Erianin against Staphylococcus aureus Infection via Inhibiting Sortase A
Abstract
1. Introduction
2. Results
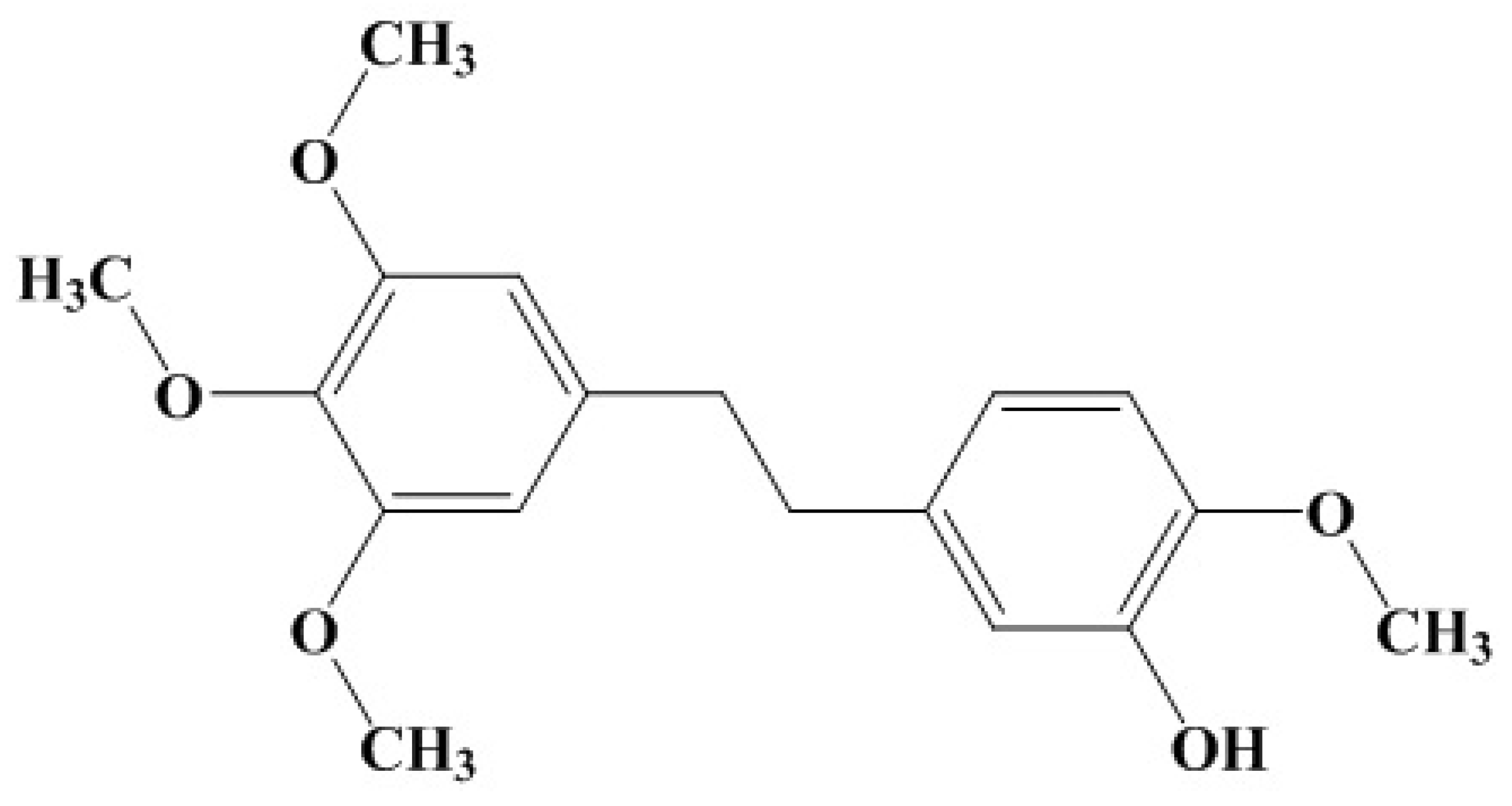
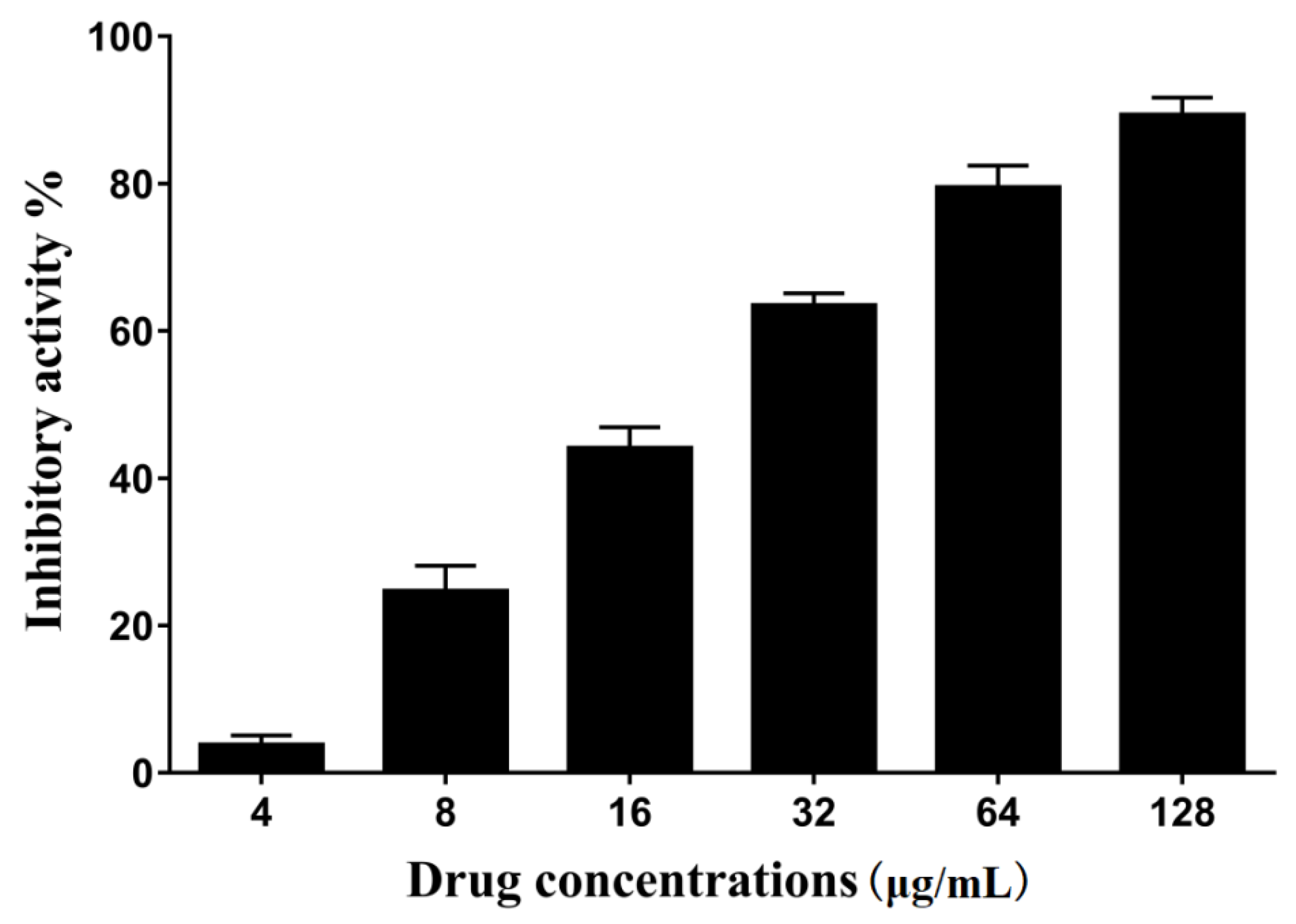
2.1. The Activity of Erianin against S. aureus SrtA
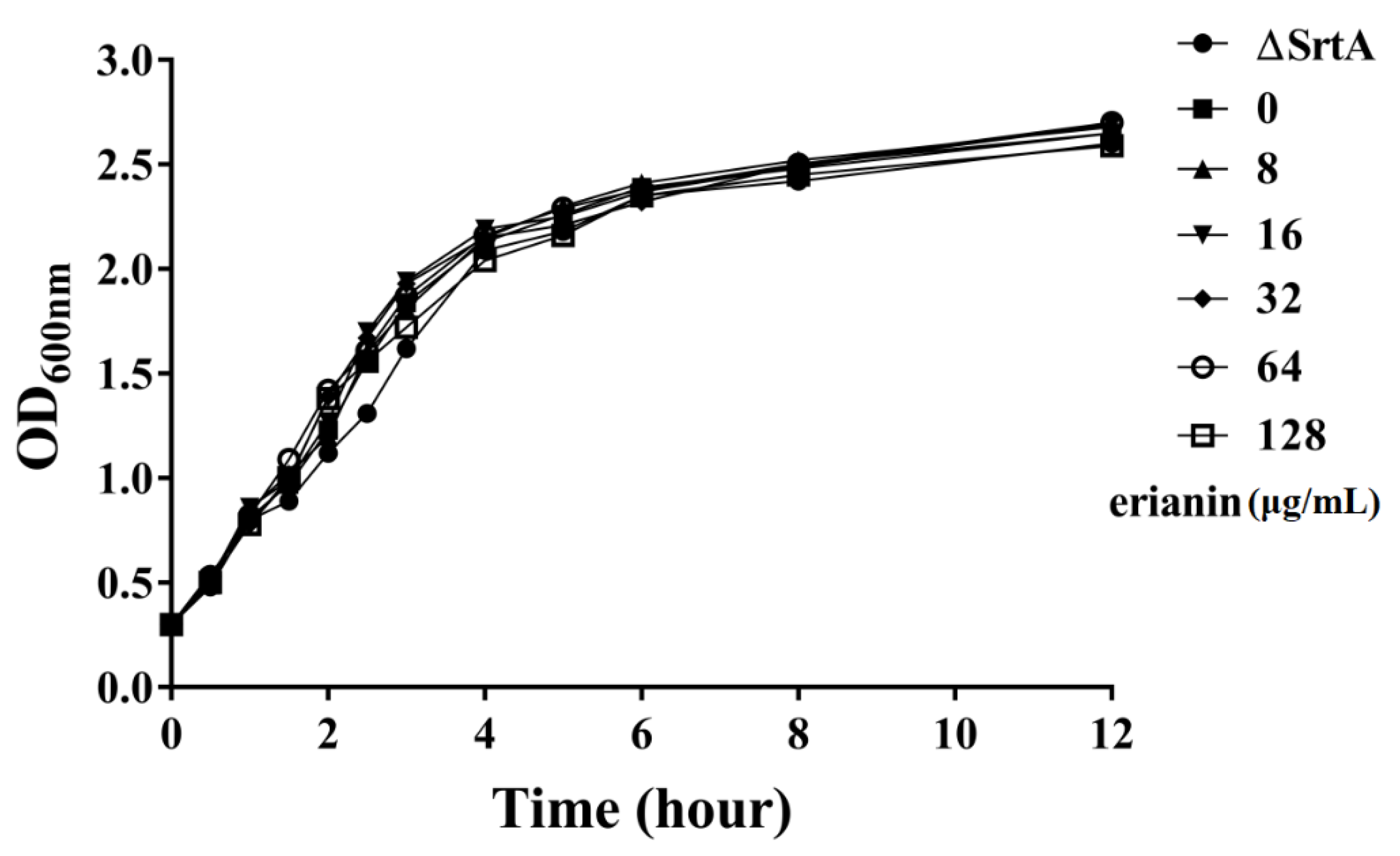
2.2. The Influence of Erianin on S. aureus Growth
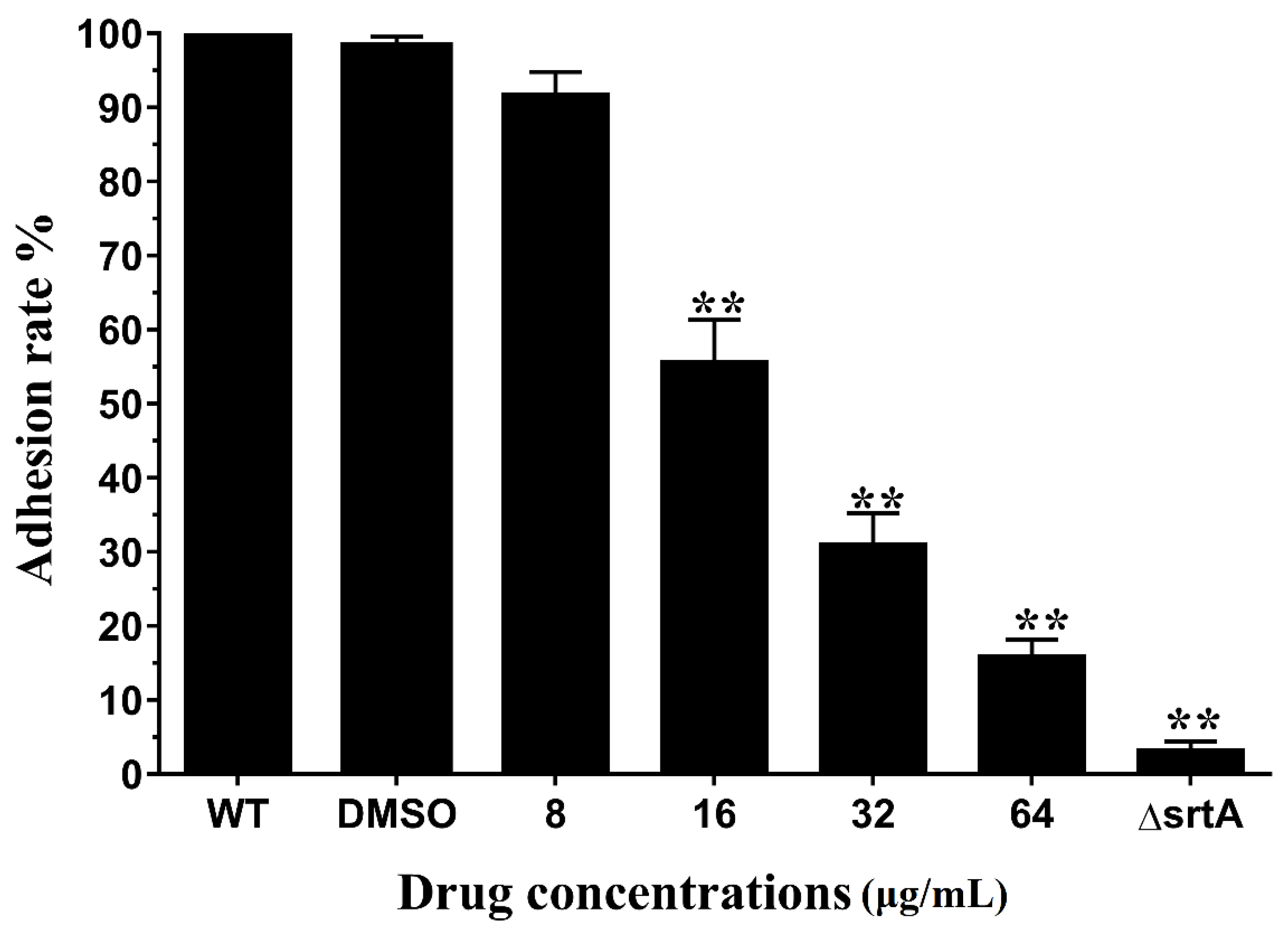
2.3. Erianin Reduced the Activity of S. aureus Adhesion to Fibrinogen
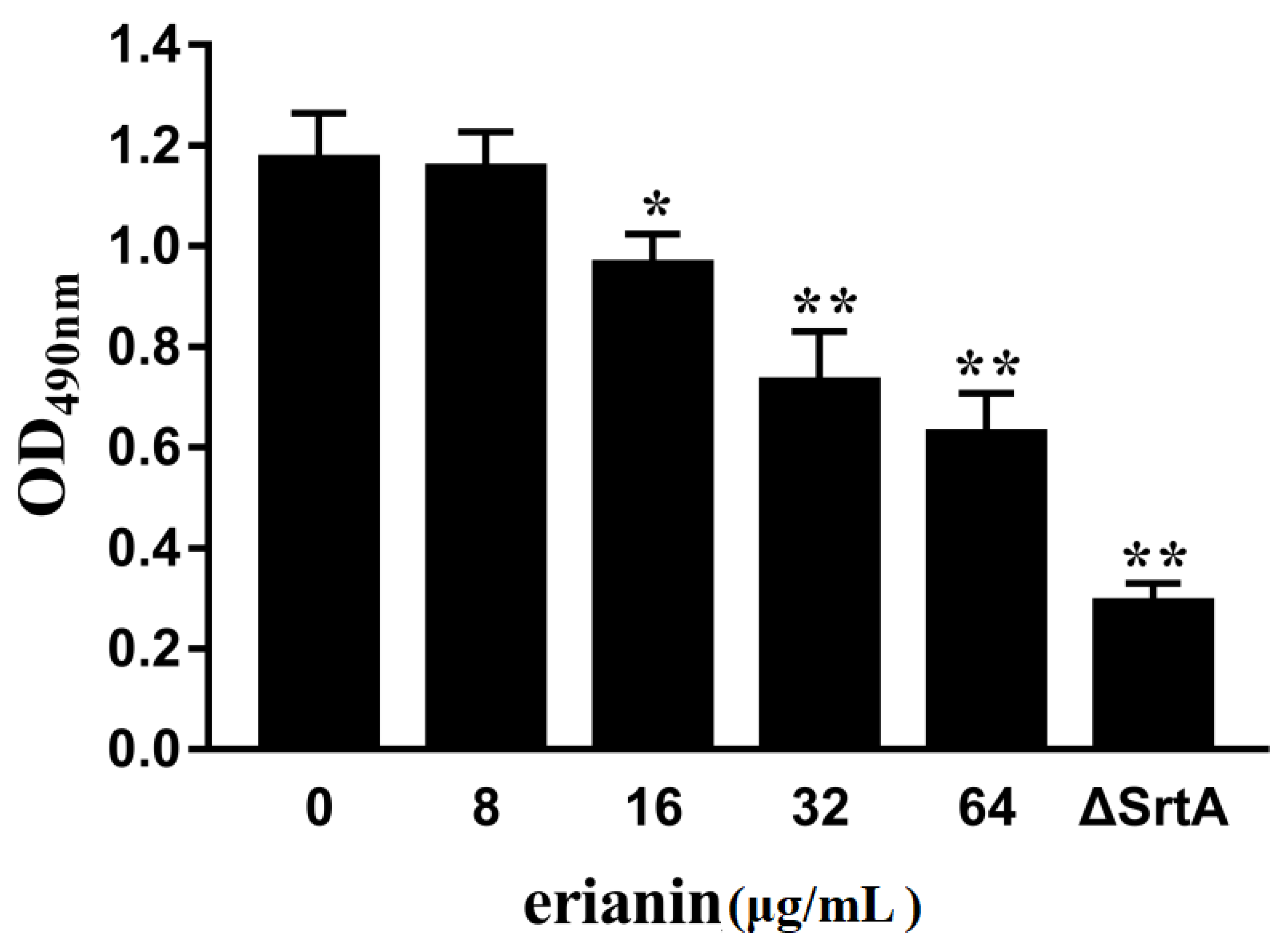
2.4. Erianin Decreased Biofilm Formation
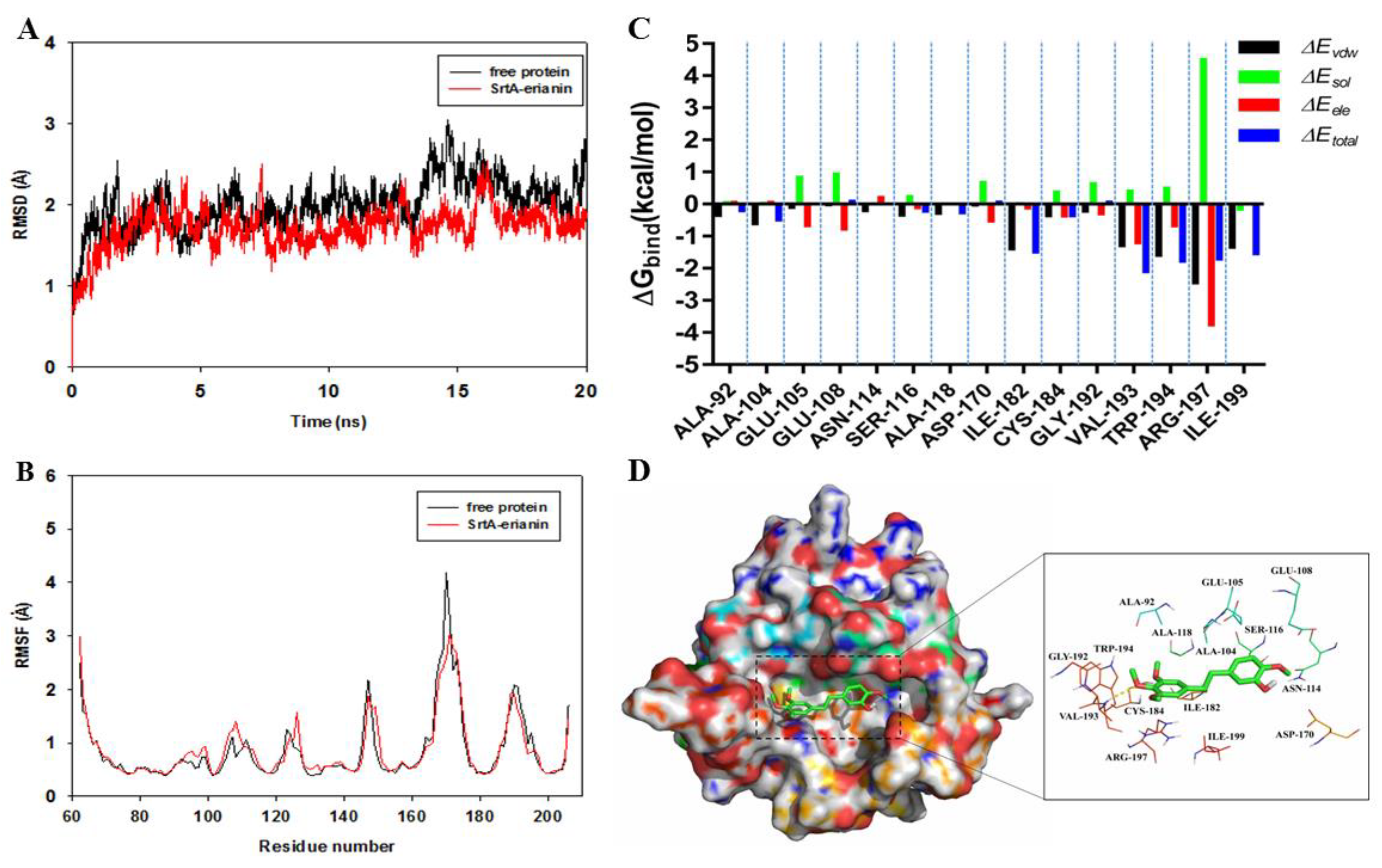
2.5. Mechanism Underlying the Binding of Erianin to SrtA
2.6. Identification of the Site of Erianin Binding to SrtA
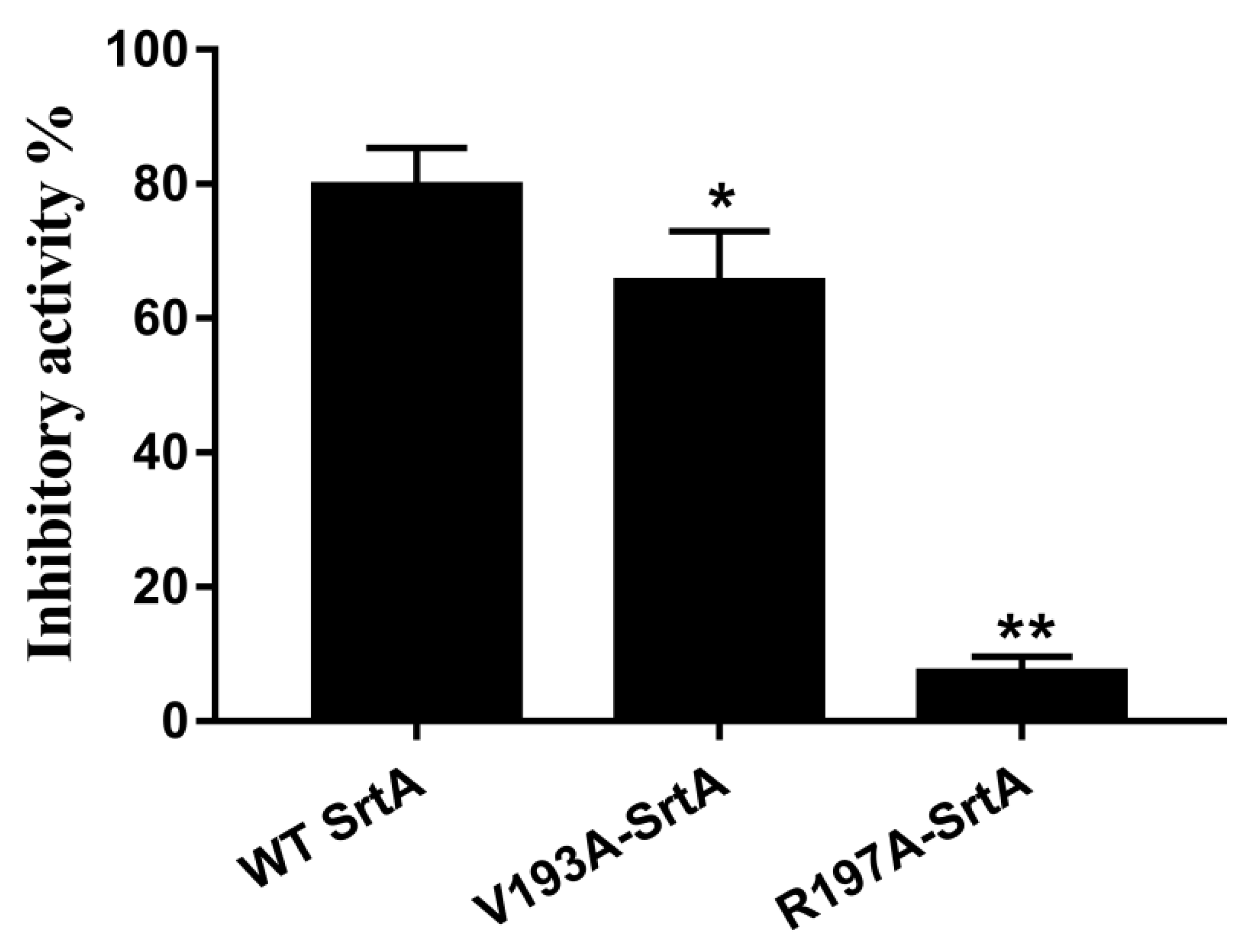
2.7. Confirmation of the Molecular Basis of Erianin against SrtA
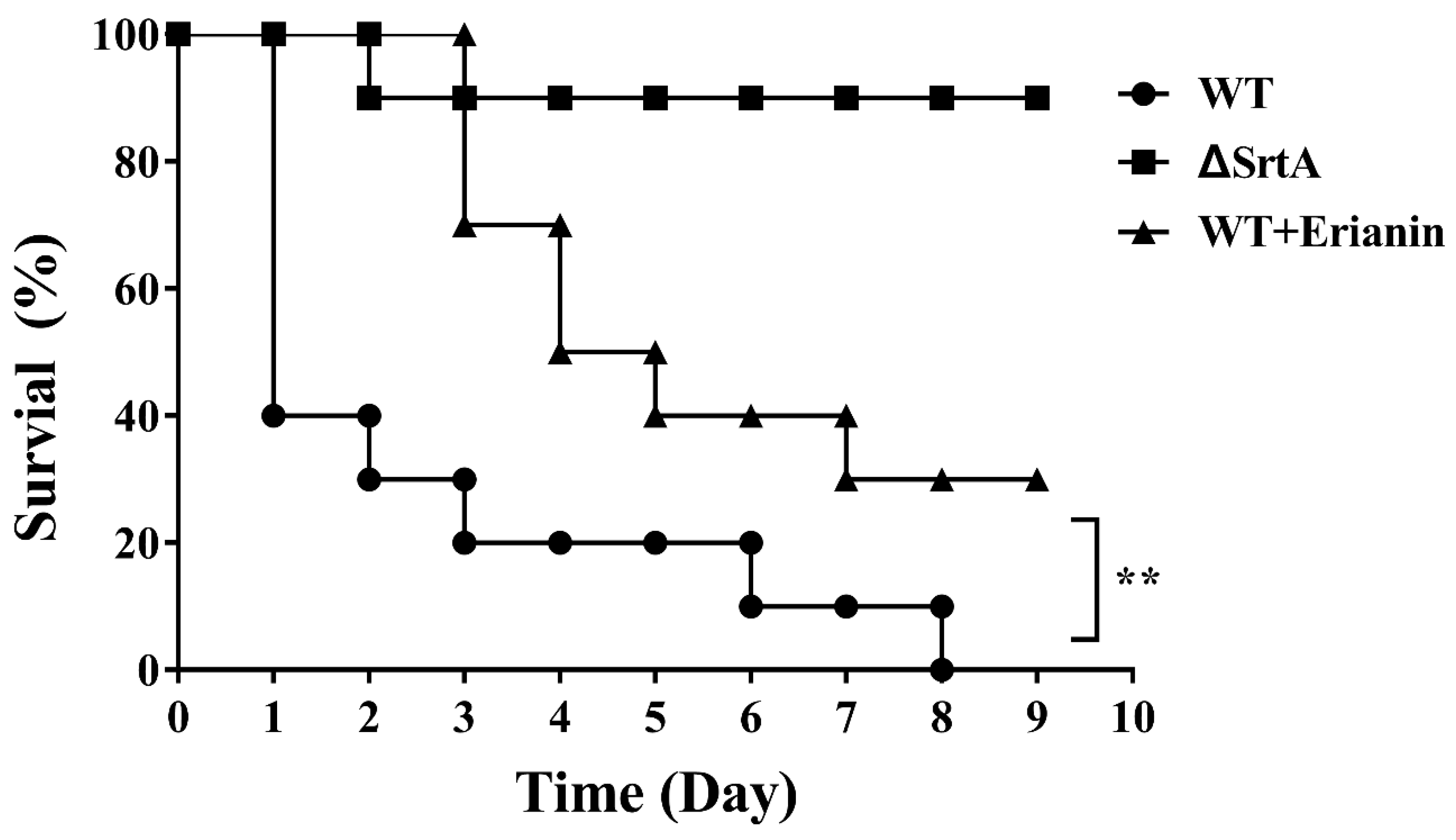
2.8. Erianin Protected Mice from Fatal S. aureus Infection
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of WT-SrtA, V193A-SrtA and R197A-SrtA
4.2. Determination of Mutant and WT SrtA Activity
4.3. Susceptibility Testing and Growth Curve Assay
4.4. Fibrinogen-Binding Assay
4.5. Biofilm Formation Assay
4.6. Binding Affinity Determination of Erianin with Mutant and WT SrtA
4.7. Molecular Docking and Molecular Dynamics
4.8. Animal Experiments
