New Insights on Moojase, a Thrombin-Like Serine Protease from Bothrops moojeni Snake Venom
Abstract
1. Introduction
2. Results
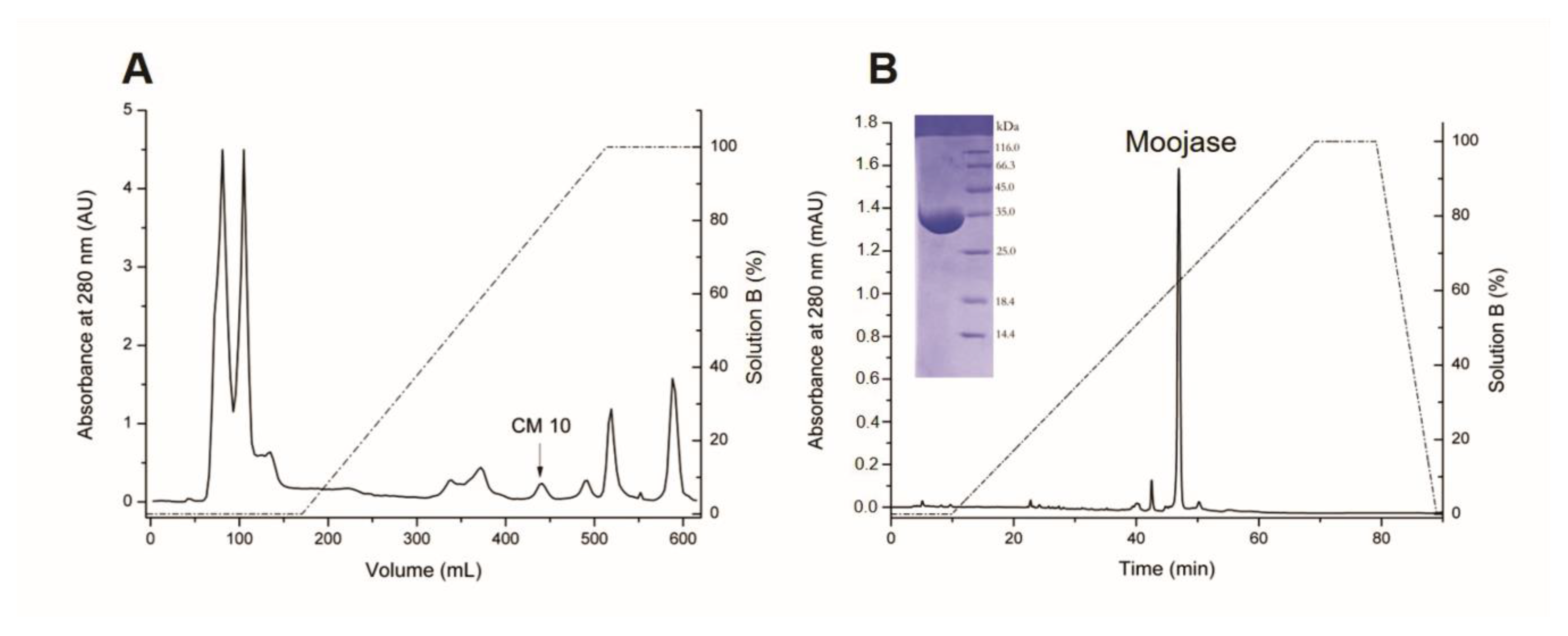
2.1. Purification of Moojase
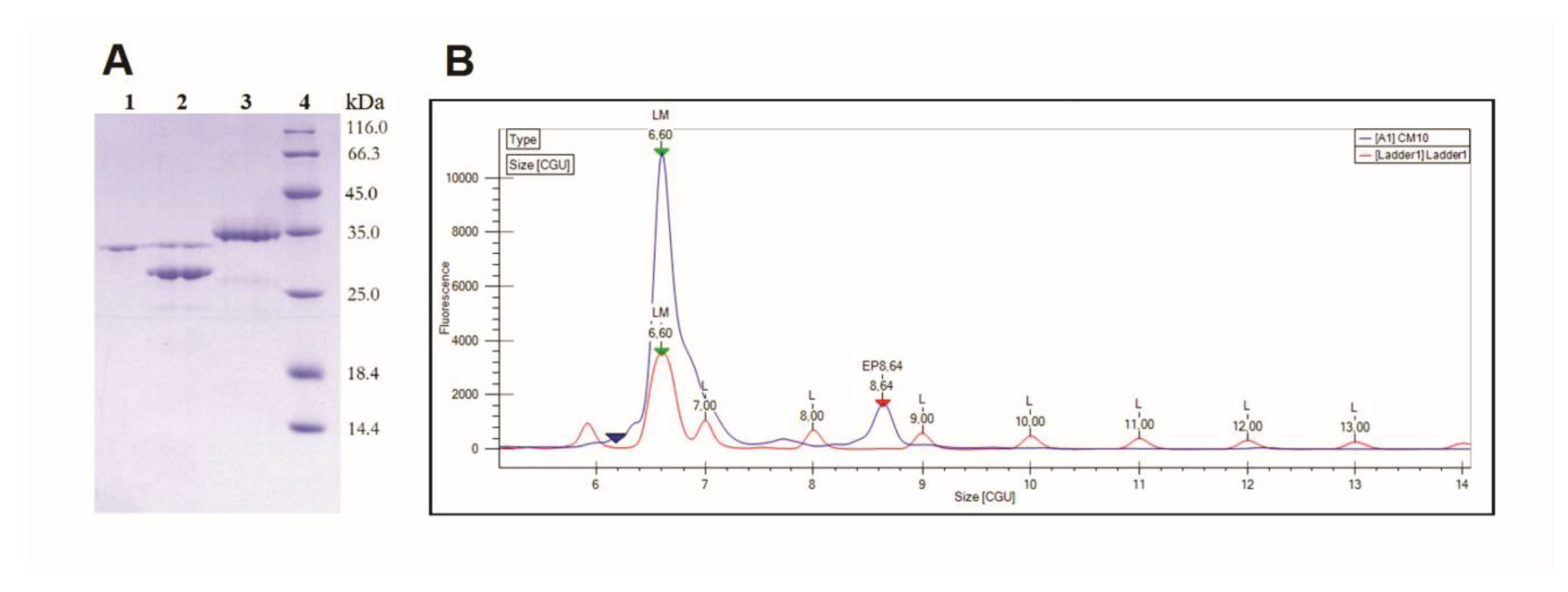
2.2. Isoelectric Focusing
2.3. Structural Characterization
2.4. Glycosylation Analyses
2.5. Coagulant Effects
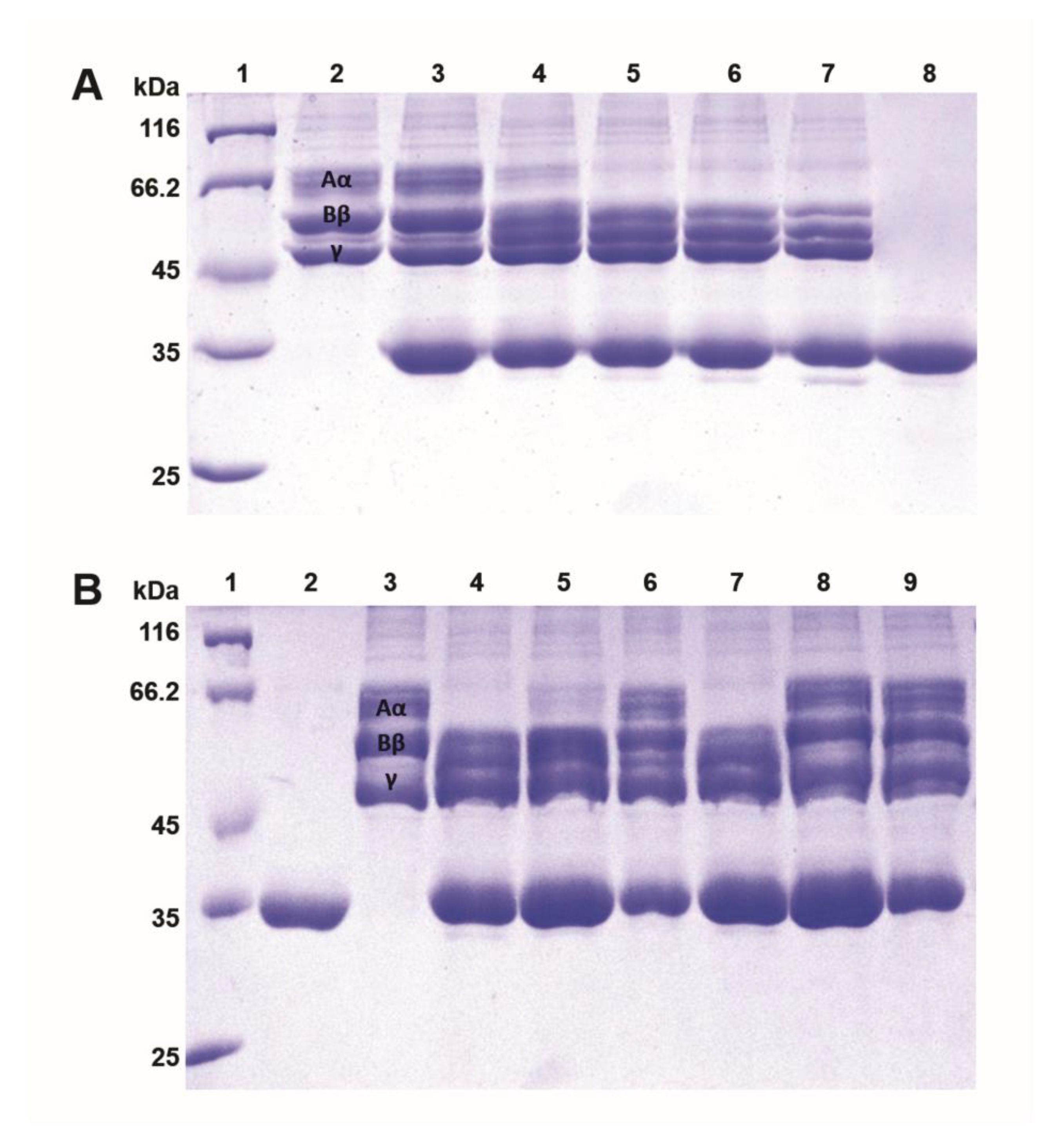
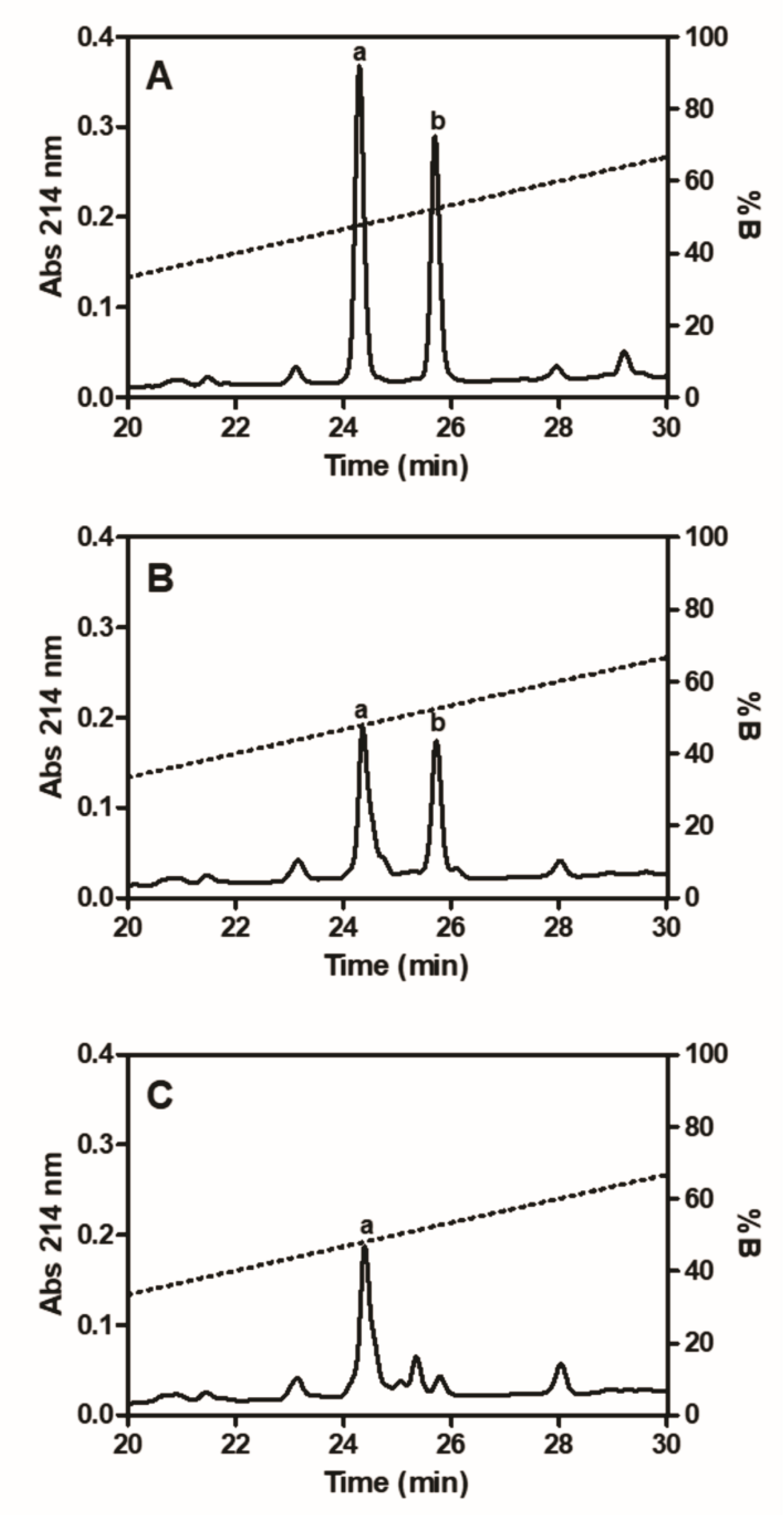
2.6. Fibrinogenolytic Effects
2.7. Fibrinolytic Effects
2.8. Induction of Platelet Aggregation
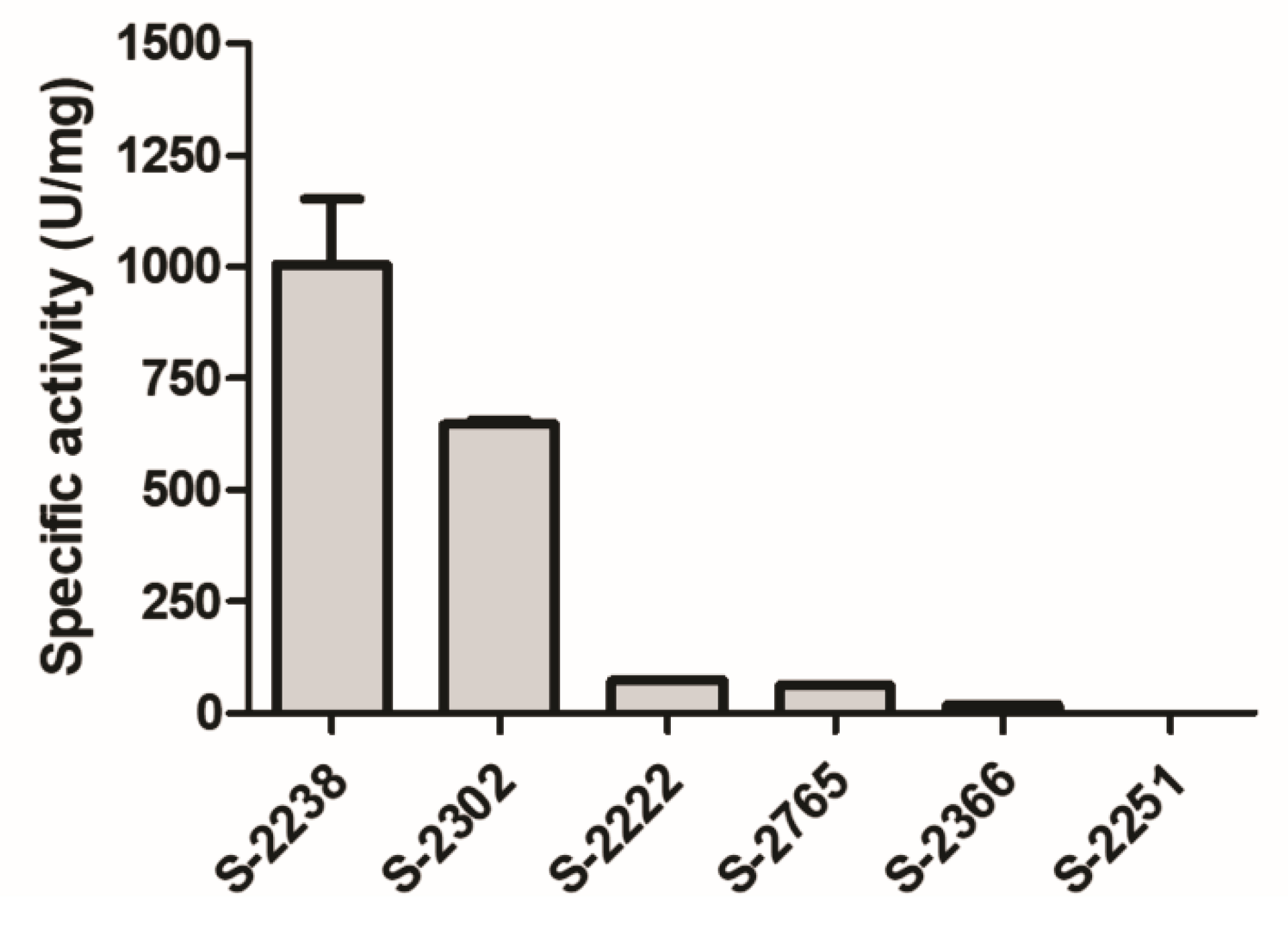
2.9. Effects on Chromogenic Substrates
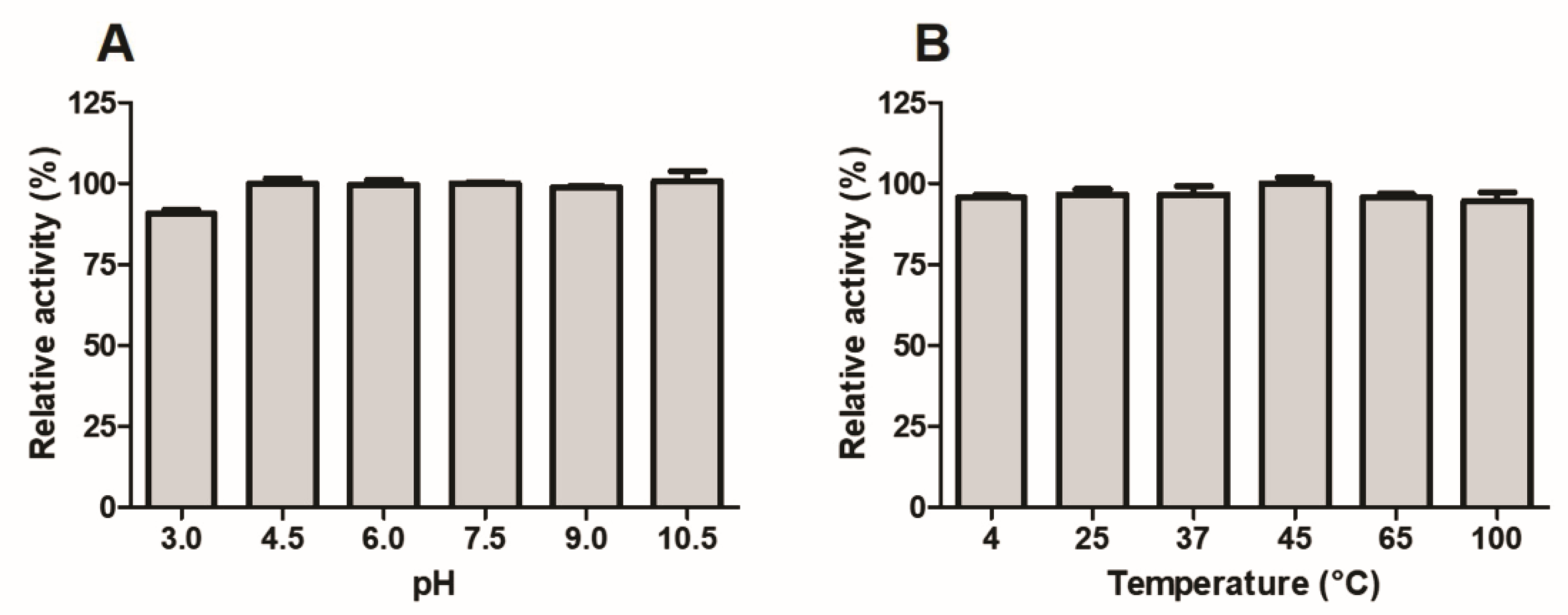
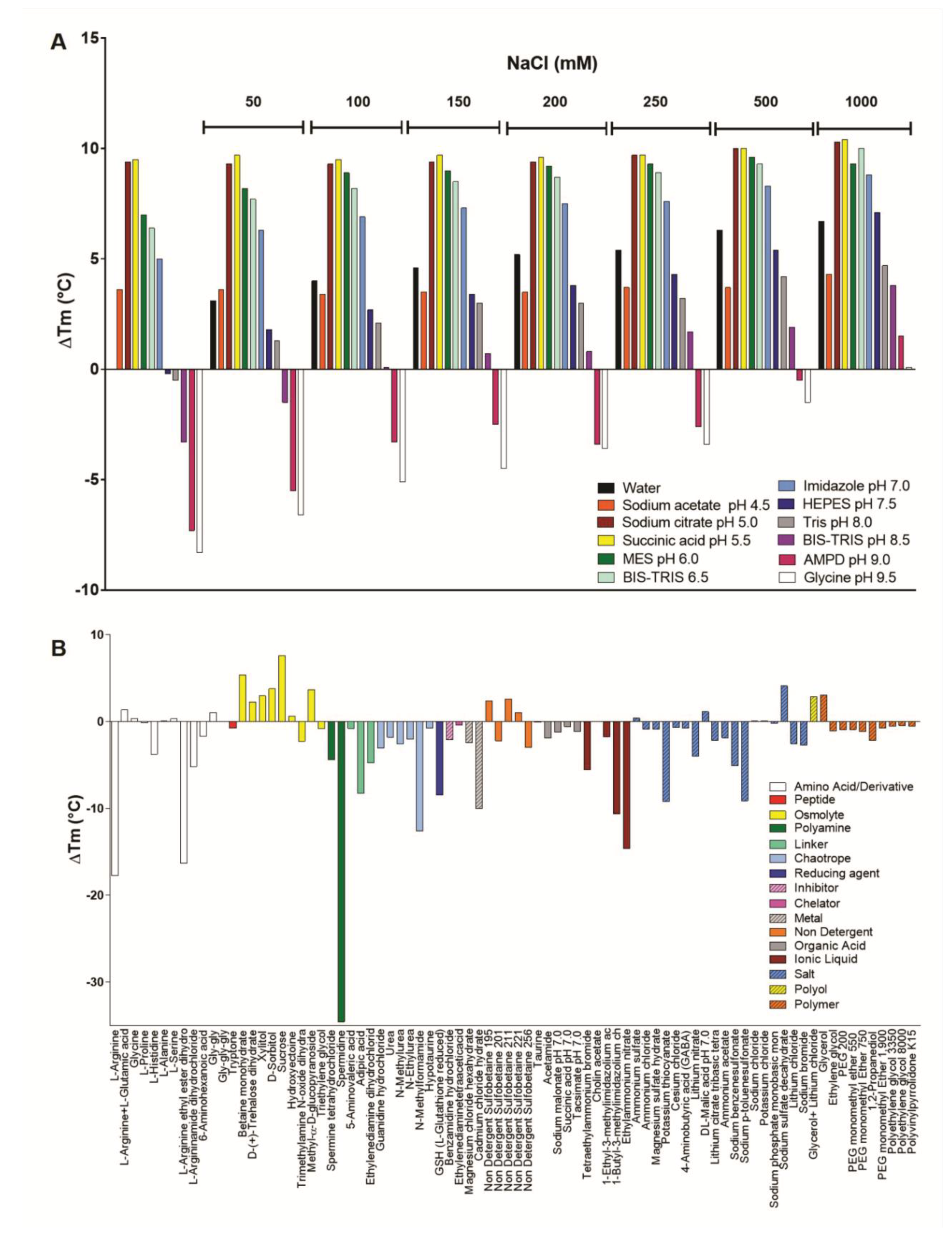
2.10. Stability Studies
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Venom
5.2. Human Plasma
5.3. Isolation of Moojase
5.4. Electrophoresis and Isoelectric Focusing
5.5. Serine Protease Activity
5.6. Structural Characterization of Moojase
5.6.1. Molecular Mass Determination
5.6.2. Amino Acid Sequencing
5.7. Deglycosylation Analysis
5.8. Coagulant Activity
5.8.1. Clotting of Human Plasma
5.8.2. Clotting of Fibrinogen Solutions
5.9. Fibrinogenolytic Activity
