Abstract
The Colombian rattlesnake Crotalus durissus cumanensis is distributed in three geographic zones of the country: the Atlantic Coast, the upper valley of the Magdalena River, and the eastern plains of the Colombian Orinoquía. Its venom induces neurological symptoms, such as eyelid ptosis, myasthenic facies, and paralysis of the respiratory muscles, which can lead to death. Identification and analysis of C. d. cumanensis showed nine groups of proteins responsible for the neurotoxic effect, of which the crotoxin complex was the most abundant (64.71%). Immunorecognition tests of C. d. cumanensis showed that the use of a commercial antivenom manufactured in Mexico resulted in immunoreactivity.
1. Introduction
The group under the generic name “rattlesnake” (Crotalus) constitutes a monophyletic group of snakes next to the genus Sistrurus. It is characterized by the presence of a cornified structure derived from the ecdysis (molting) in its tail and by its wide geographic distribution from the southeast of Canada to the north of Argentina. This genus includes the species Crotalus durissus (C. d.), with at least 11 subspecies closely related to each other and a wide distribution in the American neotropics [1,2].
C. d. cumanensis is found in Colombia, specifically in dry or semidry forest areas (up to 1000 m.a.s.l.) of the eastern plains (departments of Meta, Casanare, and Vichada), the north of the country (departments of Guajira, Bolívar, Magdalena, Atlántico, Córdoba, Sucre, and Cesar), and the upper and middle part of the Magdalena River valley (departments of Huila, Tolima, Cundinamarca, and Caldas); however, there are reports of areas above 2000 m.a.s.l. in the Sierra Nevada de Santa Marta [1] (see Figure 1).
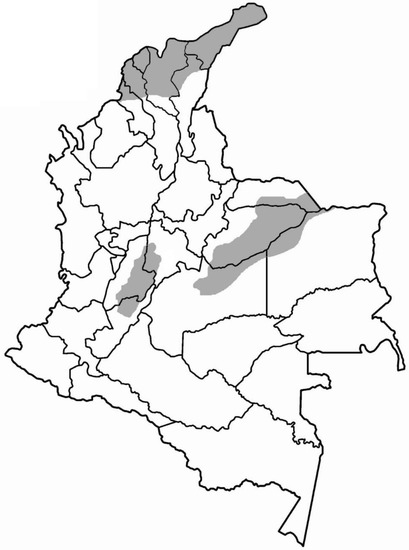
Figure 1.
Distribution of C. d. cumanensis in Colombia.
The venom of the South American rattlesnake is composed of a complex mixture of peptides, enzymes, and toxins. Regarding toxins, crotamine, gyroxine, convulxin, a thrombin-like enzyme [3,4], and the crotoxin complex (composed of two subunits, A and B), which corresponds to a heterodimeric PLA2 and can make up between 70% and 80% of the toxin content of the venom [5,6], are responsible for the high neurotoxic, nephrotoxic, and myotoxic activity [4,7,8].
Envenomation by South American rattlesnakes induces neurotoxic symptoms due to the venom’s ability to interrupt neuromuscular connectivity, leading to a progressive paralysis that generates symptoms such as palpebral ptosis, myastenic facies, and progressive flaccid paralysis, and even respiratory failure that may result in the death of the victim. In addition, envenomation is accompanied, in some cases, by nephrotoxicity due to tubular necrosis, renal failure, and rhabdomyolysis, which further complicates the clinical picture [7,9,10,11,12,13,14]. The objective of this work was to identify and characterize the protein content of C. d. cumanensis and assess the ability of immunorecognition by a commercial antivenom.
2. Results
2.1. Isolation of Fractions of C. d. cumanensis
After separation by RP-HPLC of the complete venom of C. d. cumanensis, 27 peaks were observed (Figure 2A). A similar number of peak fractions in the venom pool was observed after separation under the same conditions independently (Figure 3). The chromatographic profiles of C. d. cumanensis venom from three different Colombian locations were similar, except the peak RT at 38–40 min of the Caribbean region venom (Figure 3). The identities of some peaks were verified by MS/MS. The peak RT at 38–40 min was shown to be similar to the 9th peak of pool venom and contained crotamine and a PLA2. Minor differences in the height of the peaks were observed, suggesting that the three venoms are similar in composition but not in concentration.
