Impact of Naja nigricollis Venom on the Production of Methaemoglobin
Abstract
:1. Introduction
2. Results
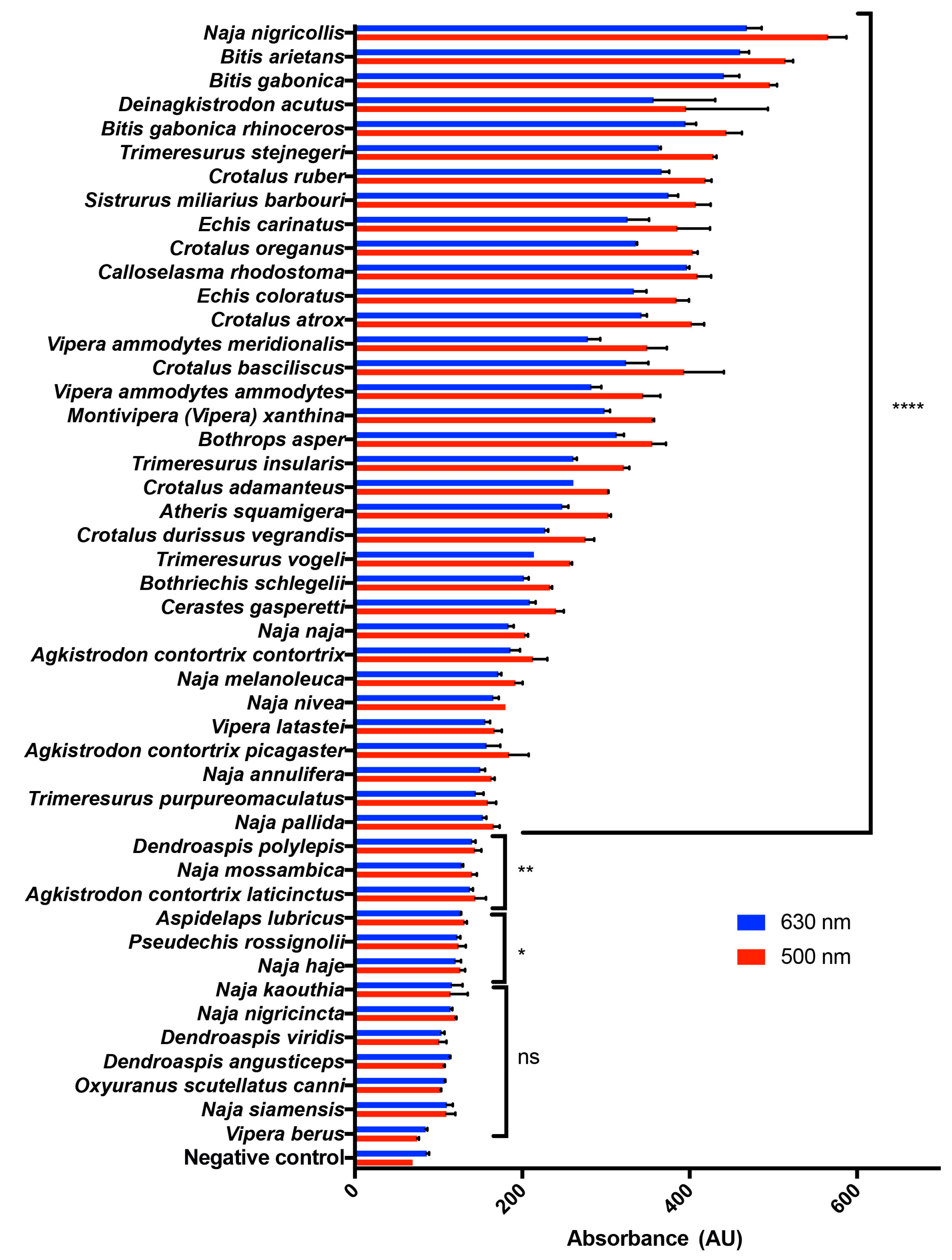
2.1. A Range of Snake Venoms Induce the Oxidation of Hb
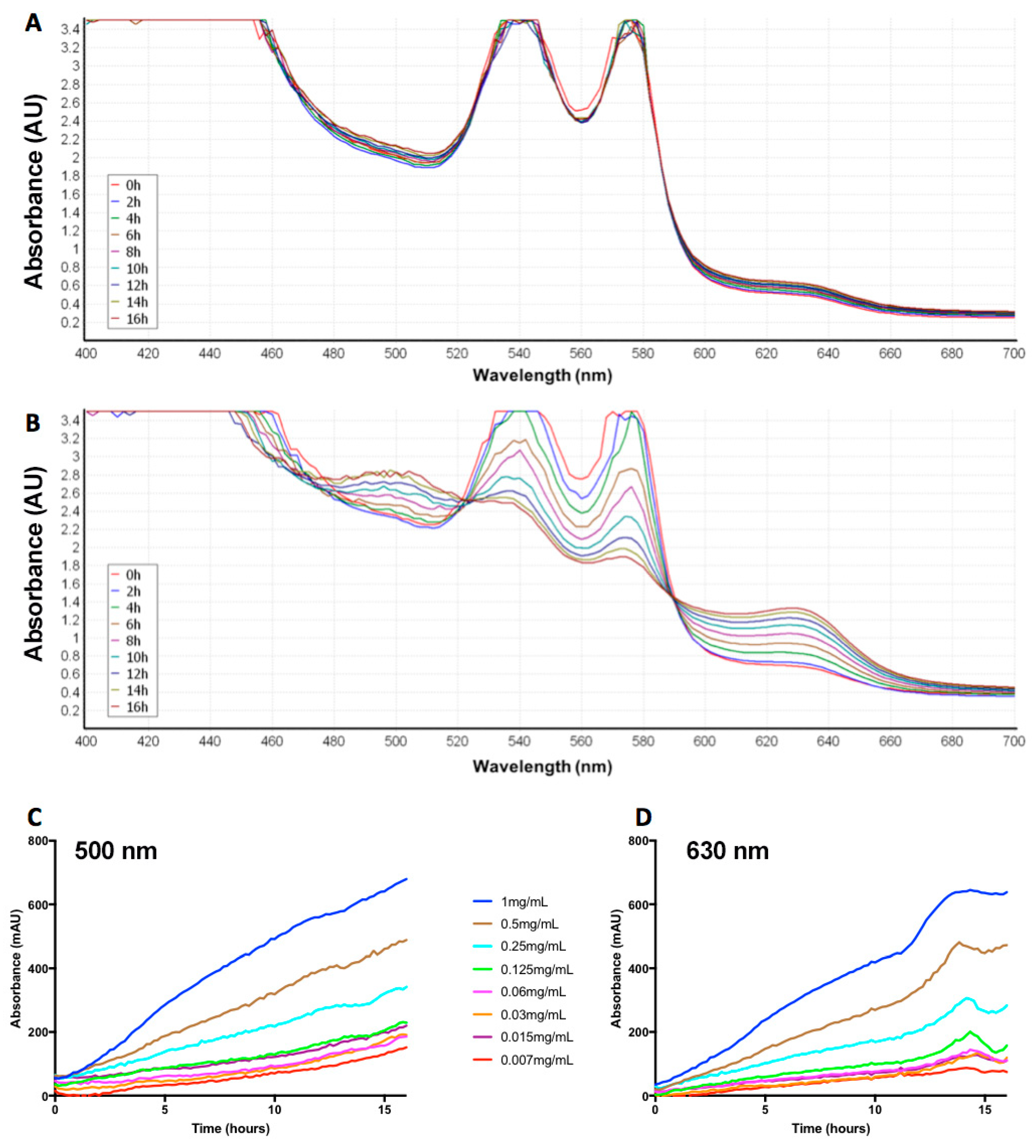
2.2. Naja Nigricollis Venom Is Significantly Increasing MetHb Production
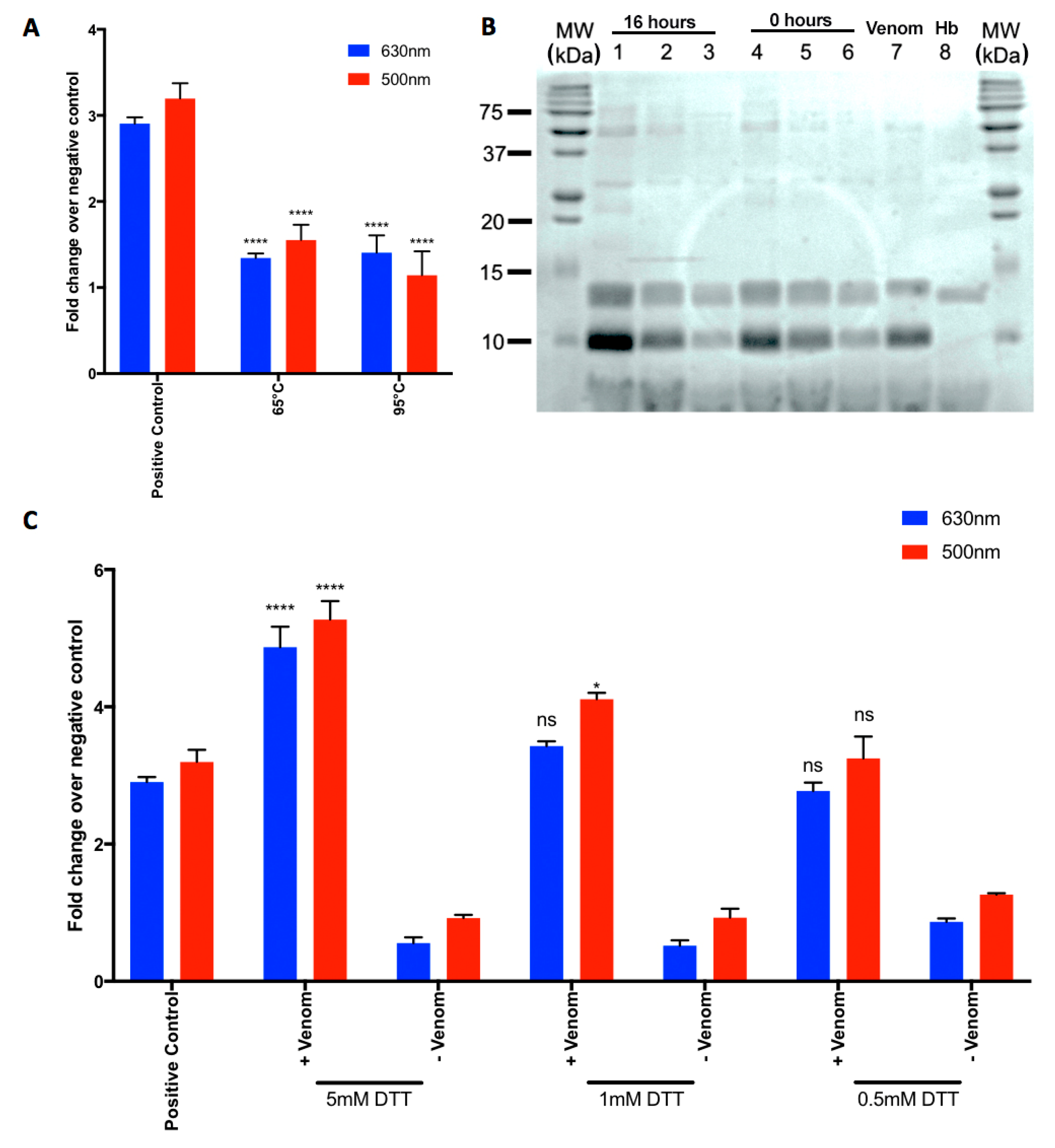
2.3. Heat-Denaturation of N. nigricollis Venom Reduces Its Effect on MetHb Production
2.4. N. nigricollis Venom Does Not Exert Direct Proteolytic Activity on Hb
2.5. A Reducing Agent Increases the N. nigricollis Venom-Induced MetHb Production
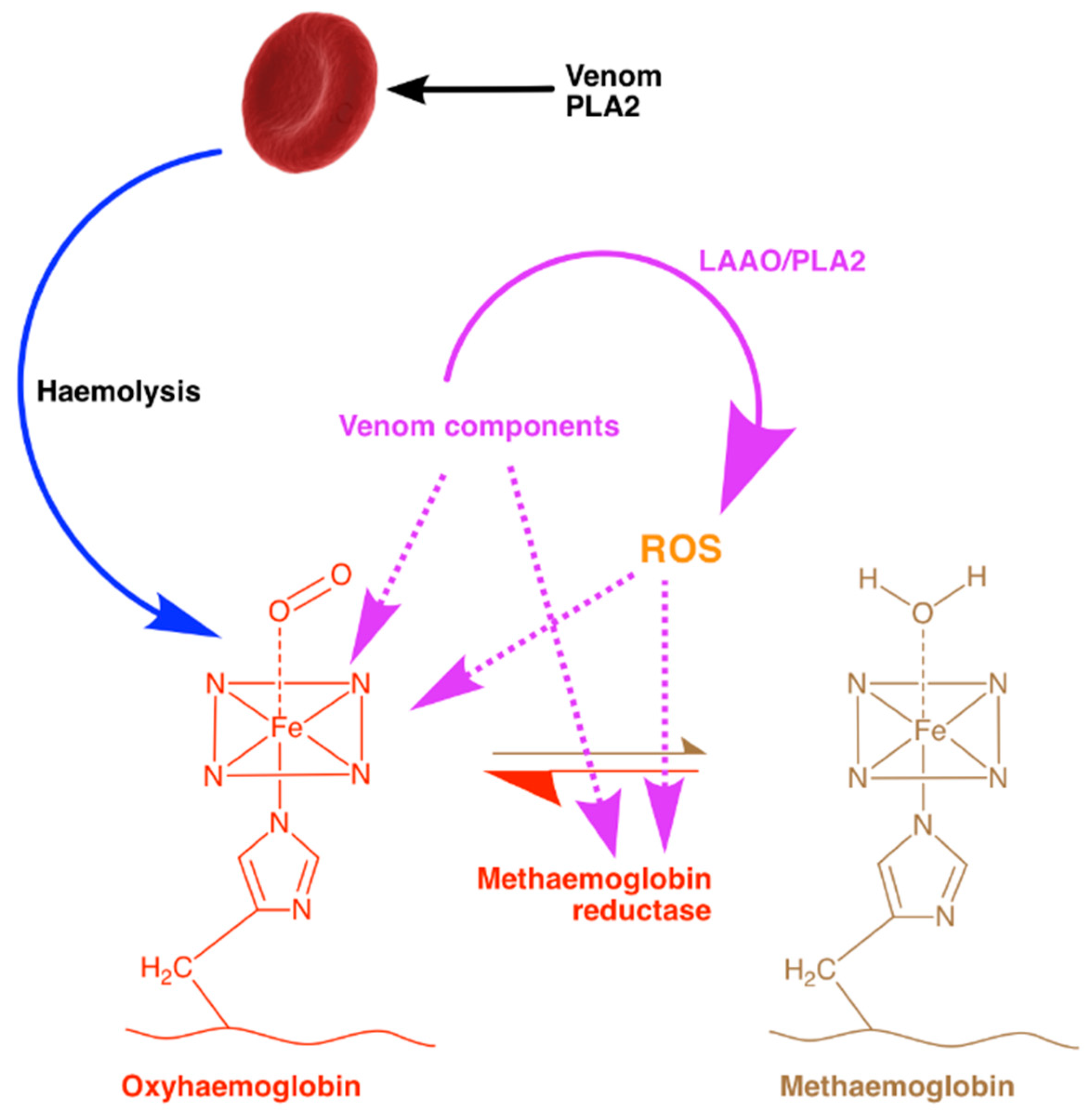
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ovine RBC Lysis and Hb Production
4.3. Screening of Venoms for MetHb Producing Activity
4.4. Time- and Concentration-Dependent Effect of N. nigricollis Venom on MetHb Production
4.5. Sodium Dodecyl Sulfate-Polyacrylamide (SDS-PAGE) Gel Electrophoresis
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.F.; Vaiyapuri, R.; Gajjeraman, P.; Hutchinson, G.; Gibbins, J.M.; Bicknell, A.B.; Vaiyapuri, S. Challenges in diagnosing and treating snakebites in a rural population of Tamil Nadu, India: The views of clinicians. Toxicon 2017, 130, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, S.; Vaiyapuri, R.; Ashokan, R.; Ramasamy, K.; Nattamaisundar, K.; Jeyaraj, A.; Chandran, V.; Gajjeraman, P.; Baksh, M.F.; Gibbins, J.M. Snakebite and its socio-economic impact on the rural population of Tamil Nadu, India. PLoS ONE 2013, 8, e80090. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Morita, T. Snake venom components affecting blood coagulation and the vascular system: Structural similarities and marked diversity. Curr. Pharm. Des. 2007, 13, 2872–2886. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S., Jr.; Swenson, S. Snake venom metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef]
- Herrera, C.; Escalante, T.; Voisin, M.-B.; Rucavado, A.; Morazán, D.; Macêdo, J.K.A.; Calvete, J.J.; Sanz, L.; Nourshargh, S.; Gutiérrez, J.M. Tissue localization and extracellular matrix degradation by PI, PII and PIII snake venom metalloproteinases: Clues on the mechanisms of venom-induced hemorrhage. PLoS Negl. Trop. Dis. 2015, 9, e0003731. [Google Scholar] [CrossRef]
- de Queiroz, M.R.; de Sousa, B.B.; da Cunha Pereira, D.F.; Mamede, C.C.N.; Matias, M.S.; de Morais, N.C.G.; de Oliveira Costa, J.; de Oliveira, F. The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon 2017, 133, 33–47. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Roweth, H.; Ali, M.S.; Unsworth, A.J.; Stainer, A.R.; Flora, G.D.; Crescente, M.; Jones, C.I.; Moraes, L.A.; Gibbins, J.M. Pharmacological actions of nobiletin in the modulation of platelet function. Br. J. Pharmacol. 2015, 172, 4133–4145. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Hutchinson, E.G.; Ali, M.S.; Dannoura, A.; Stanley, R.G.; Harrison, R.A.; Bicknell, A.B.; Gibbins, J.M. Rhinocetin, a venom-derived integrin-specific antagonist inhibits collagen-induced platelet and endothelial cell functions. J. Biol. Chem. 2012, 287, 26235–26244. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Koh, C.Y. Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: Definition and nomenclature of interaction sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Donghui, M.; Armugam, A.; Jeyaseelan, K. Cytotoxic potency of cardiotoxin from naja sputatrix: Development of a new cytolytic assay. Biochem. J. 2002, 366, 35–43. [Google Scholar]
- Gillissen, A.; Theakston, R.D.G.; Barth, J.; May, B.; Krieg, M.; Warrell, D.A. Neurotoxicity, haemostatic disturbances and haemolytic anaemia after a bite by a tunisian saw-scaled or carpet viper (echis ‘pyramidum’-complex): Failure of antivenom treatment. Toxicon 1994, 32, 937–944. [Google Scholar] [CrossRef]
- Santhosh, M.S.; Sundaram, M.S.; Sunitha, K.; Kemparaju, K.; Girish, K.S. Viper venom-induced oxidative stress and activation of inflammatory cytokines: A therapeutic approach for overlooked issues of snakebite management. Inflamm. Res. 2013, 62, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Katkar, G.D.; Sundaram, M.S.; Hemshekhar, M.; Sharma, D.R.; Santhosh, M.S.; Sunitha, K.; Rangappa, K.S.; Girish, K.S.; Kemparaju, K. Melatonin alleviates echis carinatus venom-induced toxicities by modulating inflammatory mediators and oxidative stress. J. Pineal. Res. 2014, 56, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Sebastin Santhosh, M.; Hemshekhar, M.; Thushara, R.M.; Devaraja, S.; Kemparaju, K.; Girish, K.S. Vipera russelli venom-induced oxidative stress and hematological alterations: Amelioration by crocin a dietary colorant. Cell Biochem. Funct. 2013, 31, 41–50. [Google Scholar] [CrossRef]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606. [Google Scholar] [CrossRef]