4.9. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M. Complicated skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus: Epidemiology, risk factors, and presentation. Surg. Infect. 2008, 9 Suppl. 1, s3–s10. [Google Scholar] [CrossRef] [PubMed]
- Muhlen, S.; Dersch, P. Anti-virulence strategies to target bacterial infections. Curr. Top. Microbiol. Immunol. 2016, 398, 147–183. [Google Scholar] [PubMed]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, B.; Wang, D.; Wang, L.; Deng, X.; Bi, C.; Xiong, Y.; Wu, Q.; Cui, Y.; Zhang, Y.; et al. Role of sortase A in the pathogenesis of Staphylococcus aureus-induced mastitis in mice. FEMS Microbiol. Lett. 2014, 351, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, J.; Hu, Y.; Wang, P.; Zou, L. Staphylococcus epidermidis deltasortase A strain elicits protective immunity against Staphylococcus aureus infection. Anton. Leeuwenhoek 2017, 110, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Ton-That, H.; Su, K.; Schneewind, O. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, G.; Ton-That, H.; Schneewind, O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 1999, 285, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, G.; Jensen, E.R.; Lenoy, E.; Schneewind, O. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 2000, 97, 5510–5515. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.J.; Lenoy, E.; Murphy, T.; Tardio, L.; Burgio, P.; Projan, S.J.; Schneewind, O.; Alksne, L. Effect of srtA and srtB gene expression on the virulence of Staphylococcus aureus in animal models of infection. J. Antimicrob. Chemother. 2004, 53, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Kubica, M.; Guzik, K.; Koziel, J.; Zarebski, M.; Richter, W.; Gajkowska, B.; Golda, A.; Maciag-Gudowska, A.; Brix, K.; Shaw, L.; et al. A potential new pathway for Staphylococcus aureus dissemination: The silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE 2008, 3, e1409. [Google Scholar] [CrossRef] [PubMed]
- Suree, N.; Yi, S.W.; Thieu, W.; Marohn, M.; Damoiseaux, R.; Chan, A.; Jung, M.E.; Clubb, R.T. Discovery and structure-activity relationship analysis of Staphylococcus aureus sortase A inhibitors. Bioorg. Med. Chem. 2009, 17, 7174–7185. [Google Scholar] [CrossRef] [PubMed]
- Ton-That, H.; Mazmanian, S.K.; Alksne, L.; Schneewind, O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Cysteine 184 and histidine 120 of sortase form a thiolate-imidazolium ion pair for catalysis. J. Biol. Chem. 2002, 277, 7447–7452. [Google Scholar] [CrossRef] [PubMed]
