Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer
Abstract
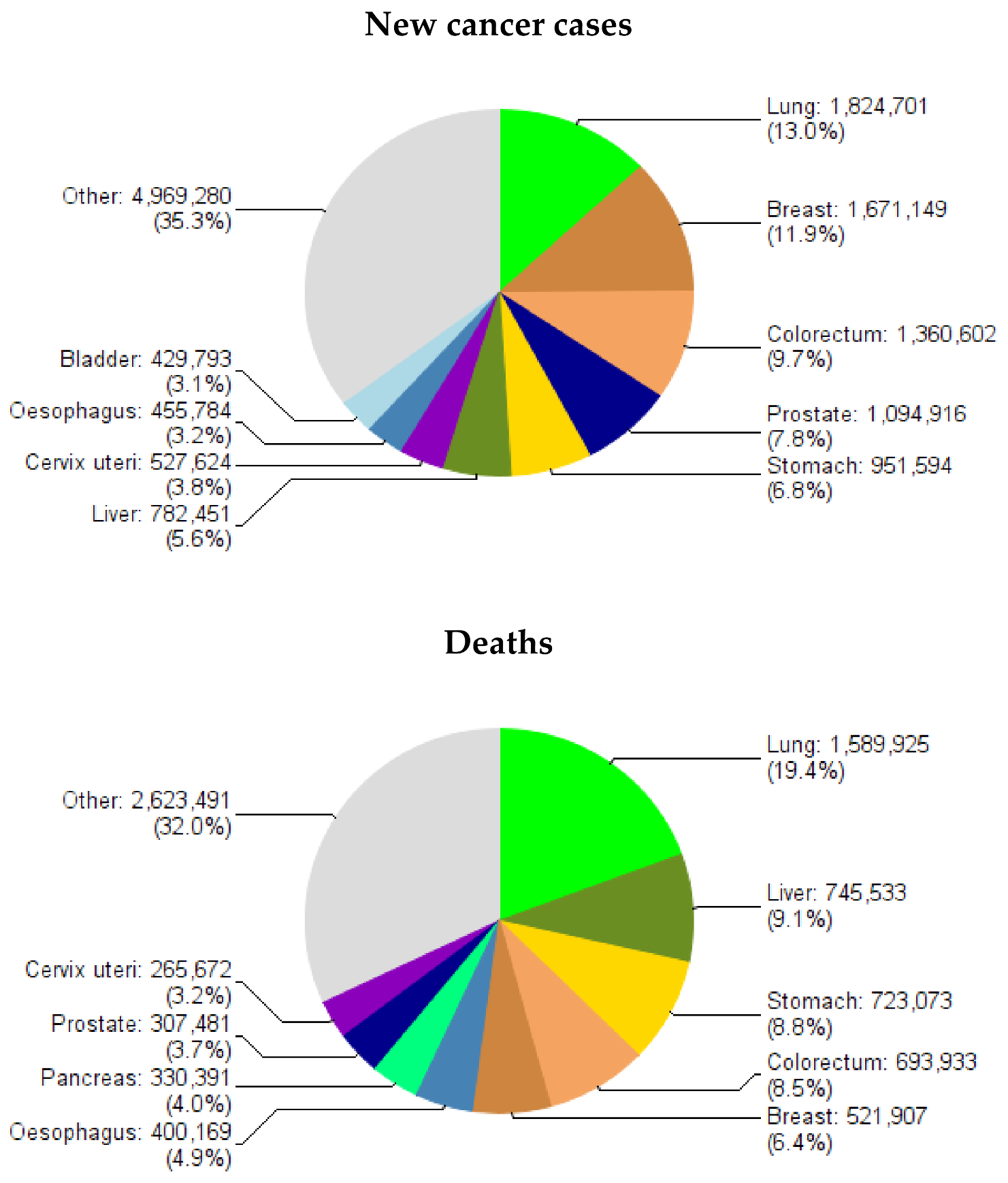
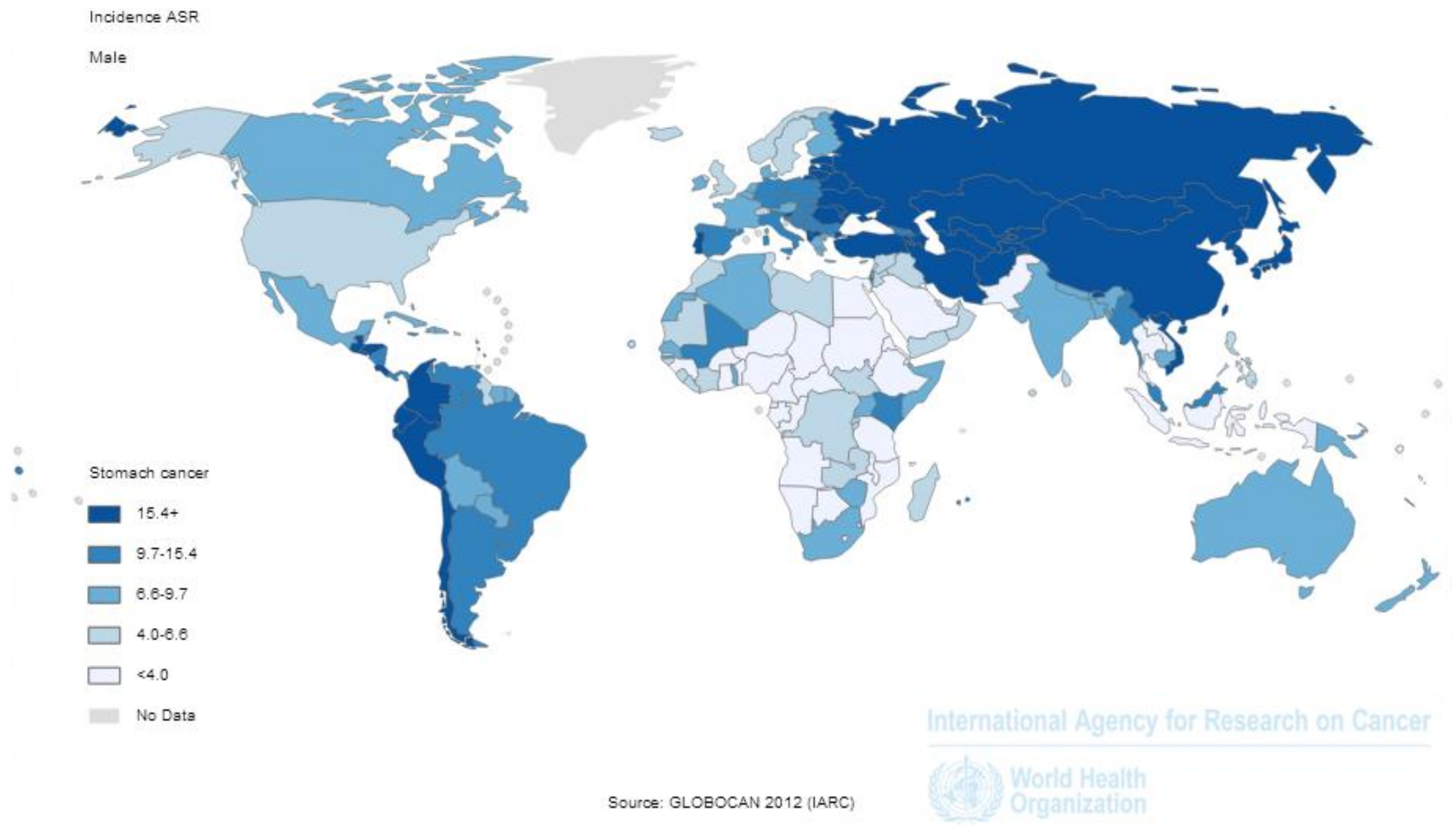
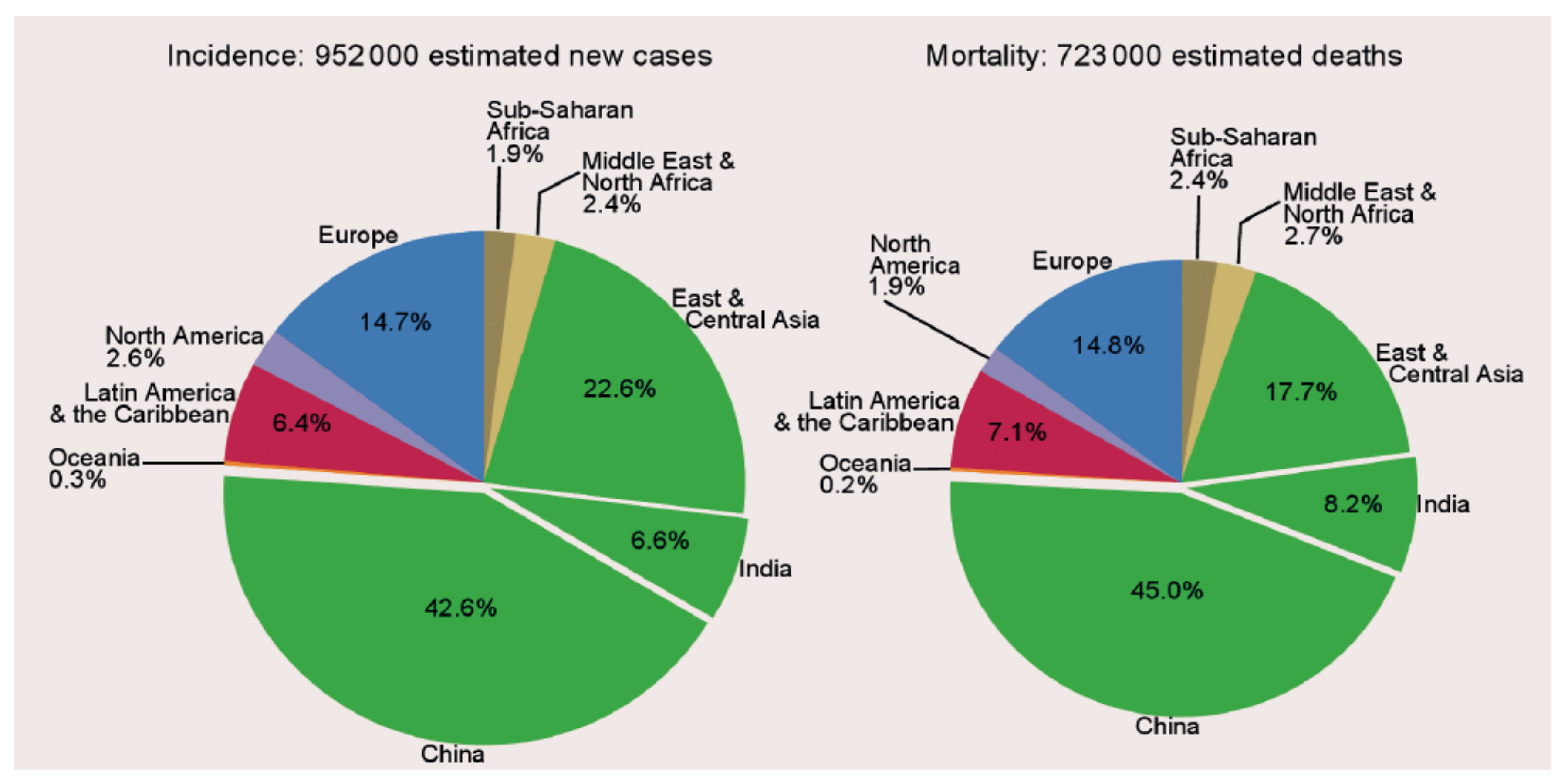
1. Introduction: The Global Burden of Gastric Cancer
2. The Association of Gastric Cancer with Helicobacter pylori Infection
2.1. Evaluation by International Agency for Research on Cancer (IARC) Working Groups
2.2. Quantification of Risk—Comparison of Western Blot and Serology Data