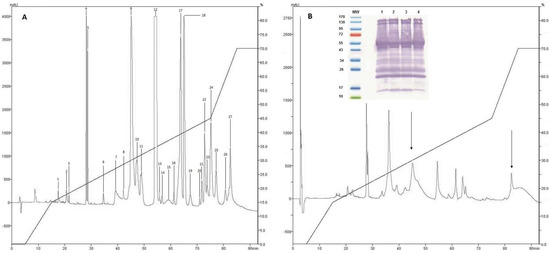
Figure 2.
Chromatographic elution profiles by RP-HPLC at 215 nm on a C-18 column. (A) Venom pool of C. d. cumanensis. (B) Complete C. d. cumanensis after being treated with Antivipmyn Tri®. Internal graphic immunoblot of C. d. cumanensis of Colombia. MW: molecular weight marker. 1. Venom pool of C. d. cumanensis. 2. Venom of C. d. cumanensis from the Caribbean region. 3. Venom of C. d. cumanensis from Tolima and Cundinamarca. 4. Venom of C. d. cumanensis from Meta.
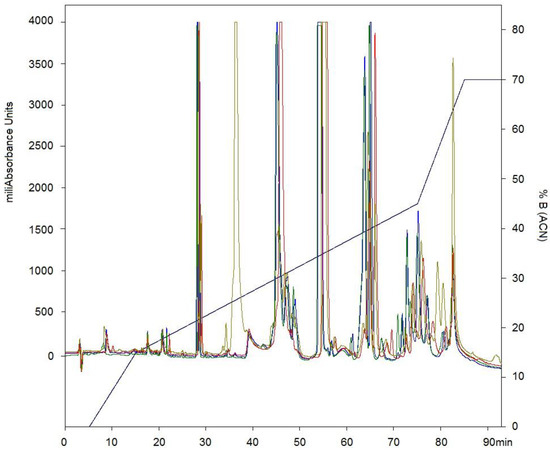
Figure 3.
Chromatographic elution profiles by RP-HPLC at 215 nm on a C-18 column. Line blue pool of venom of C. d. cumanensis. Line green venom of C. d. cumanensis from Meta. Line gray venom of C. d. cumanensis from the Caribbean region. Line red venom of C. d. cumanensis from Tolima and Cundinamarca.
2.2. Western Blotting and Immunodepletion
Recognition of the venom proteins of C. d. cumanensis by the antivenom Antivipmyn Tri® (Instituto Bioclón S.A. de C.V, México City, México) demonstrated that the venoms of the three zones studied (Meta, Cundinamarca-Tolima, and Caribbean coast) did not show significant differences compared to the pool (Figure 2B).
Analysis of the immunodepletion of the complete venom of C. d. cumanensis using Antivipmyn Tri® showed reduction in all fractions, which was more significant in fractions containing the complex crotoxin and disintegrins (fractions 1–6). Fractions 20–27 were drastically reduced, which indicates that recognition of proteins of high molecular mass was more effective compared to the crotamine (low molecular mass) present in fractions 8 and 9 (see Figure 2).
2.3. Identification of Proteins
Twenty-seven fractions were analyzed by LC/MS digested with trypsin in solution and were subjected to tandem mass analysis, which identified nine groups of proteins whose molecular mass was determined by mass spectrometry–electrospray (ESI) (Table 1). Analysis of the different fractions obtained showed that 64.71% of the venom corresponded to the crotoxin complex (crotoxin A and B), while 13.7% corresponded to disintegrins, as shown in Figure 4.

Table 1.
Identities of the fractions isolated by RP-HPLC from C. d. cumanensis, as shown in Figure 2. Molecular mass determined by nESI, monoisotopic mass of the peptides and their charge, and sequences determined by MS/MS in tandem.
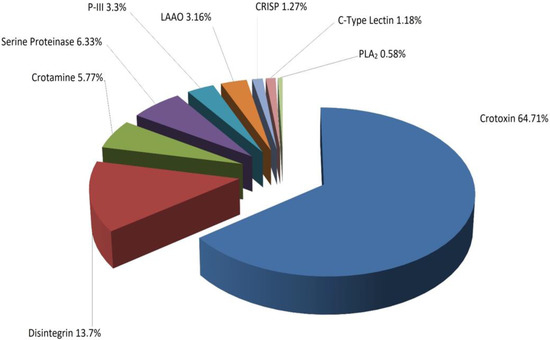
Figure 4.