- Alves, E.C.; Sachett, J.D.A.G.; Sampaio, V.S.; Sousa, J.D.D.B.; Oliveira, S.S.D.; Nascimento, E.F.D.; Santos, A.D.S.; da Silva, I.M.; da Silva, A.M.M.; Wen, F.H.; et al. Predicting acute renal failure in bothrops snakebite patients in a tertiary reference center, western brazilian amazon. PLoS ONE 2018, 13, e0202361. [Google Scholar] [CrossRef]
- Sharma, R.D.; Katkar, G.D.; Sundaram, M.S.; Paul, M.; NaveenKumar, S.K.; Swethakumar, B.; Hemshekhar, M.; Girish, K.S.; Kemparaju, K. Oxidative stress-induced methemoglobinemia is the silent killer during snakebite: A novel and strategic neutralization by melatonin. J. Pineal Res. 2015, 59, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.R.; Burin, S.M.; Menaldo, D.L.; de Castro, F.A.; Sampaio, S.V. Snake venom l-amino acid oxidases: An overview on their antitumor effects. J. Venom. Anim. Toxins 2014, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-K.; Ferraro, B.; Moore, K.; Halliwell, B. Role of haptoglobin in free hemoglobin metabolism. Redox Rep. 2001, 6, 219–227. [Google Scholar] [CrossRef]
- Moseley, M.J.; Oenning, V.; Melnik, G. Methemoglobinemia. AJN 1999, 99, 47. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, K.; Hemshekhar, M.; Thushara, R.; Santhosh, M.S.; Sundaram, M.S.; Kemparaju, K.; Girish, K. Inflammation and oxidative stress in viper bite: An insight within and beyond. Toxicon 2015, 98, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, D.; Muñoz, J.M.; Barraza-Garza, G.; Cruz-Peréz, M.S.; Gatica-Colima, A.; Alvarez-Parrilla, E.; Plenge-Tellechea, L.F. Rattlesnake crotalus molossus nigrescens venom induces oxidative stress on human erythrocytes. J. Venom. Anim. Toxins 2017, 23, 24. [Google Scholar] [CrossRef]