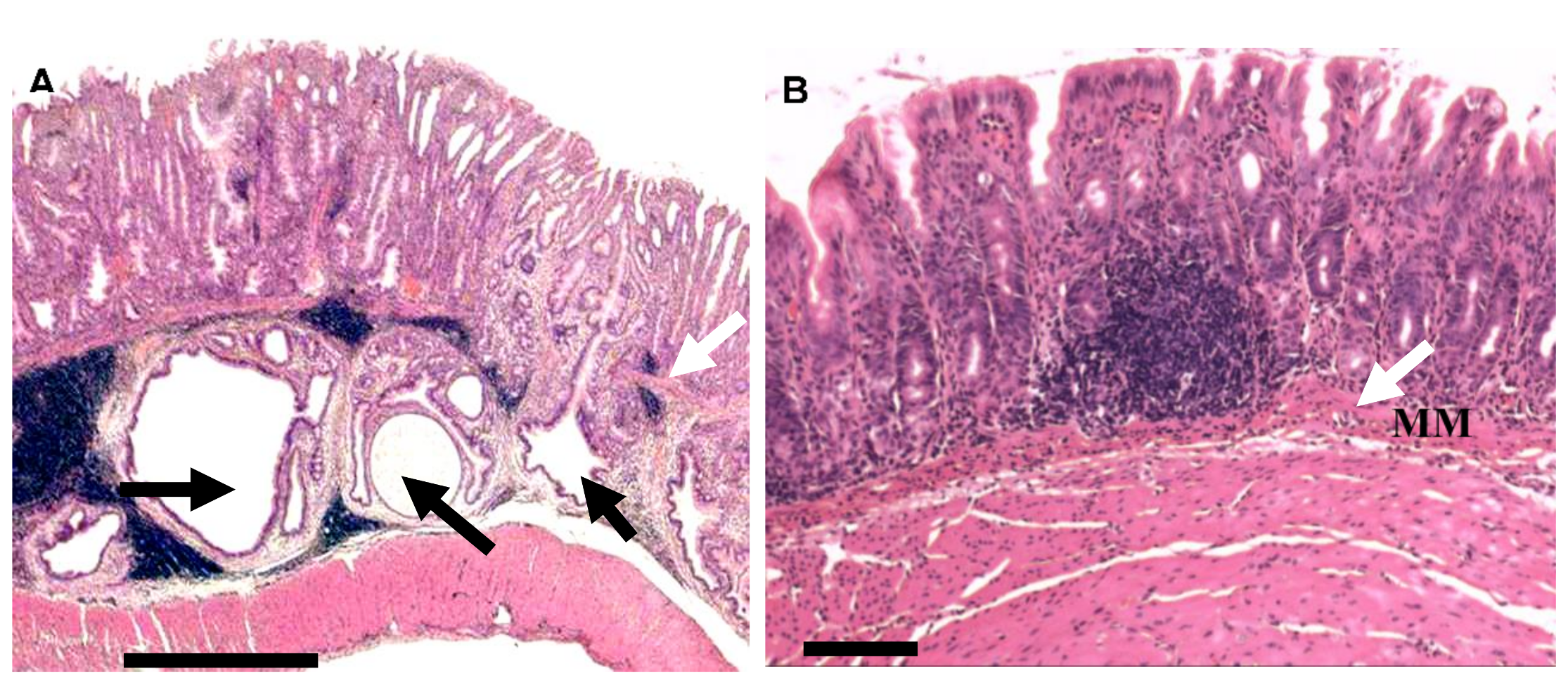
2.3. Other Evidence: Animal Studies/Mechanistic Understanding
3. H. pylori Infection: Geographic and Time Trends
4. H. pylori Strain Variation and Gastric Cancer Risk
4.1. CagA and the Cag Pathogenicity Island
4.2. CagA Serology and Corpus Atrophic Gastritis
4.3. CagA Seropositivity and Risk of Non-Cardia Gastric Cancer
4.4. Cholesterol Reduction Impacts on H. pylori-Induced Gastric Cancer Risk.
4.5. Lack of Association of CagA Seropositivity with Oesophageal Adenocarcinoma
4.6. Microbiological Evidence Linking CagA/cag PAI Strains with Gastric Cancer
4.7. Environmental Factors Regulating H. pylori CagA Expression
5. H. pylori Eradication for Gastric Cancer Prevention
5.1. Critical Evidence: Prospective Studies and Trials
5.2. Strategies for Eradication of H. pylori Infection and Their Evaluation
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available online: http://globocan.iarc.fr (accessed on 28 February 2017).
