Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens)
Abstract
1. Introduction
2. Results
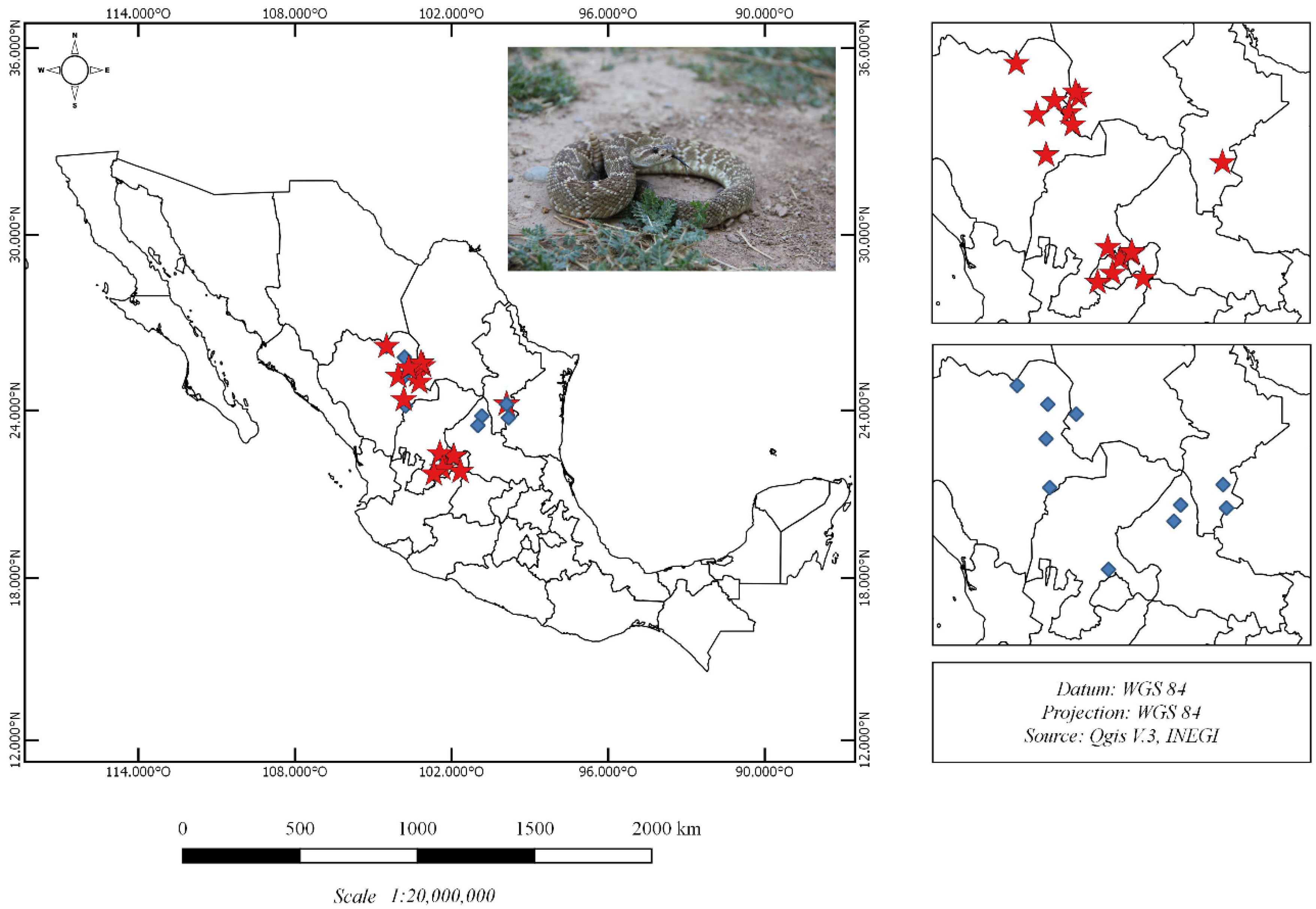
2.1. Crotalus Molossus Nigrescens Samples
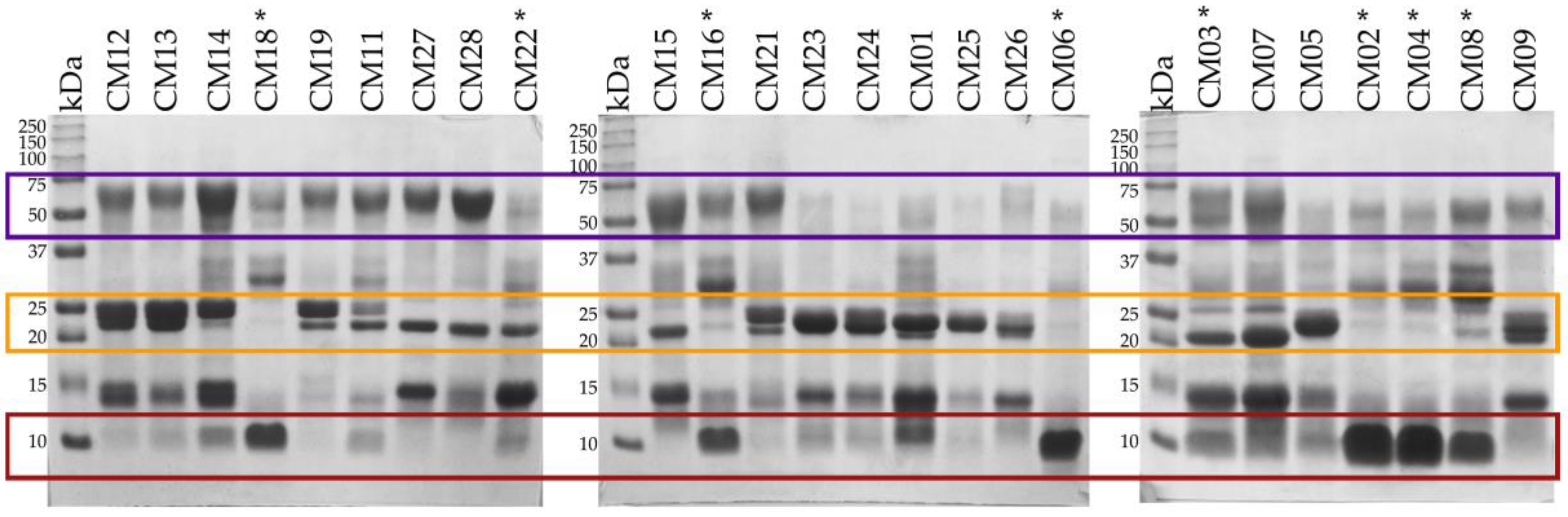
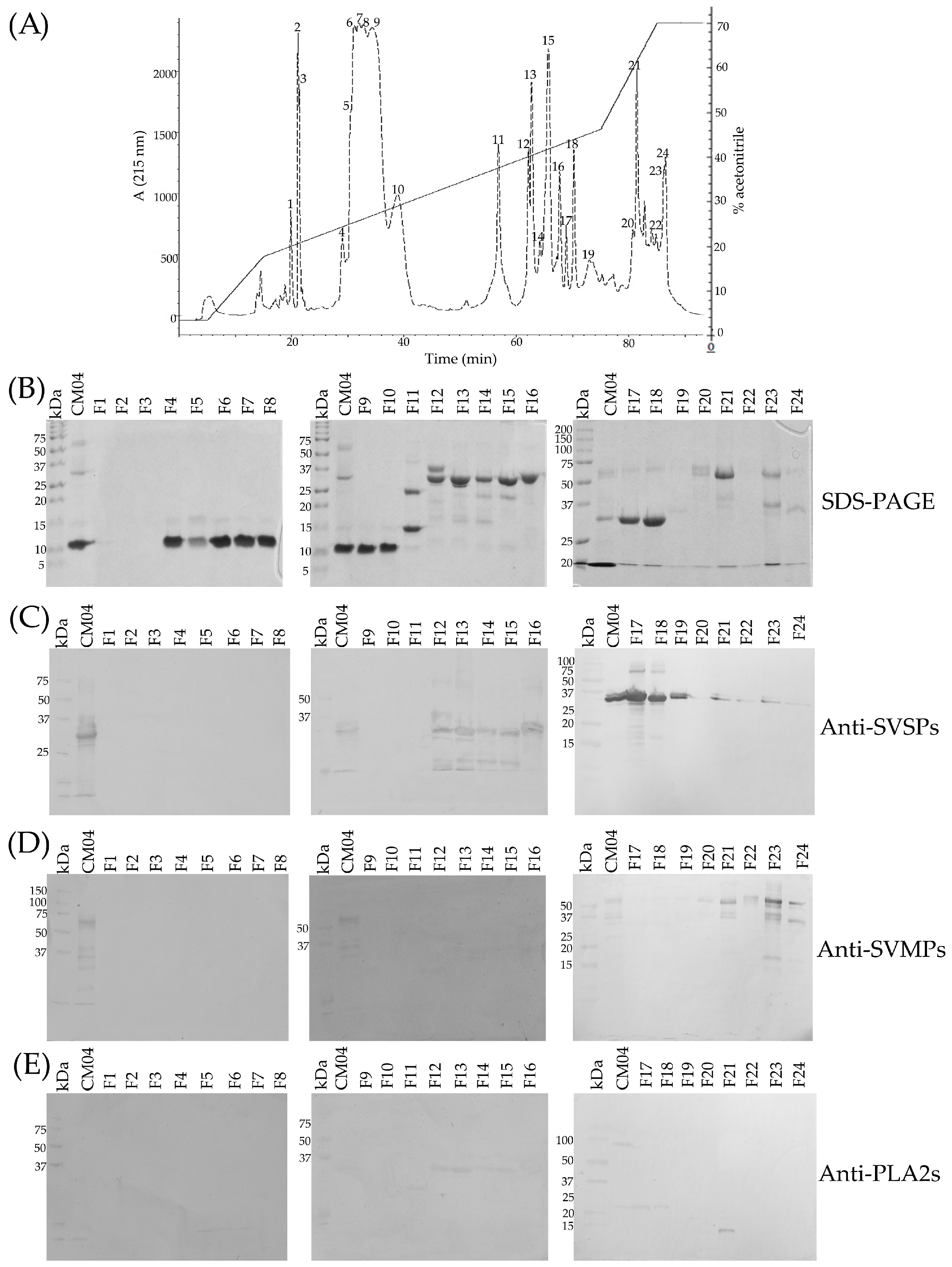
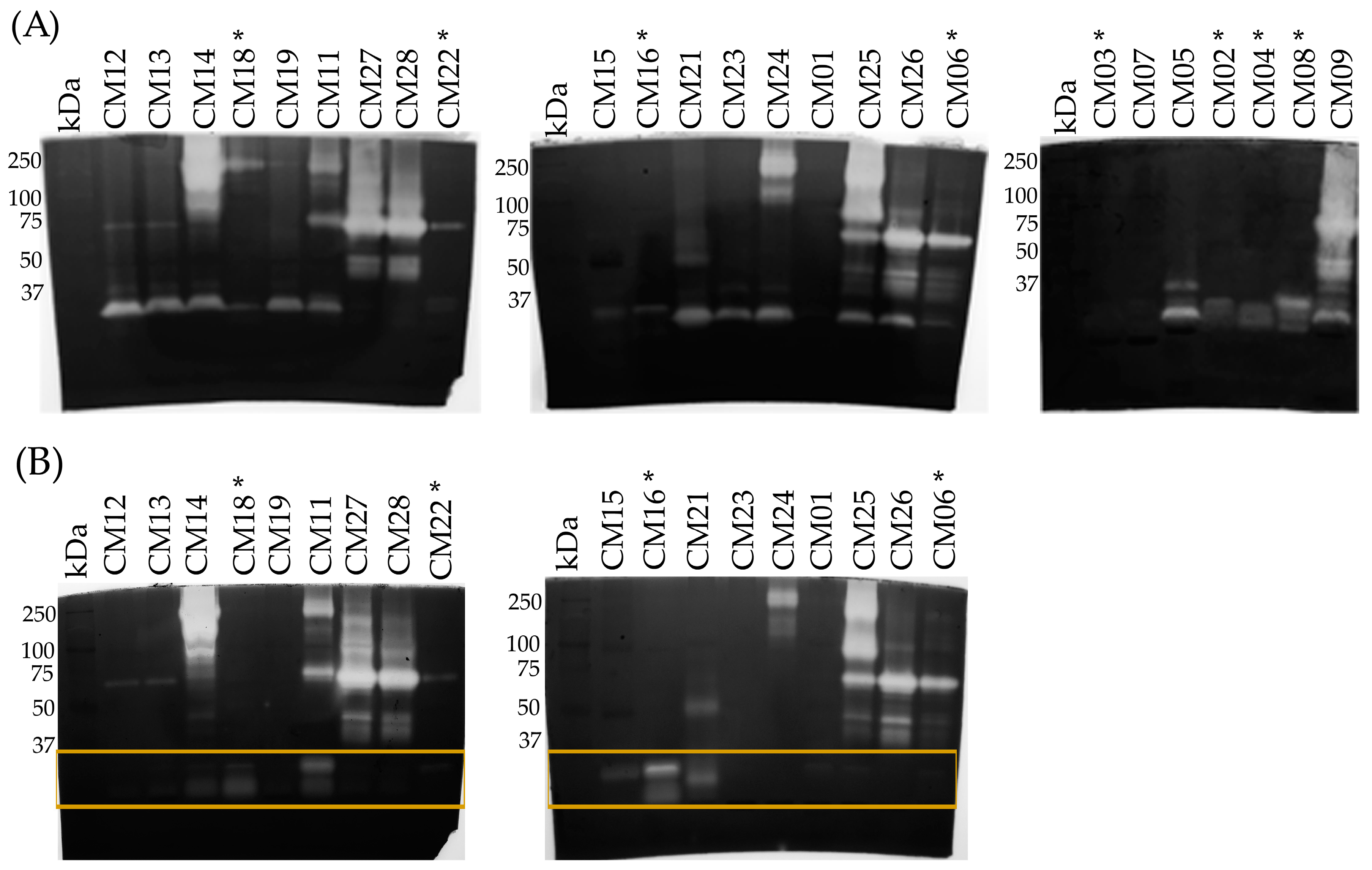
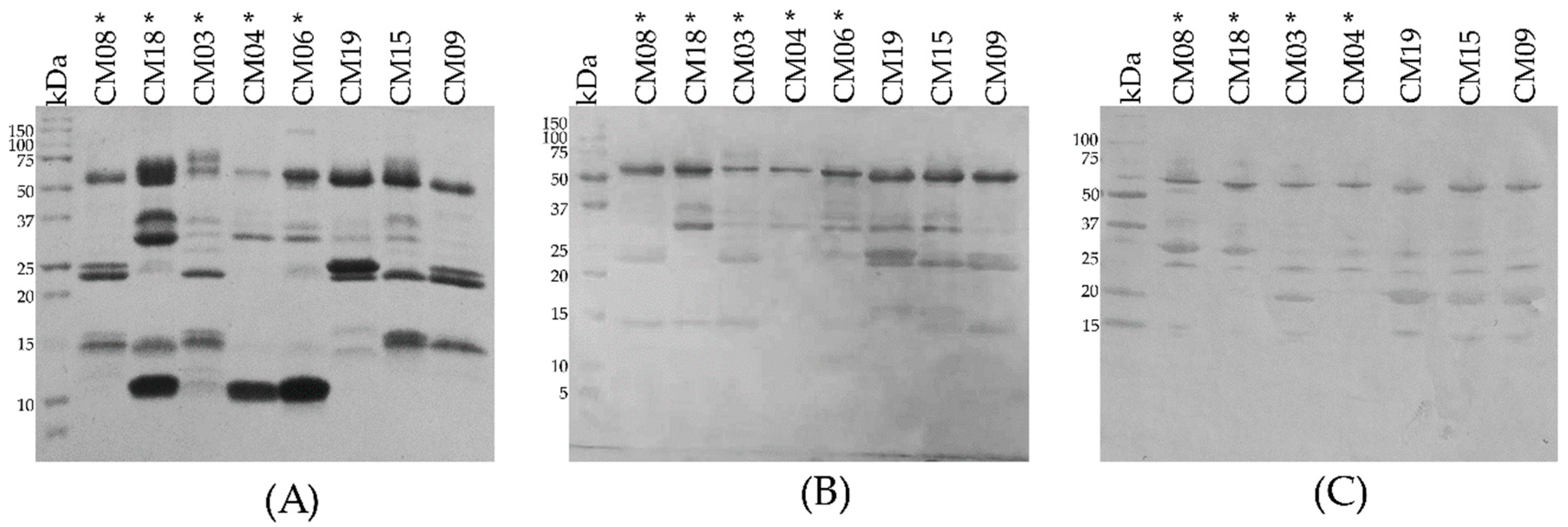
2.2. SDS-PAGE
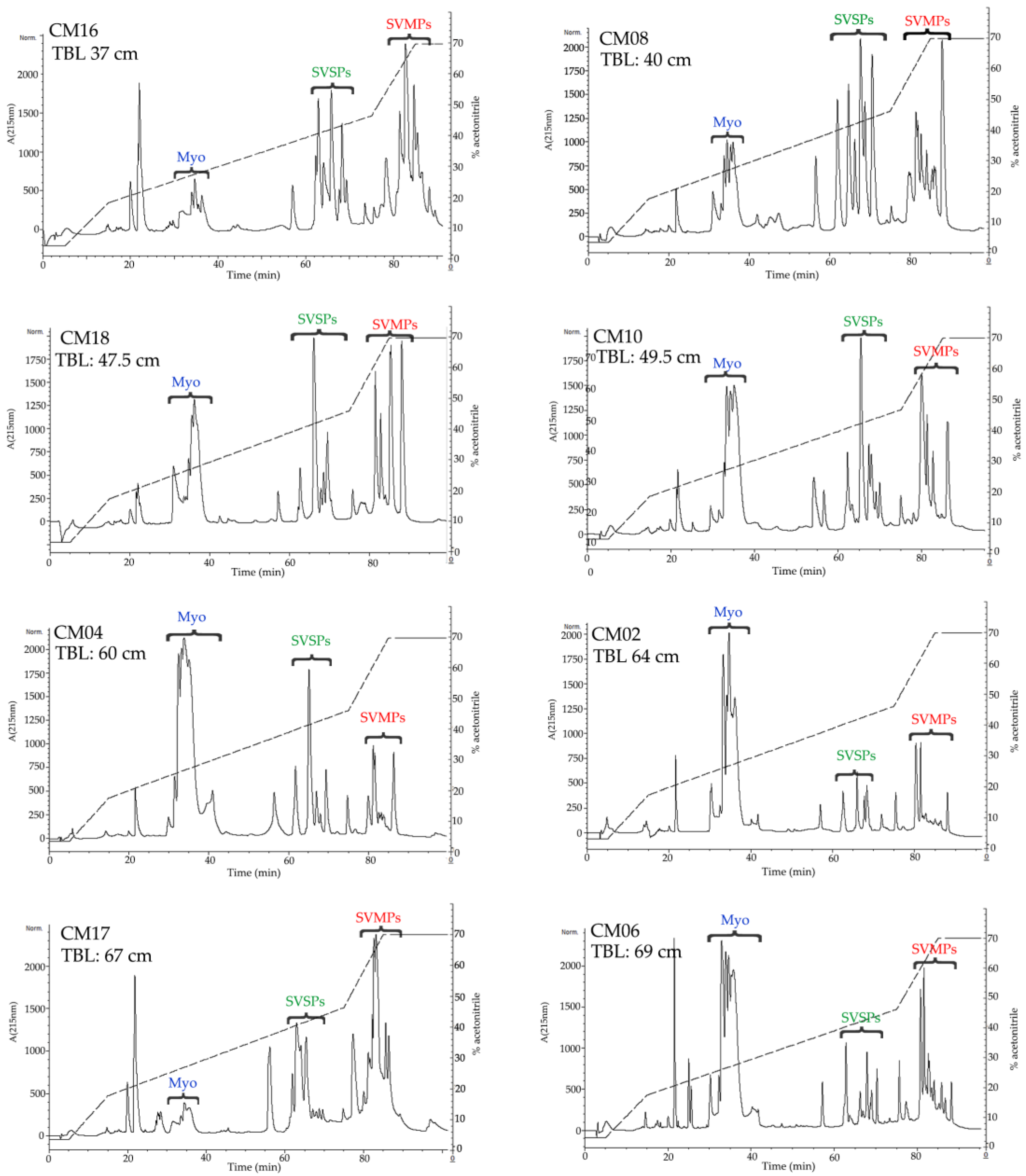
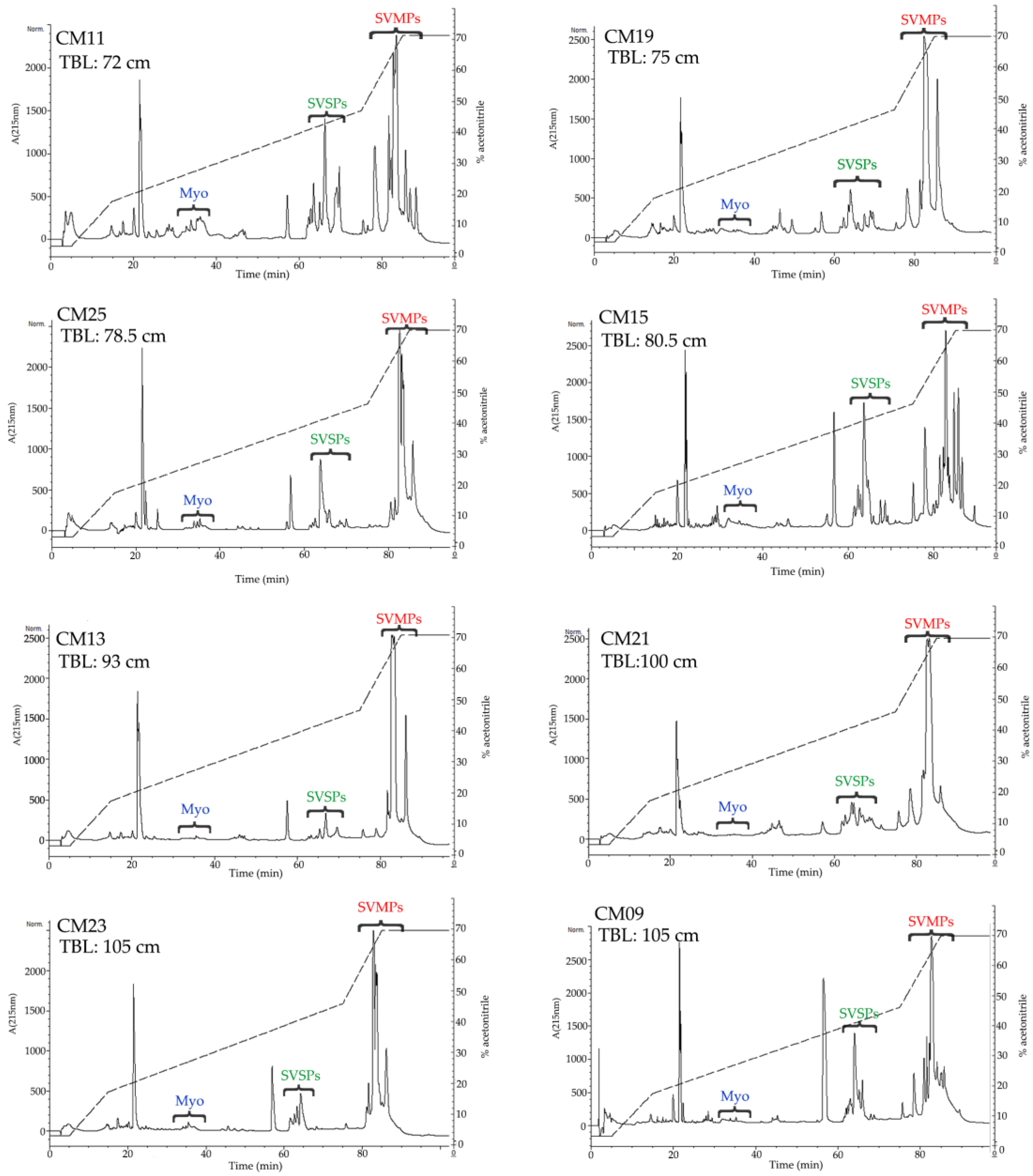
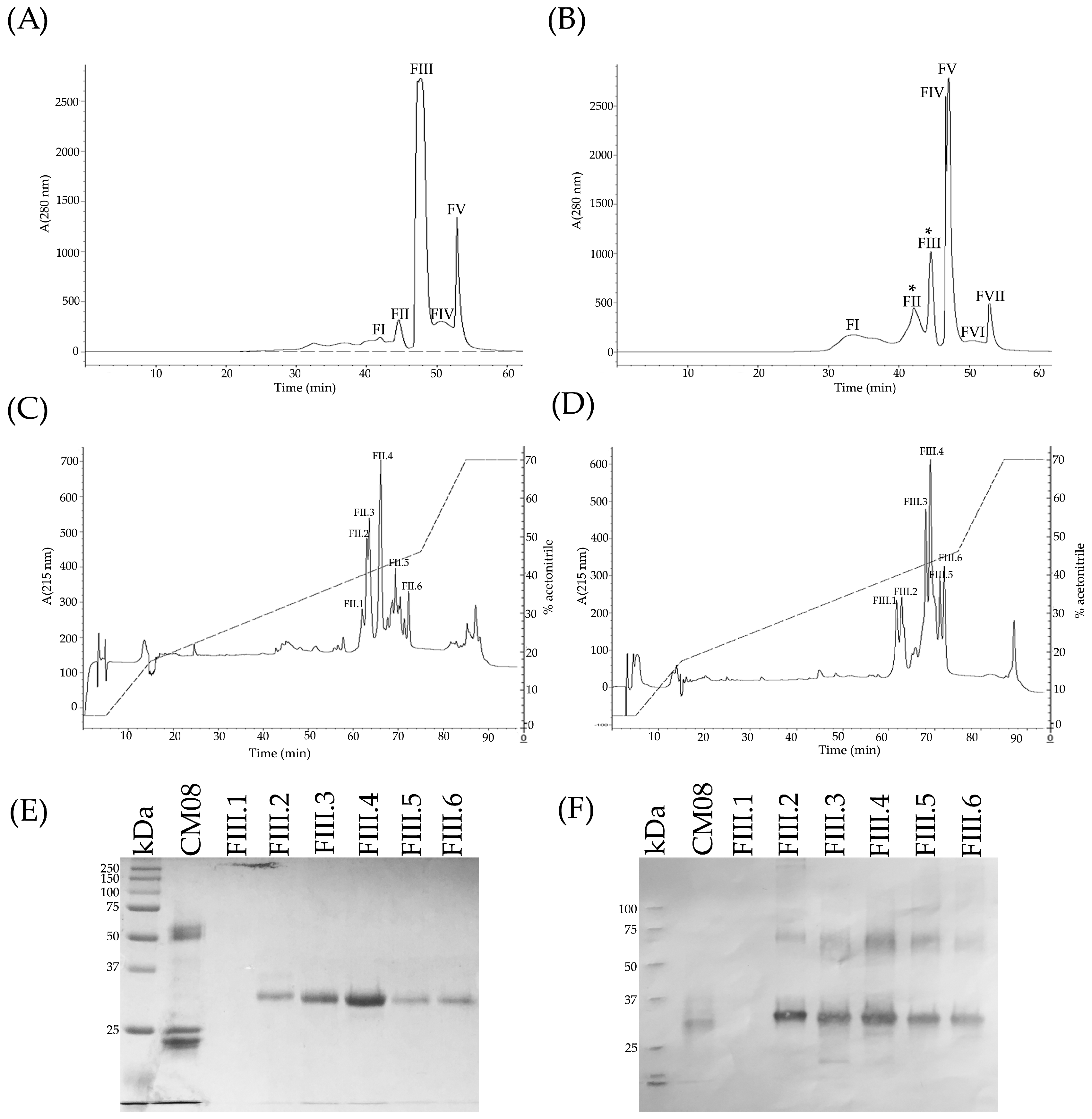
2.3. Determination of the Primary Components in C. m. nigrescens Venoms
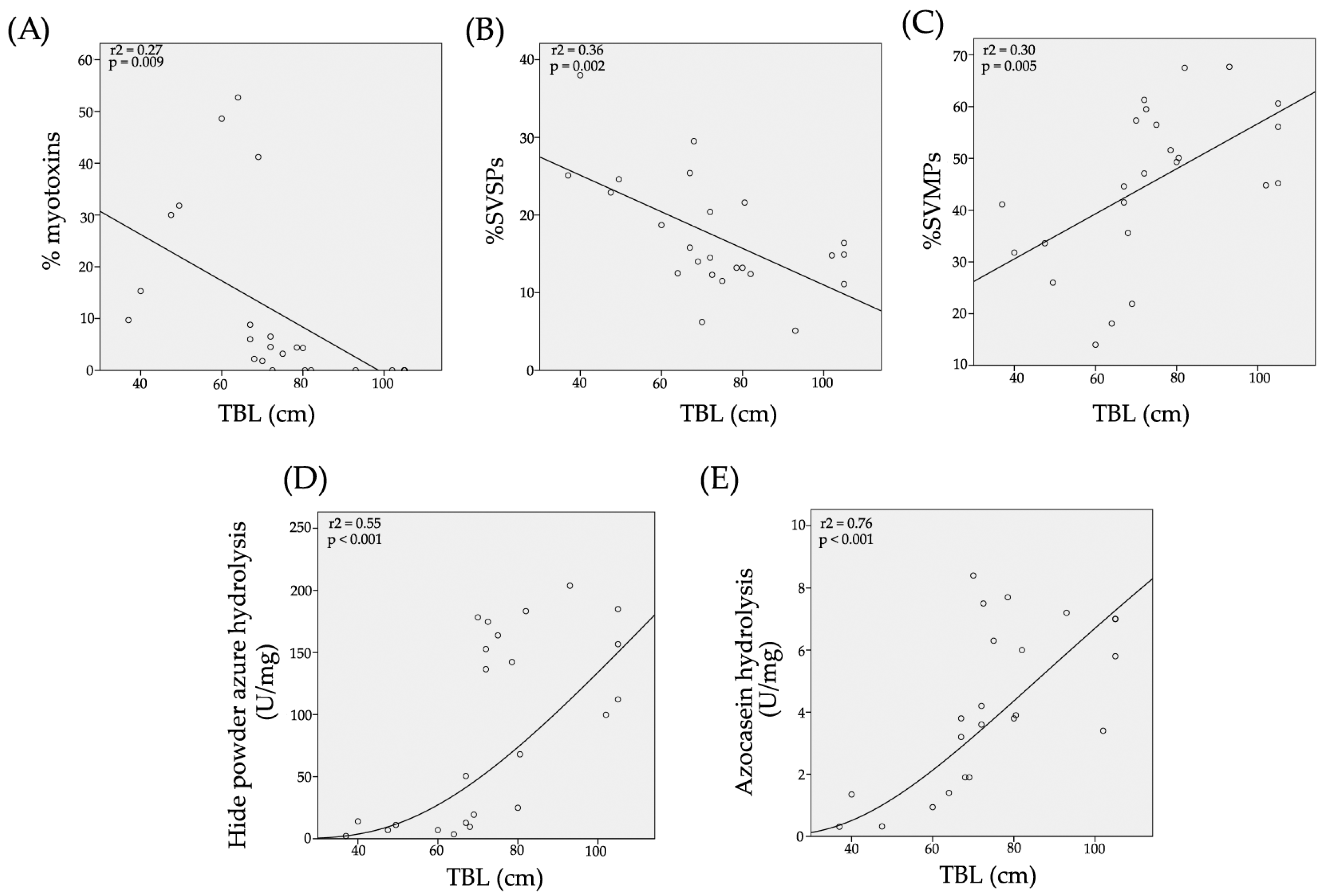
2.4. Hide Powder Azure (HPA) Hydrolysis
2.5. Azocasein Hydrolysis
2.6. Gelatin Hydrolysis
2.7. Minimum Coagulant Dose (MCD-P)
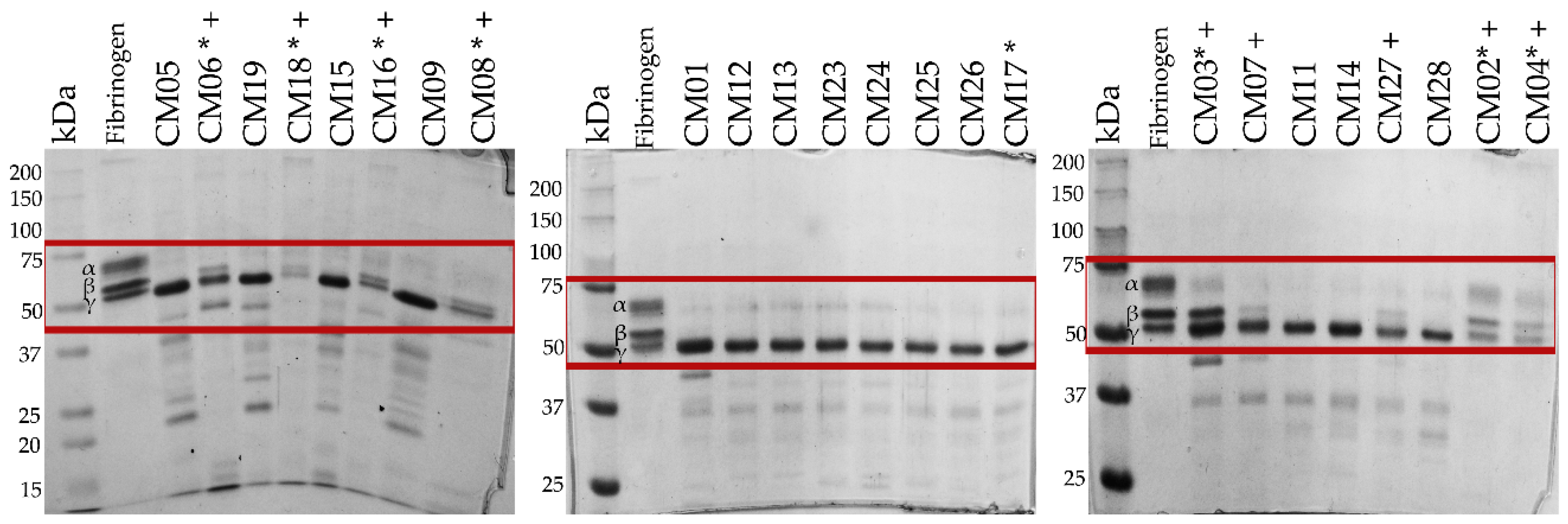
2.8. Fibrinogenolytic Activity
2.9. Median Lethal Dose (LD50)
2.10. Minimum Hemorrhagic Dose (MHD)
2.11. Toxic Components in Juvenile C. m. nigrescens Venoms
2.12. Detection of Crotoxin-Like Neurotoxins at the Protein Level
2.13. Neutralization Studies
2.14. Immune Recognition of Antivenom
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Crotalus Molossus Nigrescens Sampling
5.3. Protein Concentration Determination
5.4. SDS-PAGE
5.5. Reverse-Phased High Performance Liquid Chromatography HPLC
5.6. Identification of Protein Families in Representative C. m. nigrescens Venom by Western Blot
5.7. Molecular Mass and N-Terminal Sequence Determination of Crotamine-Like Myotoxins
5.8. Hide Powder Azure (HPA) Hydrolysis
5.9. Azocasein Hydrolysis
5.10. Gelatinolytic Activity
5.11. Minimum Coagulant Dose Plasma (MCD-P)
5.12. Fibrinogenolytic Activity
5.13. Enzymatic Inhibition Analysis
5.14. Median Lethal Dose (LD50)
5.15. Minimum Hemorrhagic Dose (MHD)
5.16. Detection of Crotoxin-Like Neurotoxins at the Protein Level
5.17. Neutralization Studies
5.18. Immune Recognition of Antivenom
5.19. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Booth, C.L. Evolutionary significance of ontogenetic colour change in animals. Biol. J. Linn. Soc. 1990, 40, 125–163. [Google Scholar] [CrossRef]
- Lind, A.J.; Welsh, H.H.J. Ontogenetic changes in foraging behaviour and habitat use by the Oregon garter snake, Thamnophis atratus hydrophilus. Anim. Behav. 1994, 48, 1261–1273. [Google Scholar] [CrossRef]
- Brodin, T.; Johansson, F. Conflicting selection pressures on the growth/predation risk trade-off in a damselfly. Ecology 2004, 85, 2927–2932. [Google Scholar] [CrossRef]
- Natusch, D.J.D.; Lyons, J.A. Relationships between ontogenetic changes in prey selection, head shape, sexual maturity, and colour in an Australasian python (Morelia viridis). Biol. J. Linn. Soc. 2012, 107, 269–276. [Google Scholar] [CrossRef]
- Herrel, A.; Joachim, R.; Vanhooydonck, B.; Irschick, D.J. Ecological consequences of ontogenetic changes in head shape and bite performance in the Jamaican lizard Anolis lineatopus. Biol. J. Linn. Soc. 2006, 89, 443–454. [Google Scholar] [CrossRef]
- Webber, M.M.; Jezkova, T.; Rodríguez-Robles, J.A. Feeding Ecology of Sidewinder Rattlesnakes, Crotalus cerastes (Viperidae). Herpetologica 2016, 72, 324–330. [Google Scholar] [CrossRef]
- Holycross, A.T.; Mackessy, S.P. Variation in the diet of Sistrurus catenatus (Massasauga), with emphasis on Sistrurus catenatus edwardsii (Desert Massasauga). J. Herpetol. 2002, 36, 454–464. [Google Scholar] [CrossRef]