5.10. Identification of Fibrinopeptides
5.11. Fibrinolytic Activity
5.12. Platelet Aggregation Assays
5.13. Amidolytic Activity on Chromogenic Substrates
5.14. Stability Studies
5.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amorim, F.G.; Morandi-Filho, R.; Fujimura, P.T.; Ueira-Vieira, C.; Sampaio, S.V. New findings from the first transcriptome of the Bothrops moojeni snake venom gland. Toxicon 2017, 140, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Isabel, T.F.; Costa, G.N.; Pacheco, I.B.; Barbosa, L.G.; Santos-Junior, C.D.; Fonseca, F.P.; Boldrini França, J.; Henrique-Silva, F.; Yoneyama, K.A.; Rodrigues, R.S.; et al. Expression and partial biochemical characterization of a recombinant serine protease from Bothrops pauloensis snake venom. Toxicon 2016, 115, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.; Maroun, R.C. Snake venom serine proteinases: Sequence homology vs. substrate specificity, a paradox to be solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.; de Sousa, B.B.; Mamede, C.C.; de Morais, N.C.; de Queiroz, M.R.; da Cunha Pereira, D.F.; Matias, M.S.; Homi Brandeburgo, M.I. Biochemical and functional characterization of BmooSP, a new serine protease from Bothrops moojeni snake venom. Toxicon 2016, 111, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.C.; Rodrigues, C.R. Current status of snake venom thrombin-like enzymes. Toxin Rev. 2006, 25, 19. [Google Scholar] [CrossRef]
- Menaldo, D.L.; Bernardes, C.P.; Santos-Filho, N.A.; Moura, L.e.A.; Fuly, A.L.; Arantes, E.C.; Sampaio, S.V. Biochemical characterization and comparative analysis of two distinct serine proteases from Bothrops pirajai snake venom. Biochimie 2012, 94, 2545–2558. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Bernardes, C.P.; Pereira, J.C.; Silveira, D.S.; Mamede, C.C.; Stanziola, L.; Oliveira, F.; Pereira-Crott, L.S.; Faccioli, L.H.; Sampaio, S.V. Effects of two serine proteases from Bothrops pirajai snake venom on the complement system and the inflammatory response. Int. Immunopharmacol. 2013, 15, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.C.; Zingali, R.B.; Albuquerque, M.G.; Pujol-Luz, M.; Rodrigues, C.R. Snake venom thrombin-like enzymes: From reptilase to now. Cell. Mol. Life Sci. 2004, 61, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Oyama, E.; Fukuda, T.; Takahashi, H. Amino