- Bray, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Zanetti, R.; Ferlay, J. (Eds.) (2017) Cancer Incidence in Five Continents, Vol. XI (Electronic Version). Lyon: International Agency for Research on Cancer. Available online: http://ci5.iarc.fr (accessed on 18 February 2018).
- Crew, K.D.; Neugut, A.I. Epidemiology of gastric cancer. World J. Gastroenterol. 2006, 12, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Bray, F.; Steliarova-Foucher, E.; Forman, D. (2014) Cancer Incidence in Five Continents, CI5plus. IARC CancerBase No. 9 Lyon: International Agency for Research on Cancer; 2014. Available online: http://ci5.iarc.f (accessed on 28 March 2017).
- Colquhoun, A.; Arnold, M.; Ferlay, J.; Goodman, K.J.; Forman, D.; Soerjomataram, I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 2015, 64, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, Liver Flukes and Helicobacter pylori. 1994. Available online: http://monographs.iarc.fr/ENG/Monographs/vol61/ (accessed on 22 March 2018).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens. Part B: Biological Agents. 2012. Available online: http://monographs.iarc.fr/ENG/Monographs/vol100B/mono100B.pdf (accessed on 22 March 2018).
- Mitchell, H.; English, D.R.; Elliott, F.; Gengos, M.; Barrett, J.H.; Giles, G.G.; Forman, D. Immunoblotting using multiple antigens is essential to demonstrate the true risk of Helicobacter pylori infection for gastric cancer. Aliment. Pharmacol. Ther. 2008, 28, 903–910. [Google Scholar] [PubMed]
- Simán, J.H.; Engstrand, L.; Berglund, G.; Forsgren, A.; Florén, C.H. Helicobacter pylori and CagA seropositivity and its association with gastric and oesophageal carcinoma. Scand. J. Gastroenterol. 2007, 42, 933–940. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Megraud, F.; Buissonniere, A.; Barroso, L.; Agudo, A.; Duell, E.J.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Palli, D.; Krogh, V.; et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: The Eurgast-EPIC project. Ann. Oncol. 2012, 23, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.E.; Wyatt, J.I.; Sobala, G.M.; Miller, G.; Tompkins, D.S.; Primrose, J.N.; Morgan, A.G. Systemic and mucosal humoral responses to Helicobacter pylori in gastric cancer. Gut 1993, 34, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tada, M.; Nagai, H.; Sasaki, S.; Nakao, M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology 1998, 115, 642–648. [Google Scholar] [CrossRef]
- Franco, A.T.; Johnston, E.; Krishna, U.; Yamaoka, Y.; Israel, D.A.; Nagy, T.A.; Wroblewski, L.E.; Piazuelo, M.B.; Correa, P. Peek, R.M. Regulation of Gastric Carcinogenesis by Helicobacter pylori Virulence Factors. Cancer Res. 2008, 68, 79–387. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Fujioka, T.; Tokieda, M.; Satoh, R.; Nishizono, A.; Nasu, M. Development of Helicobacter pylori induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998, 58, 4255–4259. [Google Scholar] [PubMed]
- Zheng, Q.; Chen, X.Y.; Shi, Y.A.; Xiao, S.D. Development of gastric adenocarcinoma in Mongolian gerbils after long-term infection with Helicobacter pylori. J. Gastroenterol. Hepatol. 2004, 19, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, F.; Takagi, S.; Iwao, E.; Yokoyama, Y.; Haga, K.; Hanada, S. Development of poorly differentiated adenocarcinoma and carcinoid due to long-term Helicobacter pylori colonization in Mongolian gerbils. J. Gastroenterol. 1999, 34, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gallo, J.; Harris, E.J.; Krishna, U.; Washington, M.K.; Perez-Perez, G.I.; Peek, R.M. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab. Investig. 2008, 88, 328–336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franco, A.T.; Israel, D.A.; Washington, M.K.; Krishna, U.; Fox, J.G.; Rogers, A.B.; Neish, A.S.; Collier-Hyams, L.; Perez-Perez, G.I.; Hatakeyama, M.; et al. Activation of β-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA 2005, 102, 10646–10651. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Willen, R.; Svensson, M.; Ljungh, Å.; Wadstrom, T. Two-year follow-up of Helicobacter pylori infection in C57BL/6 and Balb/cA mice. APMIS 2003, 111, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.B.; Taylor, N.S.; Whary, M.T.; Stefanich, E.D.; Wang, T.C.; Fox, J.G. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005, 65, 10709–10715. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.W.; Song, Y.J.; Oh, T.Y.; Han, S.U.; Kim, Y.B.; Joo, H.J.; Cho, Y.K.; Kim, D.Y.; Cho, S.W.; et al. Long-term evaluation of mice model infected with Helicobacter pylori focus on gastric pathology including gastric cancer. Aliment. Pharmacol. Ther. 2003, 18, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hahm, K.B.; Lee, K.M.; Kim, Y.B.; Hong, W.S.; Lee, W.H.; Han, S.U.; Kim, M.W.; Ahn, B.O.; Oh, T.Y.; Lee, M.H.; et al. Conditional loss of TGF-β signalling leads to increased susceptibility to gastrointestinal carcinogenesis in mice. Aliment. Pharmacol. Ther. 2002, 16, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Kuzushita, N.; Rogers, A.B.; Monti, N.A.; Whary, M.T.; Park, M.J.; Aswad, B.I.; Shirin, H.; Koff, A.; Eguchi, H.; Moss, S.F. p27kip1 deficiency confers susceptibility to gastric carcinogenesis in Helicobacter pylori–infected mice. Gastroenterology 2005, 129, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Rogers, A.B.; Whary, M.T.; Ge, Z.; Ohtani, M.; Jones, E.K.; Wang, T.C. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2−/−C57BL6 × Sv129 Helicobacter pylori-infected mice. Am. J. Pathol. 2007, 171, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Rogers, A.B.; Ihrig, M.; Taylor, N.S.; Whary, M.T.; Dockray, G.; Varro, A.; Wang, T.C. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 2003, 63, 942–950. [Google Scholar] [PubMed]
