Acid Sphingomyelinase Promotes Cellular Internalization of Clostridium perfringens Iota-Toxin
Abstract
:1. Introduction
2. Results
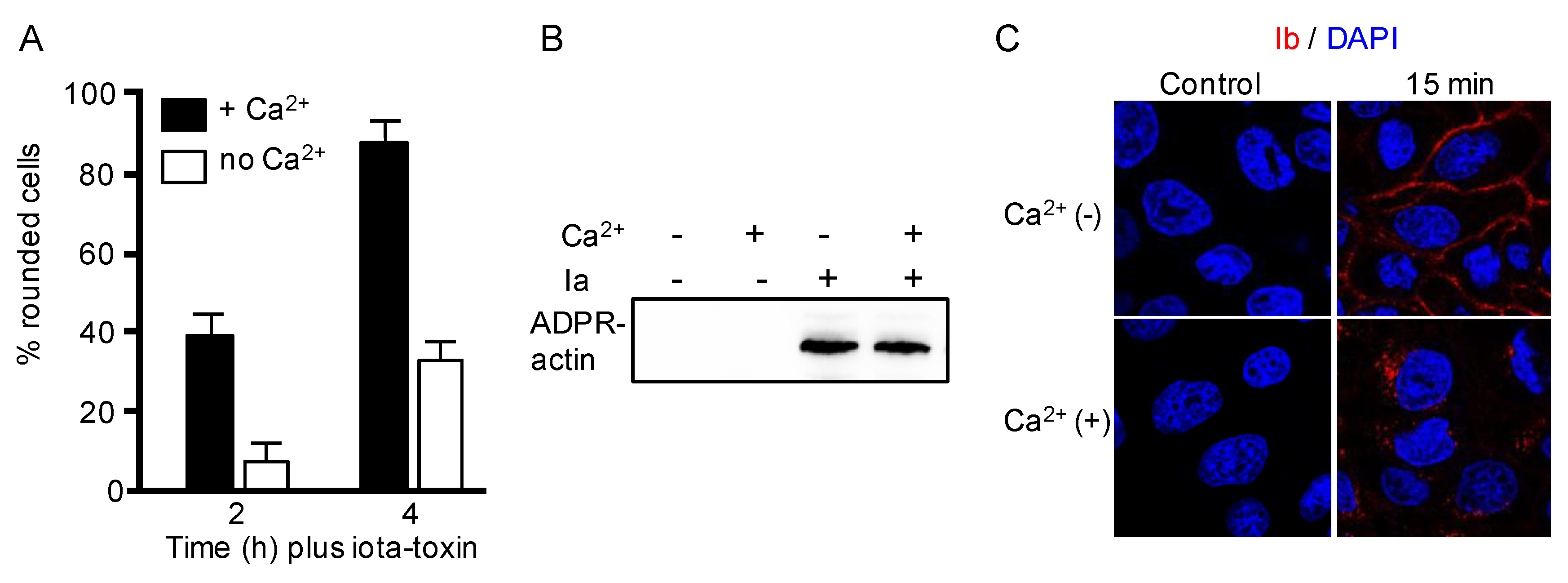
2.1. Calcium Ion Accelerates Endocytosis of Iota-Toxin
2.2. Ib Promotes Exocytosis of Lysosomal Acid Sphingomyelinase
2.3. Role of Acid Sphingomyelinase on Cellular Uptake of Iota-Toxin
2.4. Effects of ASMase-siRNA on Iota-Toxin-Caused Cytotoxicity
2.5. Iota-Toxin Causes An Increase in Ceramide
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Cell Culture Assay
5.3. Measurement of Acidic or Neutral Sphingomyelinase Activities
5.4. Assay of β-Hexosaminidase
5.5. Assay of ADP-Ribosylation
5.6. Si RNA Transfection
5.7. Immunocytochemistry
5.8. Determination of Ceramide
5.9. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sakurai, J.; Nagahama, M.; Oda, M.; Tsuge, H.; Kobayashi, K. Clostridium perfringens iota-toxins: Structure and function. Toxins (Basel) 2009, 1, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Lang, A.E.; Schwan, C.; Mannherz, H.G. Actin as target for modification by bacterial protein toxins. FEBS J. 2011, 278, 4526–4543. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Schwan, C.; Papatheodorou, P.; Lang, A.E. Bidirectional attack on the actin cytoskeleton. Bacterial protein toxins causing polymerization or depolymerization of actin. Toxicon 2012, 60, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Stiles, B.G.; Pradhan, K.; Fleming, J.M.; Samy, R.P.; Barth, H.; Popoff, M.R. Clostridium and Bacillus binary enterotoxins: Bad for the bowels, and eukaryotic being. Toxins (Basel) 2014, 6, 2626–2656. [Google Scholar] [CrossRef] [PubMed]
- Knapp, O.; Benz, R.; Popoff, M.R. Pore-forming activity of clostridial binary toxins. Biochim. Biophys. Acta 2015, 1858, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Takehara, M.; Takagishi, T.; Seike, S.; Oda, M.; Sakaguchi, Y.; Hisatsune, J.; Ochi, S.; Kobayashi, K.; Nagahama, M. Cellular entry of Clostridium perfringens iota-toxin and Clostridium botulinum C2 toxin. Toxins (Basel) 2017, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, H.; Nagahama, M.; Oda, M.; Iwamoto, S.; Utsunomiya, H.; Marquez, V.E.; Katunuma, N.; Nishizawa, M.; Sakurai, J. Structural basis of actin recognition and arginine ADP-ribosylation by Clostridium perfringens iota-toxin. Proc. Natl. Acad. Sci. USA 2008, 105, 7399–7404. [Google Scholar] [CrossRef] [PubMed]
- Tsurumura, T.; Tsumori, Y.; Qiu, H.; Oda, M.; Sakurai, J.; Nagahama, M.; Tsuge, H. Arginine ADP-ribosylation mechanism based on structural snapshots of iota-toxin and actin complex. Proc. Natl. Acad. Sci. USA 2013, 110, 4267–4272. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, P.; Carette, J.E.; Bell, G.W.; Schwan, C.; Guttenberg, D.; Brummelkamp, T.R.; Aktories, K. Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT). Proc. Natl. Acad. Sci. USA 2011, 108, 16422–16427. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Nagayasu, K.; Kobayashi, K.; Sakurai, J. Binding component of Clostridium perfringens iota-toxin induces endocytosis in Vero cells. Infect. Immun. 2002, 70, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Yamaguchi, A.; Hagiyama, T.; Ohkubo, N.; Kobayashi, K.; Sakurai, J. Binding and internalization of Clostridium perfringens iota-toxin in lipid rafts. Infect. Immun. 2004, 72, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Umezaki, M.; Tashiro, R.; Oda, M.; Kobayashi, K.; Shibutani, M.; Takagishi, T.; Ishidoh, K.; Fukuda, M.; Sakurai, J. Intracellular trafficking of Clostridium perfringens iota-toxin b. Infect. Immun. 2012, 80, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Umezaki, M.; Oda, M.; Kobayashi, K.; Tone, S.; Suda, T.; Ishidoh, K.; Sakurai, J. Clostridium perfringens iota-toxin b induces rapid cell necrosis. Infect. Immun. 2011, 79, 4353–4360. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Gulbins, E.; Brenner, B.; Ferlinz, K.; Sandhoff, K.; Harzer, K.; Lang, F.; Meyer, T.F. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 1997, 91, 605–615. [Google Scholar] [CrossRef]
- Utermöhlen, O.; Karow, U.; Lohler, J.; Krönke, M. Severe impairment in early host defense against Listeria monocytogenes in mice deficient in acid sphingomyelinase. J. Immunol. 2003, 170, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Jendrossek, V.; Riehle, A.; von Kurthy, G.; Berger, J.; Schwarz, H.; Weller, M.; Kolesnick, R.; Gulbins, E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 2003, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Falcone, S.; Perrotta, C.; De Palma, C.; Pisconti, A.; Sciorati, C.; Capobianco, A.; Rovere-Querini, P.; Manfredi, A.A.; Clementi, E. Activation of acid sphingomyelinase and its inhibition by the nitric oxide/cyclic guanosine 3′,5′-monophosphate pathway: Key events in Escherichia coli-elicited apoptosis of dendritic cells. J. Immunol. 2004, 173, 4452–4463. [Google Scholar] [CrossRef] [PubMed]
- Simonis, A.; Hebling, S.; Gulbins, E.; Schneider-Schaulies, S.; Schubert-Unkmeir, A. Differential activation of acid sphingomyelinase and ceramide release determines invasiveness of Neisseria meningitidis into brain endothelial cells. PLoS Pathog. 2014, 10, e1004160. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; Idone, V.; Devlin, C.; Fernandes, M.C.; Flannery, A.; He, X.; Schuchman, E.; Tabas, I.; Andrews, N.W. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 2010, 189, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Los, F.C.O.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.W.; Almeida, P.E.; Corrotte, M. Damage control: Cellular mechanisms of plasma membrane repair. Trends Cell. Biol. 2014, 24, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Takehara, M.; Takagishi, T.; Seike, S.; Miyamoto, K.; Kobayashi, K. Cellular uptake of Clostridium botulinum C2 Toxin requires acid sphingomyelinase activity. Infect. Immun. 2017, 85, e00966-16. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nagahama, M.; Ohkubo, N.; Kojima, T.; Shirai, H.; Iwamoto, S.; Oda, M.; Sakurai, J. Role of Ca2+-binding motif in cytotoxicity induced by Clostridium perfringens iota-toxin. Microb. Pathog. 2008, 44, 265–270. [Google Scholar] [CrossRef] [PubMed]