acid sequence of a kinin-releasing enzyme, KR-E-1, from the venom of Agkistrodon caliginosus (Kankoku-mamushi). Toxicon 2008, 52, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wisner, A.; Xiong, Y.; Bon, C. A novel plasminogen activator from snake venom. Purification, characterization, and molecular cloning. J. Biol. Chem. 1995, 270, 10246–10255. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.F.; Serrano, S.M.; Kuliopulos, A.; Niewiarowski, S. Interaction of viper venom serine peptidases with thrombin receptors on human platelets. FEBS Lett. 2000, 477, 199–202. [Google Scholar] [CrossRef]
- Murakami, M.T.; Arni, R.K. Thrombomodulin-independent activation of protein C and specificity of hemostatically active snake venom serine proteinases: Crystal structures of native and inhibited Agkistrodon contortrix contortrix protein C activator. J. Biol. Chem. 2005, 280, 39309–39315. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Tsuru, D.; Oda-Ueda, N.; Ohno, M.; Hattori, S.; Kim, S.T. Flavoxobin, a serine protease from Trimeresurus flavoviridis (habu snake) venom, independently cleaves Arg726-Ser727 of human C3 and acts as a novel, heterologous C3 convertase. Immunology 2002, 107, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Rosing, J.; Govers-Riemslag, J.W.; Yukelson, L.; Tans, G. Factor V activation and inactivation by venom proteases. Haemostasis 2001, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Ihara, H.; Takada, Y.; Fujie, M.; Takada, A. The cleavage and inactivation of plasminogen activator inhibitor type 1 and alpha2-antiplasmin by reptilase, a thrombin-like venom enzyme. Blood Coagul. Fibrinolysis 2000, 11, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kitano, E.S.; Garcia, T.C.; Menezes, M.C.; Tashima, A.K.; Zelanis, A.; Serrano, S.M. Cotiarinase is a novel prothrombin activator from the venom of Bothrops cotiara. Biochimie 2013, 95, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M.; Yu, H.; Liu, Z.Z.; Pei, J.Z.; Yang, Y.E.; Yan, S.X.; Zhang, C.; Zhao, W.L.; Wang, Z.Z.; Wang, Y.M.; et al. Serine protease isoforms in Gloydius intermedius venom: Full sequences, molecular phylogeny and evolutionary implications. J. Proteom. 