- Lee, C.W.; Rickman, B.; Rogers, A.B.; Ge, Z.; Wang, T.C.; Fox, J.G. Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res. 2008, 68, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lee, D.S.; Morrissey, R.; Aponte-Pieras, J.R.; Rogers, A.B.; Moss, S.F. Early or late antibiotic intervention prevents Helicobacter pylori-induced gastric cancer in a mouse model. Cancer Lett. 2015, 359, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Peek, R.M.; Crabtree, J.E. Helicobacter infection and gastric neoplasia. J. Pathol. 2006, 208, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.E.; Court, M.; Aboshkiwa, M.A.; Jeremy, A.H.T.; Dixon, M.F.; Robinson, P.A. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori in Mongolian gerbils. J. Pathol. 2004, 202, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wallasch, C.; Crabtree, J.E.; Bevec, D.; Robinson, P.A.; Wagner, H.; Ullrich, A. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem. Biophys. Res. Commun. 2002, 295, 695–701. [Google Scholar] [CrossRef]
- Keates, S.; Sougioultzis, S.; Keates, A.C.; Zhao, D.; Peek, R.M.; Shaw, L.M.; Kelly, C.P. cag + Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J. Biol. Chem. 2001, 276, 48127–48134. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.E.; Jeremy, A.H.T.; Duval, C.; Dixon, M.F.; Danjo, K.; Carr, I.M.; Pritchard, D.M.; Robinson, P.A. Effects of EGFR inhibitor on Helicobacter pylori induced gastric epithelial pathology in vivo. Pathogens 2013, 2, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.C.; Asim, M.; Verriere, T.G.; Piazuelo, M.B.; Suarez, G.; Romero-Gallo, J.; Delgado, A.G.; Wroblewski, L.E.; Barry, D.P.; Peek, R.M.; et al. Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis. Gut 2018. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, B.; Bastos, A.; Ferro, A.; Lunet, N. Prevalence of Helicobacter pylori infection worldwide: A systematic review of studies with national coverage. Dig. Dis. Sci. 2014, 59, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Brenner, H.; Arndt, V.; Stegmaier, C.; Ziegler, H.; Rothenbacher, D. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am. J. Epidemiol. 2004, 159, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.E.; Taylor, J.D.; Wyatt, J.I.; Heatley, R.V.; Shallcross, T.M.; Tompkins, D.S.; Rathbone, B.J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration and gastric pathology. Lancet 1991, 338, 332–335. [Google Scholar] [CrossRef]
- Censini, S.; Lange, N.; Xiang, Z.; Crabtree, J.E.; Ghiara, P.; Borodovsky, M.; Rappuoli, R.; Covacci, A. cag, a pathogenicity island of Helicobacter pylori encodes Type I-specific and disease associated virulence factors. Proc. Natl. Acad. Sci. USA 1996, 93, 14648–14653. [Google Scholar] [CrossRef]
- Akopyants, N.S.; Clifton, S.W.; Kersulyte, D.; Crabtree, J.E.; Youree, B.E.; Reece, C.A.; Bukanov, N.O.; Drazek, S.E.; Roe, B.A.; Berg, D.E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 1998, 28, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, N.; Yuasa, H.; Tanaka, S.; Sawa, H.; Miura, M.; Matsui, A.; Higashi, H.; Musashi, M.; Iwabuchi, K.; Suzuki, M.; et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl. Acad. Sci. USA 2008, 105, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Hatakeyama, M. Sequence polymorphisms and intrinsic structural disorder as related to pathobiological performance of the Helicobacter pylori CagA oncoprotein. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Tegtmeyer, N. Type IV secretion and signal transduction of Helicobacter pylori CagA through interaction with host cell receptors. Toxins 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Memet, S.; Huerre, M.R.; Coyle, A.J.; et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, T.; Everett, S.M.; Dixon, M.F.; Axon, A.T.R.; Crabtree, J.E. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J. Clin. Pathol. 1998, 51, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.E.; Covacci, A.; Farmery, S.; Xiang, Z.; Tompkins, D.S.; Perry, S.; Lindley, I.J.D.; Rappuoli, R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J. Clin. Pathol. 1995, 48, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Kita, M.; Kodama, T.; Sawai, N.; Tanahashi, T.; Kashima, K.; Imanishi, J. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut 1998, 42, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Gall, A.; Gaudet, R.G.; Gray-Owen, S.D.; Salama, N.R. TIFA signaling in gastric epithelial cells initiates the cag type 4 secretion system-dependent innate immune response to Helicobacter pylori infection. mBio 2017, 8, e01168-17. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Faber, E.; Bats, S.H.; Murillo, T.; Speidel, Y.; Coombs, N.; Josenhans, C. Helicobacter pylori modulates host cell responses by CagT4SS-dependent translocation of an intermediate metabolite of LPS inner core heptose biosynthesis. PLoS Pathog. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sobala, G.M.; Crabtree, J.E.; Dixon, M.F.; Schorah, C.J.; Taylor, J.D.; Rathbone, B.J.; Heatley, R.V.; Axon, A.T.R. Acute Helicobacter pylori infection: Clinical features, local and systemic immune response, gastric mucosal histology and gastric juice ascorbic acid concentrations. Gut 1991, 32, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Bugnoli, M.; Ponzetto, A.; Morgando, A.; Figura, N.; Covacci, A.; Petracca, R.; Pennatini, C.; Censini, S.; Armellini, D. Detection in an enzyme immunoassay of an immune response to a recombinant fragment of the 128 kilodalton protein (CagA) of Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Perez-Perez, G.I.; Kleanthous, H.; Cover, T.L.; Peek, R.M.; Chyou, P.H.; Stemmermann, G.N.; Nomura, A. Infection with Helicobacter pylori strains possessing CagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995, 55, 2111–2115. [Google Scholar] [PubMed]
- Kuipers, E.J.; Perez-Perez, G.I.; Meuwissen, S.G.M.; Blaser, M.J. Helicobacter pylori and atrophic gastritis: Importance of CagA status. J. Natl. Cancer Inst. 1995, 87, 1777–1780. [Google Scholar] [CrossRef] [PubMed]
- Beales, I.L.P.; Crabtree, J.E.; Scunes, D.; Covacci, A.; Calam, J. Antibodies to CagA are associated with gastric atrophy in Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 1996, 8, 645–649. [Google Scholar] [PubMed]
- Sozzi, M.; Valentini, M.; Figura, N.; De Paoli, P.; Tedeschi, R.M.; Gloghini, A.; Serraino, D.; Poletti, M.; Carbone, A. Atrophic gastritis and intestinal metaplasia in Helicobacter pylori infection: The role of CagA status. Am. J. Gastroenterol. 1998, 83, 375–379. [Google Scholar] [CrossRef] [PubMed]
- The Eurohepygast Study Group. Risk factors for atrophic gastritis in a European population: Results of the Eurohepygast study. Gut 2002, 50, 779–785. [Google Scholar]
- Webb, P.M.; Crabtree, J.E.; Forman, D. Gastric cancer, cytotoxin associated gene a positive Helicobacter pylori and serum pepsinogens: An international study. Gastroenterology 1999, 116, 269–276. [Google Scholar] [CrossRef]
- Du, Y.; Agnew, A.; Ye, X.; Robinson, P.A.; Forman, D.; Crabtree, J.E. Helicobacter pylori and Schistosoma japonicum co-infection in a Chinese population: Helminth infection alters humoral responses to H. pylori and serum pepsinogen I/II ratio. Microb. Infect. 2006, 8, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Rieder, G.; Merchant, J.L.; Haas, R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 2005, 128, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.S.; Pearce, E.J. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J. Immunol. 2004, 173, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Olbermann, P.; Josenhans, C.; Moodley, Y.; Markus, U.; Stamer, C.; Vauterin, M.; Suerbaum, S.; Achtman, M.; Linz, B. A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet. 2010, 6, e1001069. [Google Scholar] [CrossRef] [PubMed]
- Higashi, H.; Tsutsumi, R.; Fujita, A.; Yamazaki, S.; Asaka, M.; Azuma, T.; Hatakeyama, M. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA 2002, 99, 4428–4433. [Google Scholar] [CrossRef] [PubMed]
- Nagase, L.; Hayashi, T.; Senda, T.; Hatakeyama, M. Dramatic increase in SHP2 binding activity of Helicobacter pylori western CagA by EPIYA-C duplication: Its implications in gastric carcinogenesis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Senda, M.; Suzuki, N.; Nishikawa, H.; Ben, C.; Tang, C.; Nagase, L.; Inoue, K.; Senda, T.; Hatakeyama, M. Differential mechanisms for SHP2 binding and activation are exploited by geographically distinct Helicobacter pylori CagA oncoproteins. Cell Rep. 2017, 20, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.M.; Lee, J.; Stemmermann, G.N.; Nomura, R.Y.; Perez-Perez, G.I.; Blaser, M.J. Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J. Infect. Dis. 2002, 186, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Orentreich, N.; Vogelman, H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 1997, 40, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, T.; Fukada, S.; Tanaka, M.; Mikami, T.; Munakata, A.; Crabtree, J.E. CagA seropositivity associated with development of gastric cancer in a Japanese population. J. Clin. Pathol. 1998, 51, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Crabtree, J.E.; Forman, D.; Kurosawa, M.; the Research Group on Prevention of Gastric Cancer in Young Adults. Association between infections with CagA-positive or -negative strains of Helicobacter pylori and risk for gastric cancer in young adults. Research Group on Prevention of Gastric Carcinoma Among Young Adults. Am. J. Gastroenterol. 1999, 94, 3455–3559. [Google Scholar] [CrossRef] [PubMed]