Distribution of whole venom proteins of C. d. cumanensis separated by RP-HPLC and identified by nano LC-MS/MS.
3. Discussion
Snake venoms are a complex mixture of proteins that induce various signs and symptoms and, in some cases, can lead to the death of the patient by neurotoxicity, as in the case of C. d. cumanensis (rattlesnake) [4,7,8]. The use of antivenoms for more than 100 years has allowed for a reduction in the number of deaths associated with these accidents; however, the rapid initiation of symptoms and the delay in starting treatment make it difficult for antivenoms to work properly [15,16]. On the other hand, variability in the venoms is associated with a decrease in neutralizing power between different geographical zones where the same species can be found. In fact, in the Daboia russelii species, there is variation in the venom, and thus treatment with an antivenom produced with Indian species is not effective for envenoming by this snake on the island of Sri Lanka [17].
Numerous works have shown variations in the protein composition of the venoms of different snake species and intraspecifically, with respect to ontogeny, season, diet, sex, and the geographical area where the snake is sourced [18,19,20,21,22,23]. The studies have included analyzing variability with respect to the production, toxicity, cross-reactivity, and phylogenetic relationships of venoms, as well as the implications of these differences for clinical management of accidents in consideration of diagnosis, therapy, and production of antivenoms [24,25,26,27,28]. The results obtained with antivenom in immunodepletion and Western blot show immunorecognition of the proteins of C. d. cumanensis venom, although this recognition does not imply good neutralization of the effects of the venom in vivo; thus, it is necessary to carry out neutralization tests in vivo.
The rattlesnake species of South America are similar in that their venoms are neurotoxic [1]. Boldrini et al. found that three Brazilian rattlesnake subspecies showed changes in the content of the crotoxin complex, which was 67.4% for C. d collilineatus and 72.5% for C. d. cascavella [29]. These data are in agreement with those reported in this paper, which show that the content is 64.71% for the same complex. In addition, it is observed that the contents of other proteins, such as LAAO, disintegrins, and serine proteinases, are higher in C. d. cumanensis than in C. d collilineatus and C. d. cascavella, which vary by 2.6% and 0.1% for LAAO, 0.2% and 0.5% for disintegrins, and between 1.9% and 0.5% for serine proteinases [29]. From a clinical point of view, this increase in serine proteinase and disintegrins could be crucial to understanding the coagulant effect of these species due to the action they exert on fibrinogen and platelets, respectively [30].
The content of the crotoxin complex in C. d. cumanensis of Venezuela is 2.6% [31], which differs greatly with the results obtained in this work. This was probably because the lethality of C. d. cumanensis from Venezuela differs from that observed in this work, since the LD50 of the venom from Colombian snakes is lower (160 μg/kg Venezuelan, 50 μg/kg Colombian) [32]; however, it is almost twice as much [32] as that of C. d. terrificus of Brazil (65 μg/kg). Previously, Cespedes et al. reported an LD50 for the venom of C. d. cumanensis of 47 µg/kg for females and 41 µg/kg for juveniles. These results are consistent with those described in this study; however, the males were shown to be less lethal [33].
These differences could be consistent with the content of the crotoxin complex in the different subspecies, or by variations in the venoms that come from different geographical zones. Nonetheless, the same subspecies [1] can have variations according to geographical distribution, age, and some other characteristics that would explain these differences [18,19,20,21,22,23]. Calvete et al. suggested that C. d. cumanensis represents the evolutionary transition between northern hemorrhagic rattlesnakes and southern neurotoxic rattlesnakes; however, the results on concentration of crotoxin and lethal dose in this study do not support this evolutionary hypothesis [31].
For development of the work, as well as experimental phases with animals in the treatment of systemic hemorrhage, Antivipmyn Tri® was used, since it demonstrated safety and efficacy for bites by B. asper in Colombia [34,35]. Using Western blot, this antivenom demonstrated a high degree of recognition of the proteins in the poisons and a decrease in the proteins of different chromatographic fractions obtained by RP-HPLC, showing that both in vivo and in vitro tests can be used as evaluation methods. Antivenoms can be used to determine the cross-seroreactivity of antivenoms, although preclinical efficacy must ultimately be demonstrated by neutralization tests of toxic effects in animal models.