2017, 164, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, J.R.; Brust, A.; Jin, A.H.; Alewood, P.F.; Dutertre, S.; Lewis, R.J. Cone snail venomics: From novel biology to novel therapeutics. Future Med. Chem. 2014, 6, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, C.M.; Prieto da Silva, Á.R.d.B.; Magalhães, G.S. Serine proteases—Cloning, Expression and Potential Applications. In An Integrated View of the Molecular Recognition and Toxinology-From Analytical Procedures to Biomedical Applications; Radis-Baptista, G., Ed.; InTech: London, UK, 2013. [Google Scholar]
- PentaPharm. Haemocoagulase. Available online: https://www.pentapharm.com/content.cfm?nav=21&content=40 (accessed on 27 November 2018).
- PentaPharm. Defibrase. Available online: https://www.pentapharm.com/content.cfm?nav=21&content=39 (accessed on 27 November 2018).
- Fox, J.W.; Serrano, S.M. Approaching the golden age of natural product pharmaceuticals from venom libraries: an overview of toxins and toxin-derivatives currently involved in therapeutic or diagnostic applications. Curr. Pharm. Des. 2007, 13, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- PentaPharm. Pefakit Reptilase Time. Available online: https://www.pentapharm.com/content.cfm?nav=11&content=26 (accessed on 27 November 2018).