- Palli, D.; Masala, G.; Del Giudice, G.; Plebani, M.; Basso, D.; Berti, D.; Numans, M.E.; Ceroti, M.; Peeters, P.H.; Bueno de Mesquita, H.B.; et al. CagA+ Helicobacter pylori infection and gastric cancer risk in the EPIC-EURGAST study. Int. J. Cancer 2007, 120, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Tatemichi, M.; Hamada, G.S.; Nishimoto, I.N.; Kowalski, L.P.; Iriya, K.; Rodrigues, J.J.G.; Tsugane, S. Ethnic difference in serology of Helicobacter pylori CagA between Japanese and non-Japanese Brazilians for non-cardia gastric cancer. Cancer Sci. 2003, 94, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Cullings, H.; Fujiwara, S.; Hattori, N.; Matsuura, S.; Hakoda, M.; Akahoshi, M.; Kodama, K.; Tahara, E. Low-positive antibody titer against Helicobacter pylori cytotoxin-associated gene A (CagA) may predict future gastric cancer better than simple seropositivity against H. pylori CagA or against H. pylori. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Zheng, G.F.; Sumanac, K.; Irvine, E.J.; Hunt, R.H. Meta-analysis of the relationship between CagA serpositivity and gastric cancer. Gastroenterology 2003, 125, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Matsunari, O.; Watada, M.; Yamaoka, Y. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol. 2010, 5, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.L.; Liao, W.C.; Lin, H.J.; Hsu, Y.M.; Lin, C.L.; Chen, Y.A.; Feng, C.L.; Chen, C.J.; Kao, M.C.; Lai, C.H.; et al. Statins attenuate Helicobacter pylori CagA translocation and reduce incidence of gastric cancer: In vitro and population-based case-control studies. PLoS ONE 2016, 11, e0146432. [Google Scholar] [CrossRef]
- Lai, C.H.; Chang, Y.C.; Du, S.Y.; Wang, H.J.; Kuo, C.H.; Fang, S.H.; Fu, H.W.; Lin, H.H.; Chiang, A.S.; Wang, W.C. Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells. Infect. Immun. 2008, 76, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Held, M.; Lagergren, J.; Engstrand, L.; Blot, W.J.; McLaughlin, J.K.; Nyrén, O. Helicobacter pylori infection and gastric atrophy: Risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J. Natl. Cancer Inst. 2004, 96, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Crabtree, J.E.; Bernstein, L.; Hawtin, P.; Cockburn, M.; Tseng, C.C.; Forman, D. Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and the esophagus. Int. J. Cancer 2003, 103, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Blaser, M.J.; Blot, W.J.; Gammon, M.D.; Vaughan, T.L.; Risch, H.A.; Perez-Perez, G.I.; Schoenberg, J.B.; Stanford, J.L. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998, 58, 588–590. [Google Scholar] [PubMed]
- Bahmanyar, S.; Zendehdel, K.; Nyrén, O.; Ye, W. Risk of oesophageal cancer by histology among patients hospitalised for gastroduodenal ulcers. Gut 2007, 56, 464–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamaoka, Y.; Kodama, T.; Gutierrez, O.; Kim, J.G.; Kashima, K.; Graham, D.Y. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: Studies in four different countries. J. Clin. Microbiol. 1999, 37, 2274–2279. [Google Scholar] [PubMed]
- Yamaoka, Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Suzuki, R.; Yamaoka, Y. The significance of virulence factors in Helicobacter pylori. J. Dig. Dis. 2013, 14, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Vilaichone, R.K.; Mahachai, V.; Tumwasorn, S.; Wu, J.Y.; Graham, D.Y.; Yamaoka, Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: A cultural cross roads. Helicobacter 2004, 9, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kodama, M.; Murakami, K.; Matsunari, O.; Mizukami, K.; Inoue, K.; Uchida, M.; Okimoto, T.; Fujioka, T.; Uchida, T.; et al. Impact of Helicobacter pylori cagA diversity on gastric mucosal damage: An immunohistochemical study of East-Asian-type CagA. J. Gastroenterol. Hepatol. 2011, 26, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Yamakawa, A.; Yamazaki, S.; Ohtani, M.; Ito, Y.; Muramatsu, A.; Suto, H.; Yamazaki, Y.; Keida, Y.; Higashi, H.; et al. Distinct diversity of the cag pathogenicity island among Helicobacter pylori strains in Japan. J. Clin. Microbiol. 2004, 42, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 2004, 4, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Karita, M.; Tummuru, M.K.; Wirth, H.P.; Blaser, M.J. Effect of growth phase and acid shock on Helicobacter pylori cagA expression. Infect. Immun. 1996, 64, 4501–4507. [Google Scholar] [PubMed]
- Noto, J.M.; Gaddy, J.A.; Lee, J.Y.; Piazuelo, M.B.; Friedman, D.B.; Colvin, D.C.; Romero-Gallo, J.; Suarez, G.; Loh, J.; Slaughter, J.C.; et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J. Clin. Investig. 2013, 123, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.T.; Torres, V.J.; Cover, T.L. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007, 67, 4709–4715. [Google Scholar] [CrossRef] [PubMed]
- Allan, E.; Clayton, C.L.; McLaren, A.; Wallace, D.M.; Wren, B.W. Characterization of the low-pH responses of Helicobacter pylori using genomic DNA arrays. Microbiology 2001, 147, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.M.; Hoffmann, S.; Darfeuille, F.; Reignier, J.; Findeiss, S.; Sittka, A.; Chabas, S.; Reiche, K.; Hackermuller, J.; Reinhardt, R.; et al. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 2010, 464, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; El-Zimaity, H.M.; Gutierrez, O.; Figura, N.; Kim, J.G.; Kodama, T.; Kashima, K.; Graham, D.Y. Relationship between the cagA 3’ repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology 1999, 117, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Radin, J.N.; Loh, J.T.; Zhang, F.; Washington, M.K.; Peek, R.M., Jr.; Algood, H.M.; Cover, T.L. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect. Immun. 2013, 81, 2258–2267. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Nunes, A.; Agudo, A.; Aranda, N.; Arija, V.; Cross, A.J.; Molina, E.; Sanchez, M.J.; Bueno-de-Mesquita, H.B.; Siersema, P.; Weiderpass, E. Body iron status and gastric cancer risk in the EURGAST study. Int. J. Cancer 2015, 137, 2904–2914. [Google Scholar] [CrossRef] [PubMed]