- McNeil, P.L.; Miyake, K.; Vogel, S.S. The endomembrane requirement for cell surface repair. Proc. Natl. Acad. Sci. USA 2003, 100, 4592–4597. [Google Scholar] [CrossRef] [PubMed]
- Walev, I.; Bhakdi, S.C.; Hofmann, F.; Djonder, N.; Valeva, A.; Aktories, K.; Bhakdi, S. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc. Natl. Acad. Sci. USA 2001, 98, 3185–3190. [Google Scholar] [CrossRef] [PubMed]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Schissel, S.L.; Schuchman, E.H.; Williams, K.J.; Tabas, I. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J. Biol. Chem. 1996, 271, 18431–18436. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.M.; Angelova, M.I.; Kinnunen, P.K. Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys. J. 2000, 78, 830–838. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Draeger, A.; Babiychuk, E.B. Ceramide in plasma membrane repair. Handb. Exp. Pharmacol. 2013, 216, 341–353. [Google Scholar] [CrossRef]
- Papatheodorou, P.; Hornuss, D.; Nölke, T.; Hemmasi, S.; Castonguay, J.; Picchianti, M.; Aktories, K. Clostridium difficile binary toxin CDT induces clustering of the lipolysis-stimulated lipoprotein receptor into lipid rafts. MBio 2013, 4, e00244-13. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, P.; Wilczek, C.; Nölke, T.; Guttenberg, G.; Hornuss, D.; Schwan, C.; Aktories, K. Identification of the cellular receptor of Clostridium spiroforme toxin. Infect. Immun. 2012, 80, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagahama, M.; Takehara, M.; Miyamoto, K.; Ishidoh, K.; Kobayashi, K. Acid Sphingomyelinase Promotes Cellular Internalization of Clostridium perfringens Iota-Toxin. Toxins 2018, 10, 209. https://doi.org/10.3390/toxins10050209
Nagahama M, Takehara M, Miyamoto K, Ishidoh K, Kobayashi K. Acid Sphingomyelinase Promotes Cellular Internalization of Clostridium perfringens Iota-Toxin. Toxins. 2018; 10(5):209. https://doi.org/10.3390/toxins10050209
Chicago/Turabian StyleNagahama, Masahiro, Masaya Takehara, Kazuaki Miyamoto, Kazumi Ishidoh, and Keiko Kobayashi. 2018. "Acid Sphingomyelinase Promotes Cellular Internalization of Clostridium perfringens Iota-Toxin" Toxins 10, no. 5: 209. https://doi.org/10.3390/toxins10050209
APA StyleNagahama, M., Takehara, M., Miyamoto, K., Ishidoh, K., & Kobayashi, K. (2018). Acid Sphingomyelinase Promotes Cellular Internalization of Clostridium perfringens Iota-Toxin. Toxins, 10(5), 209. https://doi.org/10.3390/toxins10050209