- Mackessy, S.P. Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Fry, B.G. Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Fernandez, M.L.; Quartino, P.Y.; Arce-Bejarano, R.; Fernandez, J.; Camacho, L.F.; Gutierrez, J.M.; Kuemmel, D.; Fidelio, G.; Lomonte, B. Intravascular hemolysis induced by phospholipases a2 from the venom of the eastern coral snake, micrurus fulvius: Functional profiles of hemolytic and non-hemolytic isoforms. Toxicol. Lett. 2018, 286, 39–47. [Google Scholar] [CrossRef]
- Lenske, E.; Padula, A.M.; Leister, E.; Boyd, S. Severe haemolysis and spherocytosis in a dog envenomed by a red-bellied black snake (pseudechis porphyriacus) and successful treatment with a bivalent whole equine igg antivenom and blood transfusion. Toxicon 2018, 151, 79–83. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The fenton reaction. Toxicol. Lett. 1995, 82, 969–974. [Google Scholar] [CrossRef]
- Petras, D.; Sanz, L.; Segura, Á.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.G. Snake venomics of african spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the pan-african echitab-plus-icp antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Lomonte, B.; Tsai, W.-C.; Ureña-Diaz, J.M.; Sanz, L.; Mora-Obando, D.; Sánchez, E.E.; Fry, B.G.; Gutiérrez, J.M.; Gibbs, H.L.; Sovic, M.G.; et al. Venomics of new world pit vipers: Genus-wide comparisons of venom proteomes across agkistrodon. J. Proteomics 2014, 96, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ermolinsky, B.; Garza, A.; Provenzano, D. Phospholipase A2 in the venom of three cottonmouth snakes. Toxicon 2017, 135, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.A.; Romero, M.; Núñez, J.; Chaves, F.; Borkow, G.; Ovadia, M. Skeletal muscle necrosis and regeneration after injection of BaH1, a hemorrhagic metalloproteinase isolated from the venom of the snake Bothrops asper (Terciopelo). Exp. Mol. Pathol. 1995, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P. Estimate of the burden of snakebites in sub-saharan africa: A meta-analytic approach. Toxicon 2011, 57, 586–599. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F. Neutralization of local tissue damage induced by bothrops asper (Terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef]
- Jayawardana, S.; Gnanathasan, A.; Arambepola, C.; Chang, T. Chronic musculoskeletal disabilities following snake envenoming in sri lanka: A population-based study. PLoS Negl. Trop. Dis. 2016, 10, e0005103. [Google Scholar] [CrossRef]
- Kumar, K.G.S.; Narayanan, S.; Udayabhaskaran, V.; Thulaseedharan, N.K. Clinical and epidemiologic profile and predictors of outcome of poisonous snake bites – an analysis of 1,500 cases from a tertiary care center in malabar, north kerala, india. Int. J. General Med. 2018, 11, 209–216. [Google Scholar] [CrossRef]