- Ton-That, H.; Liu, G.; Mazmanian, S.K.; Faull, K.F.; Schneewind, O. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the lpxtg motif. Proc. Natl. Acad. Sci. USA 1999, 96, 12424–12429. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; Sun, M.; He, X.; Wang, K.; Yin, Z.; Fu, H.; Li, Y.; Geng, Y.; Shu, G.; He, C.; et al. Sclareol protects Staphylococcus aureus-induced lung cell injury via inhibiting alpha-hemolysin expression. J. Microbiol. Biotechnol. 2016, 27, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ping, O.; Ruixue, Y.; Jiaqiang, D.; Kaiyu, W.; Jing, F.; Yi, G.; Xiaoli, H.; Defang, C.; Weimin, L.; Li, T.; et al. Subinhibitory concentrations of prim-o-glucosylcimifugin decrease the expression of alpha-hemolysin in Staphylococcus aureus (USA300). Evid.-Based Complement. Altern. Med. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xuewen, H.; Ping, O.; Zhongwei, Y.; Zhongqiong, Y.; Hualin, F.; Juchun, L.; Changliang, H.; Gang, S.; Zhixiang, Y.; Xu, S.; et al. Eriodictyol protects against Staphylococcus aureus-induced lung cell injury by inhibiting alpha-hemolysin expression. World J. Microbiol. Biotechnol. 2018, 34, 64. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; Chen, J.; Sun, M.; Yin, Z.; Lin, J.; Fu, H.; Shu, G.; He, C.; Lv, C.; Deng, X.; et al. Imperatorin inhibits the expression of alpha-hemolysin in Staphylococcus aureus strain baa-1717 (USA300). Anton. Leeuwenhoek 2016, 109, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef]
