization of the antigenic binding site. Biochemistry 1982, 21, 2910–2915. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Kahan, L. Production and partial characterization of monoclonal antibodies to Bothrops asper (terciopelo) myotoxin. Toxicon 1988, 26, 675–689. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Gutiérrez, J.M.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Laustsen, A.H. Recent Advances in Next Generation Snakebite Antivenoms. Trop. Med. Infect. Dis. 2018, 3, 42. [Google Scholar] [CrossRef]
- Laustsen, A.H. Guiding recombinant antivenom development by omics technologies. New Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C. Landmark Green Light for Mosquirix Malaria Vaccine. Nat. Biotechnol. 2015, 33, 1015–1016. [Google Scholar] [CrossRef] [PubMed]
- Antivenom Market By Type [Vaccines And Hyperimmune Sera (Homologous & Heterologous)], By Animal (Snakes, Scorpions, Spiders, And Others), And By Region—Global Industry Analysis, Size, Share, Growth, Trends, And Forecasts (2018–2023) Market Data Forecast. Available online: https://www.marketdataforecast.com/market-reports/global-antivenom-market-1580/ (accessed on 18 May 2018).
- Chaves, L.F.; Chuang, T.-W.; Sasa, M.; Gutiérrez, J.M. Snakebites are associated with poverty, weather fluctuations, and El Niño. Sci. Adv. 2015, 1, e1500249. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake Envenoming: A. Disease of Poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Bawaskar, H.S.; Bawaskar, P.H.; Bawaskar, P.H. Snake bite in India: A neglected disease of poverty. Lancet 2017, 390, 1947–1948. [Google Scholar] [CrossRef]
- Williams, D.J.; Gutiérrez, J.M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H. Toxin-centric development approach for next-generation antivenoms. Toxicon 2018, 150, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Rodríguez, Y.; Quesada-Bernat, S.; Pla, D. Toxin-resolved antivenomics-guided assessment of the immunorecognition landscape of antivenoms. Toxicon 2018, 148, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Lohse, B.; Lomonte, B.; Engmark, M.; Gutiérrez, J.M. Selecting key toxins for focused development of elapid snake antivenoms and inhibitors guided by a Toxicity Score. Toxicon 2015, 104, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Lomonte, B. A bright future for integrative venomics. Toxicon 2015, 107, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Gutiérrez, J.M. Priority actions and progress to substantially and sustainably reduce the mortality, morbidity and socioeconomic burden of tropical snakebite. Toxins 2016, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.K.; Næsted, H.; Müller, C.; Tolstrup, A.B.; Frandsen, T.P. Recombinant antibody mixtures: Production strategies and cost considerations. Arch. Biochem. Biophys. 2012, 526, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Windyga, J.; Trelinski, J.; von Depka Prondzinski, M.; Giagounidis, A.; Doyen, C.; Janssens, A.; Alvarez-Román, M.T.; Jarque, I.; Loscertales, J.; et al. Rozrolimupab, a mixture of 25 recombinant human monoclonal RhD antibodies, in the treatment of primary immune thrombocytopenia. Blood 2012, 120, 3670–3676. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.J.; Poulsen, T.T.; Dahlman, A.; Kjær, I.; Koefoed, K.; Sen, J.W.; Weilguny, D.; Bjerregaard, B.; Andersen, C.R.; Horak, I.D.; et al. Pan-HER, an Antibody Mixture Simultaneously Targeting EGFR, HER2, and HER3, Effectively Overcomes Tumor Heterogeneity and Plasticity. Clin. Cancer Res. 2015, 21, 4110–4122. [Google Scholar] [CrossRef] [PubMed]
- Montagut, C.; Argilés, G.; Ciardiello, F.; Poulsen, T.T.; Dienstmann, R.; Kragh, M.; Kopetz, S.; Lindsted, T.; Ding, C.; Vidal, J.; et al. Efficacy of Sym004 in Patients with Metastatic Colorectal Cancer with Acquired Resistance to Anti-EGFR Therapy and Molecularly Selected by Circulating Tumor DNA Analyses: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e175245. [Google Scholar] [CrossRef] [PubMed]