4. Conclusions
In conclusion, knowing the proteomes of the venoms of the different species on the continent would facilitate the search for “universal” antivenoms, since one could deduce or presume equivalence between venoms that could effectively protect the populations of the continent and allow for unification of production standards; however, the lack of knowledge of the venoms of the species in each country makes it difficult to fulfill this goal. For this reason, recent efforts have focused on determining the protein content of American snake species [21,29,36,37,38,39,40,41,42]. This lack of knowledge of venoms is an adverse factor that urges us to carry out tests with different regional antivenoms before suggesting the use of a common antivenom for a single genus, as seen in the different studies carried out in recent years [16,34,43,44,45,46,47].
5. Materials and Methods
5.1. Venoms, Chemical Products, and Reagents
From specimens kept in the serpentarium of the University of Antioquia (Medellin, Colombia) and included in the COLBIOFAR-149 collection registered with the Alexander Von Humboldt Research Institute of Biological Resources, venom of C. d. cumanensis was obtained by manual milking of 25 specimens from different areas of Colombia (departments of Meta, Tolima, Cundinamarca, and Magdalena). Once extracted, the venom was centrifuged (3000 rpm, 15 min), and the resulting supernatants were lyophilized and stored at −20 °C until use. Antivenin was used, and Antivipmyn Tri®, donated by the Bioclón Institute, was produced by hyperimmunization of horses with Bothrops spp., Lachesis spp., and Crotalus spp. (South American) venoms.
5.2. Isolation and Characterization of Venom Proteins
The proteins of the complete venom of C. d. cumanensis and venom from 3 Colombian locations—Meta state (east region), Caribbean region, and Tolima and Cundinamaca states (central region)—were separated by RP-HPLC on a C-18 RESTEK column (250 mm × 4.6 mm, 5 μm particle size; RESTEK, Bellefonte, PA, USA) with protein detection at 215 nm. The resulting fractions were collected and dried in a speed-vac (Eppendorf, Hamburg, Ham, Germany), and the relative abundance of proteins was estimated from the sum of the aerial chromatographic fractions obtained from the total venom [48,49].
5.3. Electrophoresis and Determination of Molecular Mass
Venom (C. d. cumanensis) and proteins from each of the obtained fractions were separated under nonreducing conditions by 12% SDS-PAGE [50] and stained with Coomassie Brilliant Blue G-250. The molecular mass of each peak was estimated according to markers (range 97.4 to 14.4 kDa; BioRad, Philadelphia, PA, USA) and confirmed by direct infusion in an ESI electrospray mass spectrometer (IonTrap 6310 series spectrometer, Agilent Technologies, Santa Clara, CA, USA). The molecular mass was deduced by deconvolution using ChemStation V software (version 5.1, Agilent Technologies, Santa Clara, CA, USA).
5.4. Identification of Proteins by HPLC-nESI-MS/MS
The isolated fractions of C. d. cumanensis were alkylated, reduced, and digested with 0.1 ng trypsin (Agilent Technologies, Santa Clara, CA, USA) at 30 °C overnight. The products of the digestion were then injected into an LC/MS/MS system (1200 series, Agilent Technologies, Santa Clara, CA, USA) on a nano C-18 column (Agilent Zorbax 300SB-C18; 150 × 0.075 mm, 3.5 μm) at a flow of 0.2 μL/min and coupled to an MSD IonTrap mass spectrometer (6310 series, Agilent Technologies, Santa Clara, CA, USA). MS/MS mass spectra were obtained under the following conditions: positive mode, dynamic range from 200 to 1200 Da, electrospray at 2 kV, 230 °C drying temperature, and actuator trap at 200 μs. The ChemStation program G2070-91126 (Agilent Technologies, Santa Clara, CA, USA) was used for deconvolution of the MS/MS spectra in the loaded state.