- Carone, S.E.I.; Menaldo, D.L.; Sartim, M.A.; Bernardes, C.P.; Caetano, R.C.; da Silva, R.R.; Cabral, H.; Barraviera, B.; Ferreira Junior, R.S.; Sampaio, S.V. BjSP, a novel serine protease from Bothrops jararaca snake venom that degrades fibrinogen without forming fibrin clots. Toxicol. Appl. Pharmacol. 2018, 357, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Patiño, A.C.; Pereañez, J.A.; Gutiérrez, J.M.; Rucavado, A. Biochemical and biological characterization of two serine proteinases from Colombian Crotalus durissus cumanensis snake venom. Toxicon 2013, 63, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Kurtović, T.; Brgles, M.; Leonardi, A.; Lang Balija, M.; Sajevic, T.; Križaj, I.; Allmaier, G.; Marchetti-Deschmann, M.; Halassy, B. VaSP1, catalytically active serine proteinase from Vipera ammodytes ammodytes venom with unconventional active site triad. Toxicon 2014, 77, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M. The long road of research on snake venom serine proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.; Mentele, R.; Sampaio, C.A.; Fink, E. Purification, characterization, and amino acid sequence of a serine proteinase, PA-BJ, with platelet-aggregating activity from the venom of Bothrops jararaca. Biochemistry 1995, 34, 7186–7193. [Google Scholar] [CrossRef] [PubMed]
- Paes Leme, A.F.; Prezoto, B.C.; Yamashiro, E.T.; Bertholim, L.; Tashima, A.K.; Klitzke, C.F.; Camargo, A.C.; Serrano, S.M. Bothrops protease A, a unique highly glycosylated serine proteinase, is a potent, specific fibrinogenolytic agent. J. Thromb. Haemost. 2008, 6, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Rodrigues, V.M.; Borges, M.H.; Soares, A.M.; Hamaguchi, A.; Giglio, J.R.; Homsi-Brandeburgo, M.I. Purification and partial characterization of a new proteolytic enzyme from the venom of Bothrops moojeni (CAISSACA). Biochem. Mol. Biol. Int. 1999, 47, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.; Matos, M.F.; Mandelbaum, F.R.; Sampaio, C.A. Basic proteinases from Bothrops moojeni (caissaca) venom--I. Isolation and activity of two serine proteinases, MSP 1 and MSP 2, on synthetic substrates and on platelet aggregation. Toxicon 1993, 31, 471–481. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, L.M.; Ullah, A.; Masood, R.; Zelanis, A.; Spencer, P.J.; Serrano, S.M.; Arni, R.K. Rapid purification of serine proteinases from Bothrops alternatus and Bothrops moojeni venoms. Toxicon 2013, 76, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Von Klobusitzky, D.; König, P. Biochemische studien über die gifte der schlangengattung Bothrops. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1939, 192, 271–275. [Google Scholar] [CrossRef]
- Ghazaryan, N.A.; Ghulikyan, L.; Kishmiryan, A.; Andreeva, T.V.; Utkin, Y.N.; Tsetlin, V.I.; Lomonte, B.; Ayvazyan, N.M. Phospholipases a2 from Viperidae snakes: Differences in membranotropic activity between enzymatically active toxin and its inactive isoforms. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Kalita, B.; Thakur, R. Two acidic, anticoagulant PLA2 isoenzymes purified from the venom of monocled cobra Naja kaouthia exhibit different potency to inhibit thrombin and factor Xa via phospholipids independent, non-enzymatic mechanism. PLoS ONE 2014, 9, e101334. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Souza, C.T.; Bello, C.A.; Richardson, M.; Oliveira, E.B.; Magalhaes, A. Resolution of isoforms of mutalysin II, the metalloproteinase from bushmaster snake venom. Toxicon 2003, 41, 1021–1031. [Google Scholar] [CrossRef]
- Hayes, M.B.; Wellner, D. Microheterogeneity of l-amino acid oxidase separation of multiple components by polyacrylamide gel electrofocusing. J. Biol. Chem. 1969, 244, 6636–6644. [Google Scholar] [PubMed]
- Walter, M.; Nyman, D.; Krajnc, V.; Duckert, F. The activation of plasma factor XIII with the snake venom enzymes ancrod and batroxobin marajoensis. Thromb. Haemost. 1977, 38, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Dempfle, C.E.; Argiriou, S.; Alesci, S.; Kucher, K.; Müller-Peltzer, H.; Rübsamen, K.; Heene, D.L. Fibrin formation and proteolysis during ancrod treatment. Evidence for des-A-profibrin formation and thrombin independent factor XIII activity. Ann. N. Y. Acad. Sci. 2001, 936, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G. Ancrod revisited: Viscoelastic analyses of the effects of Calloselasma rhodostoma venom on plasma coagulation and fibrinolysis. J. Thromb. Thrombolysis 2016, 42, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Braud, S.; Bon, C.; Wisner, A. Snake venom proteins acting on hemostasis. Biochimie 2000, 82, 851–859. [Google Scholar] [CrossRef]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake venom proteases affecting hemostasis and thrombosis. Biochim. Biophys. Acta 2000, 1477, 146–156. [Google Scholar] [CrossRef]
- Gardiner, E.E.; Andrews, R.K. The cut of the clot(h): Snake venom fibrinogenases as therapeutic agents. J. Thromb. Haemost. 2008, 6, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Pantoliano, M.W.; Petrella, E.C.; Kwasnoski, J.D.; Lobanov, V.S.; Myslik, J.; Graf, E.; Carver, T.; Asel, E.; Springer, B.A.; Lane, P.; et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J. Biomol. Screen 2001, 6, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Pádua, R.A.; Tomaleri, G.P.; Reis, R.A.; David, J.S.; Silva, V.C.; Pinheiro, M.P.; Nonato, M.C. ThermoFMN-a thermofluor assay developed for ligand-screening as an alternative strategy for drug discovery. J. Braz. Chem. Soc. 2014, 25, 1864–1871. [Google Scholar] [CrossRef]