- Sachetto, A.T.A.; Rosa, J.G.; Santoro, M.L. Rutin (quercetin-3-rutinoside) modulates the hemostatic disturbances and redox imbalance induced by bothrops jararaca snake venom in mice. PLoS Negl. Trop. Dis. 2018, 12, e0006774. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Harrison, R.A.; Bicknell, A.B.; Gibbins, J.M.; Hutchinson, G. Purification and functional characterisation of rhinocerase, a novel serine protease from the venom of bitis gabonica rhinoceros. PLoS ONE 2010, 5, e9687. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, H.F.; Hayter, P.; Ravishankar, D.; Baines, A.; Layfield, H.J.; Croucher, L.; Wark, C.; Bicknell, A.B.; Trim, S.; Vaiyapuri, S. Impact of Naja nigricollis Venom on the Production of Methaemoglobin. Toxins 2018, 10, 539. https://doi.org/10.3390/toxins10120539
Williams HF, Hayter P, Ravishankar D, Baines A, Layfield HJ, Croucher L, Wark C, Bicknell AB, Trim S, Vaiyapuri S. Impact of Naja nigricollis Venom on the Production of Methaemoglobin. Toxins. 2018; 10(12):539. https://doi.org/10.3390/toxins10120539
Chicago/Turabian StyleWilliams, Harry F., Paul Hayter, Divyashree Ravishankar, Anthony Baines, Harry J. Layfield, Lorraine Croucher, Catherine Wark, Andrew B. Bicknell, Steven Trim, and Sakthivel Vaiyapuri. 2018. "Impact of Naja nigricollis Venom on the Production of Methaemoglobin" Toxins 10, no. 12: 539. https://doi.org/10.3390/toxins10120539
APA StyleWilliams, H. F., Hayter, P., Ravishankar, D., Baines, A., Layfield, H. J., Croucher, L., Wark, C., Bicknell, A. B., Trim, S., & Vaiyapuri, S. (2018). Impact of Naja nigricollis Venom on the Production of Methaemoglobin. Toxins, 10(12), 539. https://doi.org/10.3390/toxins10120539