- Rulon, W.C. Diet of the Timber Rattlesnake, Crotalus horridus. J. Herpetol. 2002, 36, 494–499. [Google Scholar]
- Macartney, J.M. The Ecology of the Northern Pacific Rattlesnake, Crotalus Viridis Oreganus. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 1985. [Google Scholar]
- King, R.B. Predicted and observed maximum prey size—Snake size allometry. Funct. Ecol. 2002, 16, 766–772. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, V.; Debono, J.; Goldenberg, J.; Jackson, T.N.W.; Arbuckle, K.; Dobson, J.; Koludarov, I.; Li, B.; Hay, C.; Dunstan, N.; et al. Correlation between ontogenetic dietary shifts and venom variation in Australian brown snakes (Pseudonaja). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 197, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zelanis, A.; Tashima, A.K.; Rocha, M.M.T.; Furtado, M.F.; Camargo, A.C.M.; Ho, P.L.; Serrano, S.M.T. Analysis of the Ontogenetic Variation in the Venom Proteome/Peptidome of Bothrops jararaca Reveals Different Strategies to Deal with Prey. J. Proteome Res. 2010, 9, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P.; Williams, K.; Ashton, K.G. Ontogenetic Variation in Venom Composition and Diet of Crotalus oreganus concolor: A Case of Venom Paedomorphosis? Copeia 2003, 4, 769–782. [Google Scholar] [CrossRef]
- Margres, M.J.; Wray, K.P.; Seavy, M.; McGivern, J.J.; Sanader, D.; Rokyta, D.R. Phenotypic integration in the feeding system of the eastern diamondback rattlesnake (Crotalus adamanteus). Mol. Ecol. 2015, 24, 3405–3420. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.A.; Theakston, R.D.G. Changes in coagulation effects by venoms of Crotalus atrox as snakes age. Am. J. Trop. Med. Hyg. 1978, 27, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Saravia, P.; Rojas, E.; Arce, V.; Guevara, C.; López, J.C.; Chaves, E.; Velásquez, R.; Rojas, G.; Gutiérrez, J.M. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: Pathophysiological and therapeutic implications. Rev. Biol. Trop. 2002, 50, 337–346. [Google Scholar] [PubMed]
- Céspedes, N.; Castro, F.; Jiménez, E.; Montealegre, L.; Castellanos, A.; Cañas, C.; Arévalo-Herrera, M.; Herrera, S. Biochemical comparison of venoms from young Colombian Crotalus durissus cumanensis and their parents. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 268–284. [Google Scholar] [CrossRef]
- Mackessy, S.P. Venom ontogeny in the pacific rattlesnake Crotalus viridis helleri and C. v. oreganus. Copeia 1988, 1, 92–100. [Google Scholar] [CrossRef]
- Rokyta, D.R.; Margres, M.J.; Ward, M.J.; Sanchez, E.E. The genetics of venom ontogeny in the eastern diamondback rattlesnake (Crotalus adamanteus). PeerJ 2017, 5, e3249. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.; Leroy, J.; Mociño-Deloya, E.; Setser, K.; Bryson, R.; Saviola, A. Venom Ontogeny in the Mexican Lance-Headed Rattlesnake (Crotalus polystictus). Toxins 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Neri-Castro, E.; Ponce-Lopez, R. Variación ontogénica en el veneno de Crotalus simus en México. Aridociencia 2018, 3, 42–47. [Google Scholar]
- Calvete, J.J.; Sanz, L.; Cid, P.; de la Torre, P.; Flores-Díaz, M.; Dos Santos, M.C.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B.; et al. Snake Venomics of the Central American Rattlesnake Crotalus simus and the South American Crotalus durissus Complex Points to Neurotoxicity as an Adaptive Paedomorphic Trend along Crotalus Dispersal in South America. J. Proteome Res. 2010, 9, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Farstad, D.; Thomas, T.; Chow, T.; Bush, S.; Stiegler, P. Mojave rattlesnake envenomation in southern California: a review of suspected cases. Wilderness Environ. Med. 1997, 8, 89–93. [Google Scholar] [CrossRef]