- Noto, J.M.; Lee, J.Y.; Gaddy, J.A.; Cover, T.L.; Amieva, M.R.; Peek, R.M., Jr. Regulation of Helicobacter pylori virulence within the context of iron deficiency. J. Infect. Dis. 2015, 211, 1790–1794. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Forman, D.; Greenberg, E.R.; Herrero, R. Helicobacter pylori eradication in the prevention of gastric cancer: Are more trials needed? Curr. Oncol. Rep. 2013, 15, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Shiotani, A. The time to eradicate gastric cancer is now. Gut 2005, 54, 735–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—The Mastricht /Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Katelaris, P.; Sugano, K.; Ang, T.L.; Hunt, R.; Talley, N.J.; Lam, S.K.; Xiao, S.-D.; Tan, H.J.; Wu, C.-Y.; et al. Second Asia–Pacific consensus guidelines for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2009, 24, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014, 348, g3174. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chiang, T.-H.; Chou, C.-K.; Tu, Y.-K.; Liao, W.-C.; Wu, M.-S.; Graham, D.Y. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 2016, 150, 1113–1124.e1115. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.F.; Zhang, L.; Gerhard, M.; Ma, J.L.; Liu, W.D.; Ulm, K.; Wang, J.X.; Zhang, L.; Zhang, Y.; Bajbouj, M.; et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu county, China: Baseline results and factors affecting the eradication. Gut 2016, 65, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wald, N.J. (2014) The Treatment of Helicobacter pylori Infection of the Stomach in Relation to the Possible Prevention of Gastric Cancer. In: IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8). pp. 174–180. Available online: http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php (accessed on 22 March 2018).
- Leja, M.; Park, J.Y.; Murillo, R.; Liepniece-Karele, I.; Isajevs, S.; Kikuste, I.; Rudzite, D.; Krike, P.; Parshutin, S.; Polaka, I.; et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: The GISTAR study. BMJ Open 2017, 7, e016999. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Park, J.Y.; Herrero, R. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention in the Republic of Korea: A Randomized Controlled Clinical Trial. In: IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8). pp. 154–160. Available online: http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php (accessed on 22 March 2018).
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M.; Andersen, L.P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.T.; Crabtree, J.E. Immunology of Helicobacter pylori: Insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 2007, 133, 288–308. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.E. Eradication of chronic Helicobacter pylori infection by therapeutic vaccination. Gut 1998, 43, 7–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nesic, D.; Buti, L.; Lu, X.; Stebbins, C.E. Structure of H. pylori CagA oncoprotein bound to the human tumour suppressor ASPP2. Proc. Natl. Acad. Sci. USA 2014, 111, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- IARC Helicobacter Pylori Working Group (2014). Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8). Available online: http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php (accessed on 22 March 2018).
- Herrero, R.; Parsonnet, J.; Greenberg, E. Prevention of gastric cancer. JAMA 2014, 312, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P. Feasibility and Cost-Effectiveness of Population-Based Helicobacter pylori Eradication. In: IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8). pp. 111–121. Available online: http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php (accessed on 22 March 2018).


| Registry | ASR | Registry | ASR |
|---|---|---|---|
| China, Yanting County | 144.6 | Jordan, Jordanians | 5.0 |
| Japan, Yamagata | 77.4 | USA Florida, White | 4.6 |
| Republic of Korea, Daejeon | 68.8 | Philippines, Rizal | 4.2 |
| India, Mizoram | 47.2 | Saudi Arabia, Riyadh, Saudi | 3.5 |
| Chile, Bio Bio | 41.4 | USA, Utah | 3.4 |
| Belarus | 30.3 | Thailand, Khon Kaen | 3.0 |
| Colombia, Pasto | 26.5 | India, Poona | 3.0 |
| Russian Federation, Samara | 25.7 | Malaysia, Penang, Malay | 2.9 |
| USA, Los Angeles, Korean | 24.4 | Kuwait | 2.6 |
| Costa Rica | 20.9 | South Africa, Eastern Cape | 1.4 |
| Year | No. Gastric Cancers (Millions) | |
|---|---|---|
| Demographic Effect | Demographic and −2.0% APC | |
| 2012 | 0.95 | 0.95 |
| 2015 | 1.03 | 0.97 |
| 2020 | 1.17 | 1.00 |
| 2025 | 1.34 | 1.03 |
| 2030 | 1.52 | 1.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Forman, D.; Waskito, L.A.; Yamaoka, Y.; Crabtree, J.E. Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins 2018, 10, 163. https://doi.org/10.3390/toxins10040163
Park JY, Forman D, Waskito LA, Yamaoka Y, Crabtree JE. Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins. 2018; 10(4):163. https://doi.org/10.3390/toxins10040163
Chicago/Turabian StylePark, Jin Young, David Forman, Langgeng Agung Waskito, Yoshio Yamaoka, and Jean E. Crabtree. 2018. "Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer" Toxins 10, no. 4: 163. https://doi.org/10.3390/toxins10040163
APA StylePark, J. Y., Forman, D., Waskito, L. A., Yamaoka, Y., & Crabtree, J. E. (2018). Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins, 10(4), 163. https://doi.org/10.3390/toxins10040163