- Boivin, S.; Kozak, S.; Meijers, R. Optimization of protein purification and characterization using Thermofluor screens. Protein Exp. Purif. 2013, 91, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Sartim, M.A.; Pinheiro, M.P.; de Pádua, R.A.; Sampaio, S.V.; Nonato, M.C. Structural and binding studies of a C-type galactose-binding lectin from Bothrops jararacussu snake venom. Toxicon 2017, 126, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, F.; Du, F.; Wang, A.; Gao, W.; Wang, Q.; Yin, X.; Xie, T. A novel and efficient method for the immobilization of thermolysin using sodium chloride salting-in and consecutive microwave irradiation. Bioresour. Technol. 2012, 115, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Idicula-Thomas, S.; Balaji, P.V. Understanding the relationship between the primary structure of proteins and its propensity to be soluble on overexpression in Escherichia coli. Protein Sci. 2005, 14, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Cortez, L.; Sim, V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion 2014, 8, 197–202. [Google Scholar] [CrossRef]
- Street, T.O.; Bolen, D.W.; Rose, G.D. A molecular mechanism for osmolyte-induced protein stability. Proc. Natl. Acad. Sci. USA 2006, 103, 13997–14002. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.D. Ion hydration: Implications for cellular function, polyelectrolytes, and protein crystallization. Biophys. Chem. 2006, 119, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Koupaei, M.; Shareghi, B.; Saboury, A.A.; Davar, F.; Raisi, F. The effect of spermidine on the structure, kinetics and stability of proteinase K: Spectroscopic and computational approaches. RSC Adv. 2016, 6, 105476–105486. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef] [PubMed]
- Hummel, B.C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can. J. Biochem. Physiol. 1959, 37, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Edman, P.; Begg, G. A protein sequenator. Eur. J. Biochem. 1967, 1, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell. Proteom. 2012, 11, M111.010587. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.R.; Lorenzetti, R.; Rennó, A.L.; Baldissera, L.; Zelanis, A.; Serrano, S.M.; Hyslop, S. BJ-PI2, a non-hemorrhagic metalloproteinase from Bothrops jararaca snake venom. Biochim. Biophys. Acta 2012, 1820, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Edgar, W.; Prentice, C. The proteolytic action of ancrod on human fibrinogen and its polypeptide chains. Thromb. Res. 1973, 2, 85–95. [Google Scholar] [CrossRef]
- Leitão, D.P.; Polizello, A.C.; Rothschild, Z. Coagulation and fibrinolysis in capybara (Hydrochaeris hydrochaeris), a close relative of the guinea-pig (Cavia porcellus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 113–120. [Google Scholar] [CrossRef]
- Lopes-Pires, M.E.; Naime, A.C.; Almeida Cardelli, N.J.; Anjos, D.J.; Antunes, E.; Marcondes, S. PKC and AKT Modulate cGMP/PKG Signaling Pathway on Platelet Aggregation in Experimental Sepsis. PLoS ONE 2015, 10, e0137901. [Google Scholar] [CrossRef] [PubMed]
- Niesen, F.H.; Berglund, H.; Vedadi, M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2007, 2, 2212–2221. [Google Scholar] [CrossRef] [PubMed]


| Purification Step | Protein | Enzyme Activity | ||
|---|---|---|---|---|
| Total (mg) | Recovery (%) | TAME Activity (U/mg) * | Purification Factor | |
| B. moojeni venom | 200.0 | 100.0 | 1929.6 | 1 |
| CM10 (CM Sepharose) | 2.2 | 1.1 | 2120.0 | 1.09 |
| Moojase (C18) | 1.8 | 0.9 | 11,226.0 | 5.08 |
| Samples | Halo Diameter (mm) * |
|---|---|
| PBS (negative control) | 0 |
| Plasmin (20 µg) (positive control) | 9.4 ± 1.2 |
| Moojase (5 µg) | 10.9 ± 1.2 |
| Moojase (20 µg) | 14.4 ± 1.4 |
| Samples | Platelet Aggregation (%) * |
|---|---|
| ADP (20 µg) (positive control) | 42.0 ± 8.0 |
| Moojase (20 µg) | 71.7 ± 1.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, F.G.; Menaldo, D.L.; Carone, S.E.I.; Silva, T.A.; Sartim, M.A.; De Pauw, E.; Quinton, L.; Sampaio, S.V. New Insights on Moojase, a Thrombin-Like Serine Protease from Bothrops moojeni Snake Venom. Toxins 2018, 10, 500. https://doi.org/10.3390/toxins10120500
Amorim FG, Menaldo DL, Carone SEI, Silva TA, Sartim MA, De Pauw E, Quinton L, Sampaio SV. New Insights on Moojase, a Thrombin-Like Serine Protease from Bothrops moojeni Snake Venom. Toxins. 2018; 10(12):500. https://doi.org/10.3390/toxins10120500
Chicago/Turabian StyleAmorim, Fernanda G., Danilo L. Menaldo, Sante E. I. Carone, Thiago A. Silva, Marco A. Sartim, Edwin De Pauw, Loic Quinton, and Suely V. Sampaio. 2018. "New Insights on Moojase, a Thrombin-Like Serine Protease from Bothrops moojeni Snake Venom" Toxins 10, no. 12: 500. https://doi.org/10.3390/toxins10120500
APA StyleAmorim, F. G., Menaldo, D. L., Carone, S. E. I., Silva, T. A., Sartim, M. A., De Pauw, E., Quinton, L., & Sampaio, S. V. (2018). New Insights on Moojase, a Thrombin-Like Serine Protease from Bothrops moojeni Snake Venom. Toxins, 10(12), 500. https://doi.org/10.3390/toxins10120500