- Massey, D.J.; Calvete, J.J.; Sánchez, E.E.; Sanz, L.; Richards, K.; Curtis, R.; Boesen, K. Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J. Proteom. 2012, 75, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Minton, S.A.; Weinstein, S.A. Geographic and ontogenic variation in venom of the western diamondback rattlesnake (Crotalus atrox). Toxicon 1986, 24, 71–80. [Google Scholar] [CrossRef]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.K.; Herbert, S.S.; Reiling, G.C.; Gennaro, J.F. Factors that influence venom expenditure in viperids and other snake species during predatory and defensive contexts. In Biology of the Vipers; Schuett, G.W., Hoggren, M., Douglas, M.E., Greene, H.W., Eds.; Eagle Mountain Pub Lc: Eagle Mountain, UT, USA, 2002; pp. 207–233. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, H.; Yeh, L.S.L.; Barker, W.C. Protein family classification and functional annotation. Comput. Biol. Chem. 2003, 27, 37–47. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Undheim, E.A.B.; Scheib, H.; Gren, E.C.K.; Cochran, C.; Person, C.E.; Koludarov, I.; Kelln, W.; Hayes, W.K.; King, G.F.; et al. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri): Biodiscovery, clinical and evolutionary implications. J. Proteom. 2014, 99, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Alape-Girón, A.; Sanz, L.; Escolano, J.; Flores-Díaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake venomics of the lancehead pitviper Bothrops asper. Geographic, individual, and ontogenetic variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef] [PubMed]
- Borja, M.; Neri-Castro, E.; Castañeda-Gaytán, G.; Strickland, J.L.; Parkinson, C.L.; Castañeda-Gaytán, J.; Ponce-López, R.; Lomonte, B.; Olvera-Rodríguez, A.; Alagón, A.; Pérez-Morales, R. Biological and proteolytic variation in the venom of Crotalus scutulatus scutulatus from Mexico. Toxins 2018, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.L.; Mason, A.J.; Rokyta, D.R.; Parkinson, C.L. Phenotypic variation in Mojave Rattlesnake (Crotalus scutulatus) venom is driven by four toxin families. Toxins 2018, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Segura, Á.; Herrera, M.; Villalta, M.; Vargas, M.; Uscanga-Reynell, A.; de León-Rosales, S.P.; Jiménez-Corona, M.E.; Reta-Mares, J.F.; Gutiérrez, J.M.; León, G. Venom of Bothrops asper from Mexico and Costa Rica: Intraspecific variation and cross-neutralization by antivenoms. Toxicon 2012, 59, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Zelanis, A.; Travaglia-Cardoso, S.R.; De Fátima Domingues Furtado, M. Ontogenetic changes in the venom of Bothrops insularis (Serpentes: Viperidae)and its biological implication. S. Am. J. Herpetol. 2008, 3, 43–50. [Google Scholar] [CrossRef]
- Saldarriaga, M.M.; Otero, R.; Núñez, V.; Toro, M.F.; Díaz, A.; Gutiérrez, J.M. Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon 2003, 42, 405–411. [Google Scholar] [CrossRef]
