Monocentric Prospective Study into the Sustained Effect of Incobotulinumtoxin A (XEOMIN®) Botulinum Toxin in Chronic Refractory Migraine
Abstract
1. Introduction
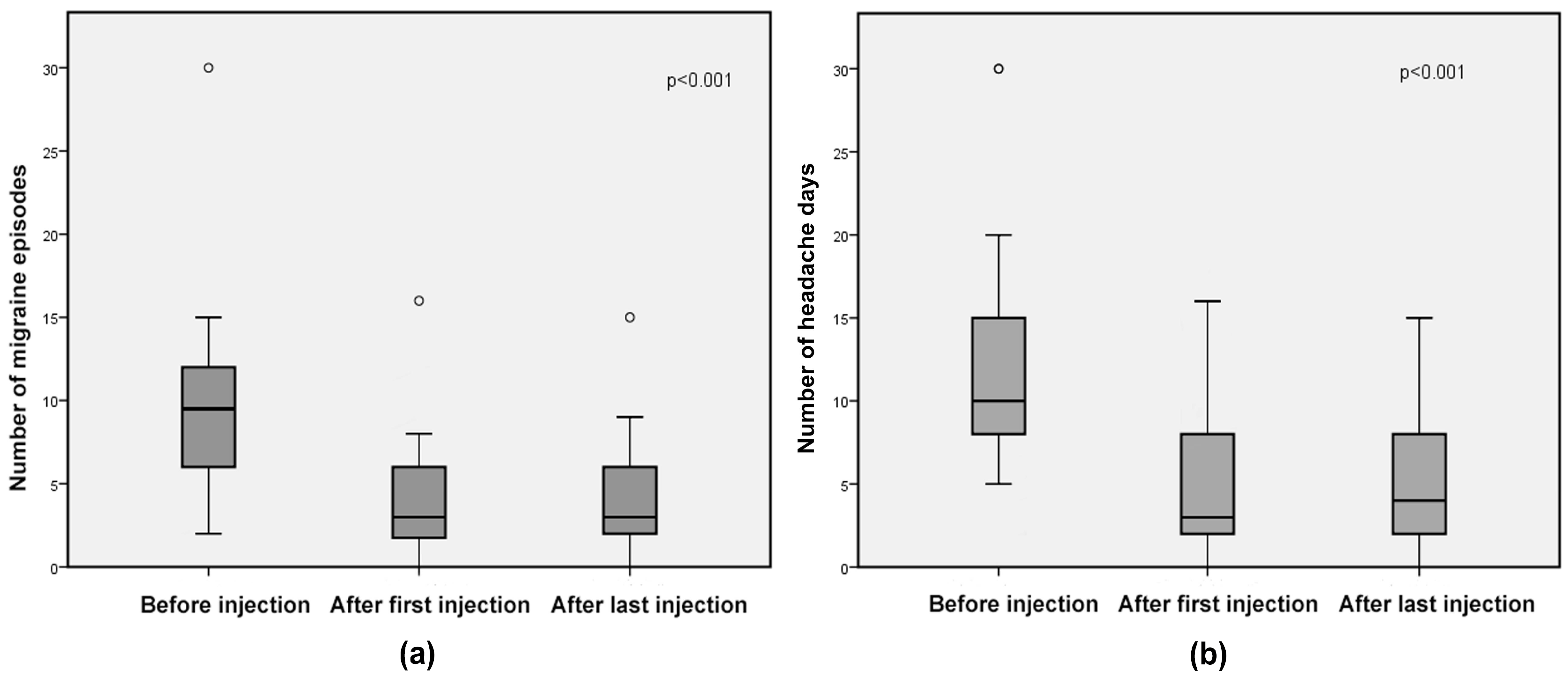
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stovner, L.; Hagen, K.; Jensen, R.; Katsarava, Z.; Lipton, R.; Scher, A.; Steiner, T.; Zwart, J.-A. The Global Burden of Headache: A Documentation of Headache Prevalence and Disability Worldwide. Cephalalgia 2007, 27, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Shamliyan, T.A.; Choi, J.-Y.; Ramakrishnan, R.; Miller, J.B.; Wang, S.-Y.; Taylor, F.R.; Kane, R.L. Preventive Pharmacologic Treatments for Episodic Migraine in Adults. J. Gen. Intern. Med. 2013, 28, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.L.; Cogbill, E.; Santana-Davila, R.; Eldredge, C.; Collier, W.; Gradall, A.; Sehgal, N.; Kuester, J. A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache. PLoS ONE 2015, 10, e0130733. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Winner, P.; Freeman, M.C.; Spierings, E.L.; Heiring, J.O.; DeGryse, R.E.; VanDenburgh, A.M.; Nolan, M.E.; Turkel, C.C. OnabotulinumtoxinA for Treatment of Chronic Migraine: Pooled Analyses of the 56-Week PREEMPT Clinical Program. Headache 2011, 51, 1358–1373. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Aurora, S.K.; Silberstein, S.D.; Lipton, R.B.; Diener, H.-C.; Brin, M.F. OnabotulinumtoxinA for Treatment of Chronic Migraine: Pooled Results From the Double-Blind, Randomized, Placebo-Controlled Phases of the PREEMPT Clinical Program. Headache 2010, 50, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Blumenfeld, A.M.; Cady, R.K.; Turner, I.M.; Lipton, R.B.; Diener, H.-C.; Aurora, S.K.; Sirimanne, M.; DeGryse, R.E.; Turkel, C.C.; et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J. Neurol. Sci. 2013, 331, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Wei, Y.; Lian, Y.; Chen, Y.; Zheng, Y. Treatment of chronic daily headache with comorbid anxiety and depression using botulinum toxin A: A prospective pilot study. Int. J. Neurosci. 2017, 127, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Yaksh, T.L. Therapeutic use of botulinum toxin in migraine: Mechanisms of action: Botulinum toxins as a prophylaxis for migraine. Br. J. Pharmacol 2014, 171, 4177–4192. [Google Scholar] [CrossRef] [PubMed]
- Luvisetto, S.; Gazerani, P.; Cianchetti, C.; Pavone, F. Botulinum Toxin Type A as a Therapeutic Agent against Headache and Related Disorders. Toxins 2015, 7, 3818–3844. [Google Scholar] [CrossRef] [PubMed]
- Shuhendler, A.J.; Lee, S.; Siu, M.; Ondovcik, S.; Lam, K.; Alabdullatif, A.; Zhang, X.; Machado, M.; Einarson, T.R. Efficacy of Botulinum Toxin Type A for the Prophylaxis of Episodic Migraine Headaches: A Meta-analysis of Randomized, Double-Blind, Placebo-Controlled Trials. Pharmacotherapy 2009, 29, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.; Göbel, H.; Jensen, R.; Elkind, A.; DeGryse, R.; Walcott, J.; Turkel, C. Botulinum Toxin Type A in the