5.5. Immunodepletion of Venom Proteins by a Mexican Polyvalent Antivenom
Two milligrams of whole venom was dissolved in 70 μL of 20 mM phosphate buffer, pH 7.0, mixed with 4 mg of Antivipmyn TRI® and incubated with gentle stirring overnight at 37 °C. Thereafter, 6 mg of rabbit anti-horse IgG antiserum (Sigma, San Luis, MO, U.S.A) in 350 μL of 20 mM phosphate buffer, pH 7.0, was added, and the mixture was incubated for another 2 h at 37 °C. Immunocomplexes were precipitated by centrifugation at 13,000 rpm for 30 min in an Eppendorf centrifuge, and the supernatant was submitted to reverse-phase separation as described for the isolation of venom proteins. HPLC fractions were characterized as described above. The control sample was subjected to the same procedure, except that antivenom IgGs were not included in the reaction mixture [21,29,36,37,38,39,40,41,42].
5.6. Search Database
The identified peptides obtained from digestion were subjected to a BLAST search [51] to compare with other snake venom protein families. This was performed in BLASTP, and search parameters included nonredundant protein sequence (nr) and snake organism.
5.7. BLAST Search of the Identified Peptides
Deconvoluted profile spectra were used to search Mascot [52] and Spectrum Mill (Agilent Technologies, Santa Clara, CA, USA) online in the National Center for Biotechnology Information nr database for protein identification. The parameters of the search included digestion with trypsin, and carbamidomethylation modified (C) as fixed modification with carbamyl (C), carbamyl (N-terminal), carboximethylation (C), oxidation (HW), and oxidation (M) as variable modifications. The minimum score for the intensity of each fraction was 50%, while monoisotopic mass and a mass tolerance of 2.5 Da were used to determine identity. Confirmation of the different peptides was carried out through the selection “Require bold red.”
Acknowledgments
This work was supported by Universidad de Antioquia and Comité Nacional para el Desarrollo de la Investigación (CONADI), Universidad Cooperativa de Colombia.
Author Contributions
J.C.Q.-C. contributed in running the laboratory work, analysis of the data and drafted the paper. L.J.V. contributed in running the laboratory work, analysis of the data and to critical reading of the manuscript. C.S. contributed to the design of the study, to the analysis of the data, and to critical reading of the manuscript. S.E.-G. contributed in running the laboratory work, and to critical reading of the manuscript. J.C.B.-S. contributed in analysis of the data and to critical reading of the manuscript. J.C.A. contributed in supervised the laboratory work, analysis of the data and to critical reading of the manuscript. All the authors have read the final manuscript and approved the submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Campbell, J.A.; Lamar, W. The Venomous Reptiles of the Western Hemisphere; Cornell University Press: New York, NY, USA, 2004; Volume II, p. 870. [Google Scholar]
- Klauber, L.M. Rattlesnakes: Their Habits, Life Histories, and Influence on Mankind; Zoological Society of San Diego: San Diego, CA, USA, 1972. [Google Scholar]
- Bucaretchi, F.; Herrera, S.R.; Hyslop, S.; Baracat, E.C.; Vieira, R.J. Snakebites by Crotalus durissus ssp. in children in Campinas, Sao Paulo, Brazil. Rev. Inst. Med. Trop. São Paulo 2002, 44, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Oshima-Franco, Y.; Hyslop, S.; Prado-Franceschi, J.; Cruz-Hofling, M.A.; Rodrigues-Simioni, L. Neutralizing capacity of antisera raised in horses and rabbits against Crotalus durissus terrificus (South American rattlesnake) venom and its main toxin, crotoxin. Toxicon 1999, 37, 1341–1357. [Google Scholar] [CrossRef]
- Bon, C. Multicomponent neurotoxic phospholipases A2. In Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism; Wiley: Chichester, UK, 1997; pp. 269–285.A2. In Venom Phospholipase A2 Enzymes: Structure, Function and Mechanism; Wiley: Chichester, UK, 1997; pp. 269–285. [Google Scholar]