- Menezes, M.C.; Furtado, M.F.; Travaglia-Cardoso, S.R.; Camargo, A.C.M.; Serrano, S.M.T. Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon 2006, 47, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.F.D.; Travaglia-Cardoso, S.R.; Rocha, M.M.T. Sexual dimorphism in venom of Bothrops jararaca (Serpentes: Viperidae). Toxicon 2006, 48, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Dagda, R.K.; Gasanov, S.; De La Oiii, Y.; Rael, E.D.; Lieb, C.S. Genetic basis for variation of metalloproteinase-associated biochemical activity in venom of the mojave rattlesnake (Crotalus scutulatus scutulatus). Biochem. Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Whittington, A.C.; Mason, A.J.; Rokyta, D.R. A Single Mutation Unlocks Cascading Exaptations in the Origin of a Potent Pitviper Neurotoxin. Mol. Biol. Evol. 2017, 35, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Dowell, N.L.; Giorgianni, M.W.; Kassner, V.A.; Selegue, J.E.; Sanchez, E.E.; Carroll, S.B. The Deep Origin and Recent Loss of Venom Toxin Genes in Rattlesnakes. Curr. Biol. 2016, 26, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Dowell, N.L.; Giorgianni, M.W.; Griffin, S.; Kassner, V.A.; Selegue, J.E.; Sanchez, E.E.; Carroll, S.B. Extremely Divergent Haplotypes in Two Toxin Gene Complexes Encode Alternative Venom Types within Rattlesnake Species. Curr. Biol. 2018, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wagstaff, S.C.; Wuster, W.; Cook, D.A.N.; Bolton, F.M.S.; King, S.I.; Pla, D.; Sanz, L.; Calvete, J.J.; Harrison, R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar] [CrossRef] [PubMed]
- García, L.T.; Parreiras, E.; Silva, L.T.; Ramos, O.H.P.; Carmona, A.K.; Bersanetti, P.A.; Selistre-De-Araujo, H.S. The effect of post-translational modifications on the hemorrhagic activity of snake venom metalloproteinases. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 138, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Borja, M.; Lazcano, D.; Martínez-Romero, G.; Morlett, J.; Sánchez, E.; Cepeda-Nieto, A.C.; Garza-García, Y.; Zugasti-Cruz, A. Intra-specific variation in the protein composition and proteolytic activity of venom of Crotalus lepidus morulus from the Northeast of Mexico. Copeia 2013, 4, 707–716. [Google Scholar] [CrossRef]
- Castro, E.N.; Lomonte, B.; del Carmen Gutiérrez, M.; Alagón, A.; Gutiérrez, J.M. Intraspecies variation in the venom of the rattlesnake Crotalus simus from Mexico: Different expression of crotoxin results in highly variable toxicity in the venoms of three subspecies. J. Proteom. 2013, 87, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Borja, M.; Castañeda, G.; Espinosa, J.; Neri, E.; Carbajal, A.; Clement, H.; García, O.; Alagon, A. Mojave Rattlesnake (Crotalus scutulatus scutulatus) with Type B Venom from Mexico. Copeia 2014, 1, 7–13. [Google Scholar] [CrossRef]
- Durban, J.; Sanz, L.; Trevisan-Silva, D.; Neri-Castro, E.; Alagón, A.; Calvete, J.J. Integrated venomics and venom gland transcriptome analysis of juvenile and adult mexican rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus revealed miRNA-modulated ontogenetic shifts. J. Proteome Res. 2017, 16, 3370–3390. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.P.; Rautsaw, R.M.; Strickland, J.L.; Holding, M.L.; Hogan, M.P.; Mason, A.J.; Rokyta, D.R.; Parkinson, C.L. Comparative venom-gland transcriptomics and venom proteomics of four Sidewinder Rattlesnake (Crotalus cerastes) lineages reveal little differential expression despite individual variation. Sci. Rep. 2018, 8, 15534. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R. Reproduction in the Blacktatl Rattlesnake, Crotalus molossus (Serpentes: Viperidae). Tex. J. Sci. 1999, 5, 323–328. [Google Scholar]