Prophylactic Treatment of Chronic Tension-Type Headache: A Multicentre, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study. Cephalalgia 2006, 26, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Durham, P.L.; Cady, R. Insights into the Mechanism of OnabotulinumtoxinA in Chronic Migraine: November/December 2011. Headache 2011, 51, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Frevert, J.; Dressler, D. Complexing proteins in botulinum toxin type A drugs: A help or a hindrance? Biol. Targets Ther. 2010, 325. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.; Dodick, D.; Aurora, S.; Turkel, C.; DeGryse, R.; Lipton, R.; Silberstein, S.; Brin, M. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, R.; Lim, J.; Ashley Blake, P.; Lessig, S. IncobotulinumtoxinA for Migraine: A Retrospective Case Series. Clin. Ther. 2015, 37, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Ranoux, D.; Martiné, G.; Espagne-Dubreuilh, G.; Amilhaud-Bordier, M.; Caire, F.; Magy, L. OnabotulinumtoxinA injections in chronic migraine, targeted to sites of pericranial myofascial pain: An observational, open label, real-life cohort study. J. Headache Pain 2017, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]


| Demographic Features | |
| Sex: men/women | 8/53 |
| Age: years, mean ± SD | 50 ± 10 |
| Headache type | |
| Isolated chronic migraine | 20 (33%) |
| Chronic migraine + tension-type headache | 18 (29%) |
| Chronic migraine + medication overuse headache | 12 (20%) |
| Episodic disabling migraine | 11 (18%) |
| XEOMIN® responders | 44 (73%) |
| Mean number of injections | 3.5 (2–13) |
| Mean duration of treatment (months) | 21 (6–68) |
| Mean duration of effect (weeks) | 13.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, I.; Renard, D.; Le Floch, A.; De Verdal, M.; Bouly, S.; Wacongne, A.; Lozza, A.; Castelnovo, G. Monocentric Prospective Study into the Sustained Effect of Incobotulinumtoxin A (XEOMIN®) Botulinum Toxin in Chronic Refractory Migraine. Toxins 2018, 10, 221. https://doi.org/10.3390/toxins10060221
Ion I, Renard D, Le Floch A, De Verdal M, Bouly S, Wacongne A, Lozza A, Castelnovo G. Monocentric Prospective Study into the Sustained Effect of Incobotulinumtoxin A (XEOMIN®) Botulinum Toxin in Chronic Refractory Migraine. Toxins. 2018; 10(6):221. https://doi.org/10.3390/toxins10060221
Chicago/Turabian StyleIon, Ioana, Dimitri Renard, Anne Le Floch, Marie De Verdal, Stephane Bouly, Anne Wacongne, Alessandro Lozza, and Giovanni Castelnovo. 2018. "Monocentric Prospective Study into the Sustained Effect of Incobotulinumtoxin A (XEOMIN®) Botulinum Toxin in Chronic Refractory Migraine" Toxins 10, no. 6: 221. https://doi.org/10.3390/toxins10060221
APA StyleIon, I., Renard, D., Le Floch, A., De Verdal, M., Bouly, S., Wacongne, A., Lozza, A., & Castelnovo, G. (2018). Monocentric Prospective Study into the Sustained Effect of Incobotulinumtoxin A (XEOMIN®) Botulinum Toxin in Chronic Refractory Migraine. Toxins, 10(6), 221. https://doi.org/10.3390/toxins10060221