- Sampaio, S.C.; Hyslop, S.; Fontes, M.R.; Prado-Franceschi, J.; Zambelli, V.O.; Magro, A.J.; Brigatte, P.; Gutierrez, V.P.; Cury, Y. Crotoxin: Novel activities for a classic beta-neurotoxin. Toxicon 2010, 55, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Marques, M.M.; Cupo, P.; Coimbra, T.M.; Hering, S.E.; Rossi, M.A.; Laure, C.J. Myonecrosis, myoglobinuria and acute renal failure induced by South American rattlesnake (Crotalus durissus terrificus) envenomation in Brazil. Toxicon 1985, 23, 631–636. [Google Scholar] [CrossRef]
- Martins, A.M.; Toyama, M.H.; Havt, A.; Novello, J.C.; Marangoni, S.; Fonteles, M.C.; Monteiro, H.S. Determination of Crotalus durissus cascavella venom components that induce renal toxicity in isolated rat kidneys. Toxicon 2002, 40, 1165–1171. [Google Scholar] [CrossRef]
- Cupo, P.; Azevedo-Marques, M.M.; Hering, S.E. Clinical and laboratory features of South American rattlesnake (Crotalus durissus terrificus) envenomation in children. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 924–929. [Google Scholar] [CrossRef]
- De Rezende, N.A.; Torres, F.M.; Dias, M.B.; Campolina, D.; Chavez-Olortegui, C.; Amaral, C.F. South American rattlesnake bite (Crotalus durissus sp.) without envenoming: Insights on diagnosis and treatment. Toxicon 1998, 36, 2029–2032. [Google Scholar] [CrossRef]
- Jorge, M.T.; Ribeiro, L.A. [the epidemiology and clinical picture of an accidental bite by the South American rattlesnake (Crotalus durissus)]. Rev. Inst. Med. Trop. São Paulo 1992, 34, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Pinho, F.M.; Zanetta, D.M.; Burdmann, E.A. Acute renal failure after Crotalus durissus snakebite: A prospective survey on 100 patients. Kidney Int. 2005, 67, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Pinho, F.O.; Zanetta, D.M.T.; Burdmann, E.A. Risk factors and prevalence of acute renal failure (ARF) after Crotalus snakebite (CSB): A prospective survey on 100 consecutive patients. J. Am. Soc. Nephrol. 2003, 14, 511a–512a. [Google Scholar]
- Sano-Martins, I.S.; Tomy, S.C.; Campolina, D.; Dias, M.B.; de Castro, S.C.; de Sousa-e-Silva, M.C.; Amaral, C.F.; Rezende, N.A.; Kamiguti, A.S.; Warrell, D.A.; et al. Coagulopathy following lethal and non-lethal envenoming of humans by the South American rattlesnake (Crotalus durissus) in Brazil. QJM 2001, 94, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Leon, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef]
- Leon, G.; Valverde, J.M.; Rojas, G.; Lomonte, B.; Gutierrez, J.M. Comparative study on the ability of IgG and Fab sheep antivenoms to neutralize local hemorrhage, edema and myonecrosis induced by Bothrops asper (terciopelo) snake venom. Toxicon 2000, 38, 233–244. [Google Scholar] [CrossRef]
- Theakston, R.D.G. The Kinetics of snakebite envenoming and therapy. In Proceedings of the 1st International Congress on Envenomations and their Treatments, Paris, France, 7–9 June 1995; p. 69. [Google Scholar]
- Sasa, M. Diet and snake venom evolution: Can local selection alone explain intraspecific venom variation? Toxicon 1999, 37, 249–252, author reply 253–260. [Google Scholar] [PubMed]
- Saldarriaga, M.M.; Otero, R.; Nunez, V.; Toro, M.F.; Diaz, A.; Gutierrez, J.M. Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon 2003, 42, 405–411. [Google Scholar] [CrossRef]
- Menezes, M.C.; Furtado, M.F.; Travaglia-Cardoso, S.R.; Camargo, A.C.; Serrano, S.M. Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon 2006, 47, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Antunez, J.; Fernandez, J.; Lomonte, B.; Angulo, Y.; Sanz, L.; Perez, A.; Calvete, J.J.; Gutierrez, J.M. Antivenomics of Atropoides mexicanus and Atropoides picadoi snake venoms: Relationship to the neutralization of toxic and enzymatic activities. J. Venom. Res. 2010, 1, 8–17. [Google Scholar] [PubMed]