- Platt, S.G.; Rainwater, T.R. A new maximum size record for Crotalus molossus (Baird and Girard, 1853). J. Kansas Herpetol. 2008, 28, 14–15. [Google Scholar]
- Ernst, C.H.; Ernst, E.M. Venomous Reptiles of the United States, Canada, and northern Mexico; Johns Hopkins University Press: Baltimore, MD, USA, 2011; Volume 2, ISBN 0801898765. [Google Scholar]
- Balderas-Valdivia, C.J.; Barreto-Oble, D.; Madrid-Sotelo, C.A. Contribución a la historia natural de Crotalus molossus. Biodivers. del Pedregal San Ángel 2009, 363–369. [Google Scholar]
- Werler, J.E.; Dixon, J.R. Texas Snakes: Identification, Distribution, and Natural History; University of Texas Press: Austin, TX, USA, 2000; ISBN 9780292791305. [Google Scholar]
- Ramírez, G.A.; Fletcher, P.L.; Possani, L.D. Characterization of the venom from Crotalus molossus nigrescencs Gloyd (Black tail rattlesnake): Isolation of two proteases. Toxicon 1990, 28, 285–297. [Google Scholar]
- Bober, M.A.; Glenn, J.L.; Straight, R.C.; Ownby, C.L. Detection of myotoxin a-like proteins in various snake venoms. Toxicon 1988, 26, 665–673. [Google Scholar] [CrossRef]
- Rael, E.D.; Rivas, J.Z.; Chen, T.; Maddux, N.; Huizar, E.; Lieb, C.S. Differences in fibrinolysis and complement inactivation by venom from different northern blacktailed rattlesnakes (Crotalus molossus molossus). Toxicon 1997, 35, 505–513. [Google Scholar] [CrossRef]
- Hardy, D.L.; Jeter, M.; Corrigan, J.J. Envenomation by the northern blacktail rattlesnake (Crotalus molossus molossus): report of two cases and the vitro effects of the venom on fibrinolysis and platelet aggregation. Toxicon 1982, 20, 487–493. [Google Scholar] [CrossRef]
- Yarema, M.C.; Curry, S.C. Envenomation by the Northern Blacktail rattlesnake (Crotalus molossus molossus): Case report. Pediatr. Emerg. Care 2005, 21, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.L.; Zamudio, K.R. Compartment syndrome, fasciotomy, and neuropathy after a rattlesnake envenomation: aspects of monitoring and diagnosis. Wilderness Environ. Med. 2006, 17, 36–40. [Google Scholar] [CrossRef]
- Rael, E.D.; Salo, R.J.; Zepeda, H. Monoclonal antibodies to Mojave toxin and use for isolation of cross-reacting proteins in Crotalus venoms. Toxicon 1986, 24, 661–668. [Google Scholar] [CrossRef]
- Wooldridge, B.J.; Pineda, G.; Banuelas-Ornelas, J.J.; Dagda, R.K.; Gasanov, S.E.; Rael, E.D.; Lieb, C.S. Mojave rattlesnakes (Crotalus scutulatus scutulatus) lacking the acidic subunit DNA sequence lack Mojave toxin in their venom. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 130, 169–179. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Williams, V.; White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef]
- Wray, K.P.; Margres, M.J.; Seavy, M.; Rokyta, D.R. Early significant ontogenetic changes in snake venoms. Toxicon 2015, 96, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, J.M.; Polson, A. The electrophoretic analysis of snake venoms. Arch. Biochem. 1947, 13, 253–259. [Google Scholar] [PubMed]