- Klutz, S.; Holtmann, L.; Lobedann, M.; Schembecker, G. Cost evaluation of antibody production processes in different operation modes. Chem. Eng. Sci. 2016, 141, 63–74. [Google Scholar] [CrossRef]
- Hammerschmidt, N.; Tscheliessnig, A.; Sommer, R.; Helk, B.; Jungbauer, A. Economics of recombinant antibody production processes at various scales: Industry-standard compared to continuous precipitation. Biotechnol. J. 2014, 9, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Gronemeyer, P.; Ditz, R.; Strube, J. Trends in Upstream and Downstream Process Development for Antibody Manufacturing. Bioengineering 2014, 1, 188–212. [Google Scholar] [CrossRef] [PubMed]
- Novais, J.L.; Titchener-Hooker, N.J.; Hoare, M. Economic comparison between conventional and disposables-based technology for the production of biopharmaceuticals. Biotechnol. Bioeng. 2001, 75, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Klutz, S.; Magnus, J.; Lobedann, M.; Schwan, P.; Maiser, B.; Niklas, J.; Temming, M.; Schembecker, G. Developing the biofacility of the future based on continuous processing and single-use technology. J. Biotechnol. 2015, 213, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.A.; Gottschalk, U. Single-use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 2013, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.S.; Washbrook, J.; Titchener-Hooker, N.J. Decision-Support Tool for Assessing Biomanufacturing Strategies under Uncertainty: Stainless Steel versus Disposable Equipment for Clinical Trial Material Preparation. Biotechnol. Prog. 2005, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. RSV Vaccine Research and Development Technology Roadmap: Priority Activities for Development, Testing, Licensure and Global Use of RSV Vaccines, with a Specific Focus on the Medical Need for Young Children in Low-and Middle-income Countries; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Pathmeswaran, A.; Kasturiratne, A.; Fonseka, M.; Nandasena, S.; Lalloo, D.G.; de Silva, H.J. Identifying the biting species in snakebite by clinical features: An epidemiological tool for community surveys. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Solano, G.; Pla, D.; Herrera, M.; Segura, Á.; Vargas, M.; Villalta, M.; Sánchez, A.; Sanz, L.; Lomonte, B.; et al. Preclinical Evaluation of the Efficacy of Antivenoms for Snakebite Envenoming: State-of-the-Art and Challenges Ahead. Toxins 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Sells, P.G. Animal experimentation in snake venom research and in vitro alternatives. Toxicon 2003, 42, 115–133. [Google Scholar] [CrossRef]
- Liberti, L.; Breckenridge, A.; Hoekman, J.; Leufkens, H.; Lumpkin, M.; McAuslane, N.; Stolk, P.; Zhi, K.; Rägo, L. Accelerating access to new medicines: Current status of facilitated regulatory pathways used by emerging regulatory authorities. J. Public Health Policy 2016, 37, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Doua, J.Y.; Van Geertruyden, J.-P. Registering medicines for low-income countries: How suitable are the stringent review procedures of the World Health Organization, the US Food and Drug Administration and the European Medicines Agency? Trop. Med. Int. Health 2014, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.F. Developing drug prototypes: Pharmacology replaces safety and tolerability? Nat. Rev. Drug Discov. 2010, 9, 865. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laustsen, A.H.; Dorrestijn, N. Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms. Toxins 2018, 10, 309. https://doi.org/10.3390/toxins10080309
Laustsen AH, Dorrestijn N. Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms. Toxins. 2018; 10(8):309. https://doi.org/10.3390/toxins10080309
Chicago/Turabian StyleLaustsen, Andreas Hougaard, and Netty Dorrestijn. 2018. "Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms" Toxins 10, no. 8: 309. https://doi.org/10.3390/toxins10080309
APA StyleLaustsen, A. H., & Dorrestijn, N. (2018). Integrating Engineering, Manufacturing, and Regulatory Considerations in the Development of Novel Antivenoms. Toxins, 10(8), 309. https://doi.org/10.3390/toxins10080309