- Gutierrez, J.M.; dos Santos, M.C.; Furtado Mde, F.; Rojas, G. Biochemical and pharmacological similarities between the venoms of newborn Crotalus durissus durissus and adult Crotalus durissus terrificus rattlesnakes. Toxicon 1991, 29, 1273–1277. [Google Scholar] [CrossRef]
- Currier, R.B.; Harrison, R.A.; Rowley, P.D.; Laing, G.D.; Wagstaff, S.C. Intra-specific variation in venom of the african puff adder (bitis arietans): Differential expression and activity of snake venom metalloproteinases (SVMPS). Toxicon 2010, 55, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Tun, P.; Nu Nu, L.; Aye Aye, M.; Kyi May, H.; Khin Aung, C. Biochemical and biological properties of the venom from Russell’s viper (Daboia russelli siamensis) of varying ages. Toxicon 1995, 33, 817–821. [Google Scholar] [CrossRef]
- Rael, E.D.; Rivas, J.Z.; Chen, T.; Maddux, N.; Huizar, E.; Lieb, C.S. Differences in fibrinolysis and complement inactivation by venom from different northern blacktailed rattlesnakes (Crotalus molossus molossus). Toxicon 1997, 35, 505–513. [Google Scholar] [CrossRef]
- Ogawa, T.; Nakashima, K.; Nobuhisa, I.; Deshimaru, M.; Shimohigashi, Y.; Fukumaki, Y.; Sakaki, Y.; Hattori, S.; Ohno, M. Accelerated evolution of snake venom phospholipase A2 isozymes for acquisition of diverse physiological functions. Toxicon 1996, 34, 1229–1236. [Google Scholar] [CrossRef]
- Monteiro, R.Q.; Carlini, C.R.; Guimaraes, J.A.; Bon, C.; Zingali, R.B. Distinct bothrojaracin isoforms produced by individual jararaca (Bothrops jararaca) snakes. Toxicon 1997, 35, 649–657. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Williams, V.; White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef]
- Boldrini-Franca, J.; Correa-Netto, C.; Silva, M.M.; Rodrigues, R.S.; De La Torre, P.; Perez, A.; Soares, A.M.; Zingali, R.B.; Nogueira, R.A.; Rodrigues, V.M.; et al. Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: Assessment of geographic variation and its implication on snakebite management. J. Proteom. 2010, 73, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Cid, P.; de la Torre, P.; Flores-Diaz, M.; Dos Santos, M.C.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B.; et al. Snake venomics of the central american rattlesnake crotalus simus and the South American Crotalus durissus complex points to neurotoxicity as an adaptive paedomorphic trend along Crotalus dispersal in South America. J. Proteom. Res. 2010, 9, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Saravia, P.; Rojas, E.; Arce, V.; Guevara, C.; López, J.C.; Chaves, E.; Velásquez, R.; Rojas, G.; Gutiérrez, J.M. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: Pathophysiological and therapeutic implications. Rev. Biol. Trop. 2002, 50, 337–346. [Google Scholar] [PubMed]
- Céspedes, N.; Castro, F.; Jiménez, E.; Montealegre, L.; Castellanos, A.; Cañas, C.; Arévalo-Herrera, M.; Herrera, S. Biochemical comparison of venoms from young colombian Crotalus durissus cumanensis and their parents. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 268–284. [Google Scholar] [CrossRef]
- Otero-Patino, R.; Segura, A.; Herrera, M.; Angulo, Y.; Leon, G.; Gutierrez, J.M.; Barona, J.; Estrada, S.; Pereanez, A.; Quintana, J.C.; et al. Comparative study of the efficacy and safety of two polyvalent, caprylic acid fractionated [IgG and F(ab’)2] antivenoms, in Bothrops asper bites in Colombia. Toxicon 2012, 59, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Patino, A.C.; Lopez, J.; Aristizabal, M.; Quintana, J.C.; Benjumea, D. [evaluation of the inhibitory effect of extracts from leaves of Renealmia alpinia rottb. Maas (Zingiberaceae) on the venom of Bothrops asper (mapana)]. Biomedica 2012, 32, 365–374. [Google Scholar] [PubMed]