- Oguiura, N.; Boni-Mitake, M.; Rádis-Baptista, G. New view on crotamine, a small basic polypeptide myotoxin from South American rattlesnake venom. Toxicon 2005, 46, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Hayashi, M.A.F.; Prieto Da Silva, A.R.B.; Pereira, A.; De Sá Júnior, P.L.; Zaharenko, A.J.; Rádis-Baptista, G.; Kerkis, A.; Yamane, T. State of the art in the studies on crotamine, a cell penetrating peptide from South American rattlesnake. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Zorzella Creste, C.F.; Curtolo de Barros, L.; Delazari dos Santos, L.; Pimenta, D.C.; Barraviera, B.; Ferreira, R.S. Individual venom profiling of Crotalus durissus terrificus specimens from a geographically limited region: Crotamine assessment and captivity evaluation on the biological activities. Toxicon 2013, 69, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ownby, C.L. Structure, function and biochemical aspects of the myotoxin from snake venoms. Toxicon 1998, 17, 213–238. [Google Scholar]
- Saviola, A.J.; Pla, D.; Sanz, L.; Castoe, T.A.; Calvete, J.J.; Mackessy, S.P. Comparative venomics of the Prairie Rattlesnake (Crotalus viridis viridis) from Colorado: Identification of a novel pattern of ontogenetic changes in venom composition and assessment of the immunoreactivity of the commercial antivenom CroFab®. J. Proteom. 2015, 121, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.C.; Zingali, R.B.; Albuquerque, M.G.; Pujol-Luz, M.; Rodrigues, C.R. Snake venom thrombin-like enzymes: From reptilase to now. Cell Mol. Life Sci. 2004, 61, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.; Markland, F.S. Snake venom fibrin(ogen)olytic enzymes. Toxicon 2005, 45, 1021–1039. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S. Snake venom fibrinogenolytic and fibrinolytic enzymes: An updated inventory. On behalf of the Registry of Exogenous Hemostatic Factors of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb. Haemost. 1998, 79, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.F.D.; Santos, M.C.; Kamiguti, A.S. Age-related biological activity of South American rattlesnake (Crotalus durissus terrificus) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2003, 9, 186–201. [Google Scholar] [CrossRef]
- Mackessy, S.P. Venom composition in rattlesnakes: Trends and biological significance. In The Biology of the Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 495–510. ISBN 978-159410-011-6. [Google Scholar]
- da Silva, N.J.; Aird, S.D.; Seebart, C.; Kaiser, I.I. A gyroxin analog from the venom of the bushmaster (Lachesis muta muta). Toxicon 1989, 27, 763–771. [Google Scholar] [CrossRef]
- Phillips, D.; Swenson, S.D.; Markland, F.S., Jr. Thrombin-like snake venom serine proteinases. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 139–154. ISBN 9780849391651. [Google Scholar]
- French, W.J.; Hayes, W.K.; Bush, S.P.; Cardwell, M.D.; Bader, J.O.; Rael, E.D. Mojave toxin in venom of Crotalus helleri (Southern Pacific Rattlesnake): Molecular and geographic characterization. Toxicon 2004, 44, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.L.; Straight, R.C.; Wolt, T.B. Regional variation in the presence of canebrake toxin in Crotalus horridus venom. Comp. Biochem. Physiol. Part C Pharmacol. 1994, 107, 337–346. [Google Scholar] [CrossRef]
- Bénard-Valle, M.; Neri-Castro, E.; Fry, B.; Boyer, L. Antivenom research and development. In Venomous Reptiles and Their Toxins; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 61–72. ISBN 978-0-19-930939-9. [Google Scholar]
- Chaves, F.; Gutiérrez, J.; Brenes, F. Pathological and biochemical changes induced in mice after intramuscular injection of venom from newborn specimens of the snake Bothrops asper (Terciopelo). Toxicon 1992, 30, 1099–1109. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Sanz, L.; Flores-Díaz, M.; Figueroa, L.; Madrigal, M.; Herrera, M.; Villalta, M.; León, G.; Estrada, R.; Borges, A.; et al. Impact of regional variation in Bothrops asper snake venom on the design of antivenoms: Integrating antivenomics and neutralization approaches. J. Proteome Res. 2010, 9, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venom. Bull. World Health Organ. 1983, 61, 949–956. [Google Scholar] [PubMed]
- Zaqueo, K.D.; Kayano, A.M.; Domingos, T.F.S.; Moura, L.A.; Fuly, A.L.; da Silva, S.L.; Acosta, G.; Oliveira, E.; Albericio, F.; Zanchi, F.B.; et al. BbrzSP-32, the first serine protease isolated from Bothrops brazili venom: Purification and characterization. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 195, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Gené, A.; Rojas, G.; Cerdas, L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon 1985, 23, 887–893. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Rojas, G.; Lomonte, B.; Gené, J.; Chaves, F.; Alvarado, J.; Rojas, E. Standardization of assays for testing the neutralizing ability of antivenoms. Toxicon 1990, 28, 1127–1129. [Google Scholar] [CrossRef]
| ID | Sex | TBL (cm) | Locality | % of Crotamine-Like Myotoxins in Venom | % of SVMPs in Venom | % of SVSPs in Venom | HPA Hydrolysis (U/mg) | Azocasein Hydrolysis (U/mg) | MCD-P (µg of Venom) | Fibrinogenolytic Activity |
|---|---|---|---|---|---|---|---|---|---|---|
| CM16 | F | 37 | La Ascensión, N. L. | 9.7 | 41.1 | 25.1 | 2.2 ± 0.04 | 0.31 ± 0.02 | 13.5 | α |
| CM08 | M | 40 | Ignacio López Rayón, Dgo. | 15.3 | 31.8 | 38.0 | 14.0 ± 0.3 | 1.35 ± 0.01 | 10.3 | α |
| CM18 | F | 47.5 | Genaro Codina, Zac. | 30.0 | 33.6 | 22.9 | 7.0 ± 0.3 | 0.32 ± 0.01 | 14.7 | α |
| CM10 | ND | 49.5 | Real de Catorce, S. L. P. | 31.8 | 26.0 | 24.6 | 11.1 ± 0.1 | ND | 24.5 | ND |
| CM04 | F | 60 | Mapimí, Dgo. | 48.6 | 14.0 | 18.7 | 7.0 ± 0.7 | 0.94 ± 0.08 | 416 | α |
| CM02 | M | 64 | Pedriceña, Dgo. | 52.7 | 18.1 | 12.5 | 3.6 ± 0.08 | 1.4 ± 0.06 | 36 | α |
| CM03 | F | 67 | Matamoros, Coah. | 8.8 | 44.6 | 15.8 | 12.8 ± 0.6 | 3.8 ± 0.13 | 390 | α |
| CM17 | M | 67 | Guadalupe del Carnicero, S. L. P. | 6.0 | 41.5 | 25.4 | 50.5 ± 2.9 | 3.2 ± 0.01 | 301 | α and β |
| CM22 | F | 68 | Miquihuana, Tam. | 2.2 | 35.6 | 29.5 | 9.6 ± 0.08 | 1.9 ± 0.06 | 44 | ND |
| CM06 | F | 69 | Agua Puerca, Dgo. | 41.2 | 21.9 | 14.0 | 19.4 ± 0.7 | 1.9 ± 0.03 | 151 | α |
| CM24 | M | 70 | Matamoros, Coah. | 1.8 | 57.32 | 6.22 | 178.3 ± 4.0 | 8.4 ± 0.27 | >400 * | α and β |
| CM11 | M | 72 | Tepezalá, Ags. | 6.5 | 47.1 | 20.4 | 136.5 ± 4.2 | 4.2 ± 0.18 | 43.3 | α and β |
| CM14 | M | 72 | Genaro García, Zac. | 4.5 | 61.3 | 14.5 | 152.7 ± 2.5 | 3.6 ± 0.19 | 271 * | α and β |
| CM05 | M | 72.5 | Nazas, Dgo. | 0 | 59.5 | 12.3 | 174.7 ± 2.6 | 7.5 ± 0.27 | 75 | α and β |
| CM19 | F | 75 | Genaro Codina, Zac. | 3.2 | 56.5 | 11.5 | 163.8 ± 11.0 | 6.3 ± 0.16 | >400 | α and β |
| CM25 | F | 78.5 | Agua Puerca, Dgo. | 4.4 | 51.6 | 13.2 | 142.3 ± 4.3 | 7.7 ± 0.25 | 182 | α and β |
| CM07 | M | 80 | Barreal de Guadalupe, Coah. | 4.3 | 49.3 | 13.2 | 24.9 ± 1.8 | 3.8 ± 0.15 | 350 | α |
| CM15 | M | 80.5 | La Ascensión, N. L. | 0 | 50.1 | 21.6 | 68.0 ± 1.33 | 3.9 ± 0.01 | 470 | α and β |