- Angulo, Y.; Escolano, J.; Lomonte, B.; Gutierrez, J.M.; Sanz, L.; Calvete, J.J. Snake venomics of central american pitvipers: Clues for rationalizing the distinct envenomation profiles of Atropoides nummifer and Atropoides picadoi. J. Proteom. Res. 2008, 7, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Valente, R.H.; Guimaraes, P.R.; Junqueira, M.; Neves-Ferreira, A.G.; Soares, M.R.; Chapeaurouge, A.; Trugilho, M.R.; Leon, I.R.; Rocha, S.L.; Oliveira-Carvalho, A.L.; et al. Bothrops insularis venomics: A proteomic analysis supported by transcriptomic-generated sequence data. J. Proteom. 2009, 72, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Tashima, A.K.; Sanz, L.; Camargo, A.C.; Serrano, S.M.; Calvete, J.J. Snake venomics of the brazilian pitvipers Bothrops cotiara and Bothrops fonsecai. Identification of taxonomy markers. J. Proteom. 2008, 71, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Alape-Giron, A.; Sanz, L.; Escolano, J.; Flores-Diaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake venomics of the lancehead pitviper Bothrops asper: Geographic, individual, and ontogenetic variations. J. Proteom. Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef] [PubMed]
- Angulo, Y.; Lomonte, B. Biochemistry and toxicology of toxins purified from the venom of the snake Bothrops asper. Toxicon 2009, 54, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Escolano, J.; Fernandez, J.; Sanz, L.; Angulo, Y.; Gutierrez, J.M.; Calvete, J.J. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J. Proteom. Res. 2008, 7, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Escolano, J.; Ferretti, M.; Biscoglio, M.J.; Rivera, E.; Crescenti, E.J.; Angulo, Y.; Lomonte, B.; Gutierrez, J.M.; Calvete, J.J. Snake venomics of the south and central american bushmasters. Comparison of the toxin composition of Lachesis muta gathered from proteomic versus transcriptomic analysis. J. Proteom. 2008, 71, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Bogarin, G.; Romero, M.; Rojas, G.; Lutsch, C.; Casadamont, M.; Lang, J.; Otero, R.; Gutierrez, J.M. Neutralization, by a monospecific bothrops lanceolatus antivenom, of toxic activities induced by homologous and heterologous Bothirops snake venoms. Toxicon 1999, 37, 551–557. [Google Scholar] [CrossRef]
- Otero, R.; Gutierrez, J.; Beatriz Mesa, M.; Duque, E.; Rodriguez, O.; Luis Arango, J.; Gomez, F.; Toro, A.; Cano, F.; Maria Rodriguez, L.; et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon 2002, 40, 1107–1114. [Google Scholar] [CrossRef]
- Otero, R.; Leon, G.; Gutierrez, J.M.; Rojas, G.; Toro, M.F.; Barona, J.; Rodriguez, V.; Diaz, A.; Nunez, V.; Quintana, J.C.; et al. Efficacy and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without beta-propiolactone, in the treatment of Bothrops asper bites in Colombia. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Otero-Patino, R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 2009, 54, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Otero-Patino, R.; Cardoso, J.L.; Higashi, H.G.; Nunez, V.; Diaz, A.; Toro, M.F.; Garcia, M.E.; Sierra, A.; Garcia, L.F.; Moreno, A.M.; et al. A randomized, blinded, comparative trial of one pepsin-digested and two whole IgG antivenoms for Bothrops snake bites in Uraba, Colombia. The Regional Group on Antivenom Therapy Research (REGATHER). Am. J. Trop. Med. Hyg. 1998, 58, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Perez, A.; Lomonte, B.; Sanchez, E.E.; Sanz, L. Snake venomics of crotalus tigris: The minimalist toxin arsenal of the deadliest nearctic rattlesnake venom. Evolutionary clues for generating a pan-specific antivenom against crotalid type ii venoms [corrected]. J. Proteom. Res. 2012, 11, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.C.; Chacon, A.M.; Vargas, L.; Segura, C.; Gutierrez, J.M.; Alarcon, J.C. Antiplasmodial effect of the venom of Crotalus durissus cumanensis, crotoxin complex and crotoxin B. Acta Trop. 2012, 124, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- BLAST Search. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins (accessed on 12 May 2017).
- Mascot. Matrix Science Mascot Ms/Ms Ion Search. Available online: http://www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH=MIS (accessed on 12 May 2017).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).