| CM12 | M | 82 | Genaro García, Zac. | 0 | 67.5 | 12.4 | 183.3 ± 6.5 | 6.0 ± 0.05 | 0 † | α and β |
| CM13 | M | 93 | Genaro García, Zac. | 0 | 67.7 | 5.1 | 203.8 ± 3.2 | 7.2 ± 0.21 | 0 † | α and β |
| CM27 | F | 102 | Milpillas de Arriba, Ags. | 0 | 44.8 | 14.8 | 99.8 ± 7.4 | 3.4 ± 0.20 | 33.5 | α |
| CM01 | M | 105 | Jimulco, Coah. | 0 | 56.1 | 16.4 | 112.2 ± 4.1 | 5.8 ± 0.14 | 269* | α and β |
| CM09 | M | 105 | Emiliano Zapata, Dgo. | 0 | 45.2 | 14.9 | 184.9 ± 6.3 | 7.0 ± 0.12 | 81.8 | α and β |
| CM23 | M | 105 | Matamoros, Coah. | 0 | 60.6 | 11.1 | 156.7 ± 12.0 | 7.0 ± 0.21 | 0 † | α and β |
| CM21 | M | ND | Ojuelos, Jal. | 0 | 61.8 | 14.5 | 180.3 ± 15.5 | 4.4 ± 0.10 | 177 | ND |
| CM26 | M | ND | La Loma, Dgo. | ND | ND | ND | 134.6 ± 5.6 | 6.6 ± 0.40 | 267 | α and β |
| CM28 | ND | ND | Sierra del Laurel, Ags. | 0 | 54.3 | 16.7 | 50.6 ± 4.4 | 3.8 ± 0.06 | 45.3 | α and β |
| ID | TBL (cm) | LD50 (mg/kg) | MHD (µg) | Neutralization (ED50) Antivipmyn® (µLAV/3LD50) | Neutralization (ED50) Faboterapico Polivalente Antiviperino® (µLAV/3LD50) |
|---|---|---|---|---|---|
| CM16 | 37 | 1.26 * | 6.10 | ND | ND |
| CM08 | 40 | 1.71 * | 19.30 | ND | ND |
| CM18 | 47.5 | 1.13 * | 4.0 | 222.0 (204.4–241.1) ** | 240.1 (184.1–313.1) ** |
| CM04 | 60 | 4.10 * | >25 | ND | ND |
| CM03 | 67 | 1.71 * | ND | ND | ND |
| CM06 | 69 | 1.72 * | 21.90 | 246.2 (214.1–283.0) ** | 158.0 (121.7–205.1) ** |
| CM19 | 75 | 4.20 | 10.26 | >450 | >450 |
| CM15 | 80.5 | 3.60 | 8.30 | ND | ND |
| CM09 | 105 | 4.30 | 10.95 | 322.1 (309.3–335.3) | 411.1 (401.7–420.7) |
| Fraction/Venom | Mortality with Different Amounts of Venom (µg/mice) | |||||
|---|---|---|---|---|---|---|
| CM04 (60) | CM08 (60) | CM04 (45) | CM08 (45) | CM04 (20) | CM08 (20) | |
| FI | 0/3 | 0/3 | NA | NA | NA | NA |
| FII | 0/3 | 3/3 | NA | 1/3 | NA | 0/3 |
| FIII | 3/3 | 3/3 | 1/3 | 2/3 | 0/3 | 1/3 |
| FIV | 0/3 | 3/3 | NA | 1/3 | NA | 0/3 |
| FV | 0/3 | 0/3 | NA | NA | NA | NA |
| FVI | NA | 0/3 | NA | NA | NA | NA |
| FVII | NA | 0/3 | NA | NA | NA | NA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borja, M.; Neri-Castro, E.; Pérez-Morales, R.; Strickland, J.L.; Ponce-López, R.; Parkinson, C.L.; Espinosa-Fematt, J.; Sáenz-Mata, J.; Flores-Martínez, E.; Alagón, A.; et al. Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens). Toxins 2018, 10, 501. https://doi.org/10.3390/toxins10120501
Borja M, Neri-Castro E, Pérez-Morales R, Strickland JL, Ponce-López R, Parkinson CL, Espinosa-Fematt J, Sáenz-Mata J, Flores-Martínez E, Alagón A, et al. Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens). Toxins. 2018; 10(12):501. https://doi.org/10.3390/toxins10120501
Chicago/Turabian StyleBorja, Miguel, Edgar Neri-Castro, Rebeca Pérez-Morales, Jason L. Strickland, Roberto Ponce-López, Christopher L. Parkinson, Jorge Espinosa-Fematt, Jorge Sáenz-Mata, Esau Flores-Martínez, Alejandro Alagón, and et al. 2018. "Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens)" Toxins 10, no. 12: 501. https://doi.org/10.3390/toxins10120501
APA StyleBorja, M., Neri-Castro, E., Pérez-Morales, R., Strickland, J. L., Ponce-López, R., Parkinson, C. L., Espinosa-Fematt, J., Sáenz-Mata, J., Flores-Martínez, E., Alagón, A., & Castañeda-Gaytán, G. (2018). Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens). Toxins, 10(12), 501. https://doi.org/10.3390/toxins10120501