- Gong, Y.Q.; Fan, Y.; Wu, D.Z.; Yang, H.; Hu, Z.B.; Wang, Z.T. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur. J. Cancer 2004, 40, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, P.; Liu, J.; Cao, Y. Erianin inhibits indoleamine 2, 3-dioxygenase -induced tumor angiogenesis. Biomed. Pharmacother. 2017, 88, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, T.; Gong, C.; Sheng, Y.; Lu, B.; Zhou, L.; Ji, L.; Wang, Z. Erianin inhibits high glucose-induced retinal angiogenesis via blocking erk1/2-regulated hif-1alpha-vegf/vegfr2 signaling pathway. Sci. Rep. 2016, 6, 34306. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fan, Y.; Liu, L.; Wu, D.; Chang, Z.; Wang, Z. Erianin induces a jnk/sapk-dependent metabolic inhibition in human umbilical vein endothelial cells. In Vivo 2004, 18, 223–228. [Google Scholar] [PubMed]
- Sun, J.; Fu, X.; Wang, Y.; Liu, Y.; Zhang, Y.; Hao, T.; Hu, X. Erianin inhibits the proliferation of t47d cells by inhibiting cell cycles, inducing apoptosis and suppressing migration. Am. J. Transl. Res. 2016, 8, 3077–3086. [Google Scholar] [PubMed]
- Wang, H.; Zhang, T.; Sun, W.; Wang, Z.; Zuo, D.; Zhou, Z.; Li, S.; Xu, J.; Yin, F.; Hua, Y.; et al. Erianin induces g2/m-phase arrest, apoptosis, and autophagy via the ros/jnk signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016, 7, e2247. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, J.B. Anti-prostatic activity of bifluranol, a fluorinated bibenzyl. Br. J. pharmacol. 1980, 71, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wann, E.R.; Gurusiddappa, S.; Hook, M. The fibronectin-binding mscramm fnbpa of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 2000, 275, 13863–13871. [Google Scholar] [CrossRef] [PubMed]
- Bingham, R.J.; Rudino-Pinera, E.; Meenan, N.A.; Schwarz-Linek, U.; Turkenburg, J.P.; Hook, M.; Garman, E.F.; Potts, J.R. Crystal structures of fibronectin-binding sites from Staphylococcus aureus fnbpa in complex with fibronectin domains. Proc. Natl. Acad. Sci. USA 2008, 105, 12254–12258. [Google Scholar] [CrossRef] [PubMed]
- Hansenova Manaskova, S.; Nazmi, K.; van Belkum, A.; Bikker, F.J.; van Wamel, W.J.; Veerman, E.C. Synthetic lpetg-containing peptide incorporation in the Staphylococcus aureus cell-wall in a sortase A- and growth phase-dependent manner. PLoS ONE 2014, 9, e89260. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Tsompanidou, E.; Denham, E.L.; Sibbald, M.J.; Yang, X.M.; Seinen, J.; Friedrich, A.W.; Buist, G.; van Dijl, J.M. The sortase A substrates fnbpa, fnbpb, clfa and clfb antagonize colony spreading of Staphylococcus aureus. PLoS ONE 2012, 7, e44646. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, R.; Nandakumar, M.P.; Marten, M.R.; Ross, J.M. Proteome analysis of membrane and cell wall associated proteins from Staphylococcus aureus. J. Proteome Res. 2005, 4, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Raffa, D.; Maggio, B.; Raimondi, M.V.; Schillaci, D.; Daidone, G. Sortase A inhibitors: Recent advances and future perspectives. J. Med. Chem. 2015, 58, 9108–9123. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.L.; Gaweska, H.; Kielec, J.M.; McCafferty, D.G. Engineering the substrate specificity of Staphylococcus aureus sortase A. The beta6/beta7 loop from srtb confers npqtn recognition to srta. J. Biol. Chem. 2007, 282, 6571–6581. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.L.; Lamb, E.C.; McCafferty, D.G. Mutagenesis studies of substrate recognition and catalysis in the sortase A transpeptidase from Staphylococcus aureus. J. Biol. Chem. 2008, 283, 14762–14771. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Bice, T.W.; Ton-That, H.; Schneewind, O.; Narayana, S.V. Crystal structures of Staphylococcus aureus sortase A and its substrate complex. J. Biol. Chem. 2004, 279, 31383–31389. [Google Scholar] [CrossRef] [PubMed]
- Marraffini, L.A.; Ton-That, H.; Zong, Y.; Narayana, S.V.; Schneewind, O. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. A conserved arginine residue is required for efficient catalysis of sortase A. J. Biol. Chem. 2004, 279, 37763–37770. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, U.; Ton-That, H.; Iwahara, J.; Schneewind, O.; Clubb, R.T. Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2001, 98, 6056–6061. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.X.; Eriksson, L.A. Catalytic mechanism and roles of arg197 and thr183 in the Staphylococcus aureus sortase A enzyme. J. Phys. Chem. B 2011, 115, 13003–13011. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.B.; Oh, M.N.; Kim, J.G.; Shin, D.S.; Shin, J. Inhibition of sortase-mediated Staphylococcus aureus adhesion to fibronectin via fibronectin-binding protein by sortase inhibitors. Appl. Microbiol. Biotechnol. 2006, 70, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.B.; Kim, S.H.; Lee, J.; Cho, W.J.; Lee, T.; Kim, S. Discovery of diarylacrylonitriles as a novel series of small molecule sortase A inhibitors. J. Med. Chem. 2004, 47, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, C.; Cai, H.; Liu, B.; Zhong, X.; Deng, X.; Wang, T.; Xiang, H.; Niu, X.; Wang, D. The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front. Microbiol. 2015, 6, 1031. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Valder, C.R.; Huynh, H.G.; Ren, H.; Allison, W.S. The beta g156c substitution in the f1-atpase from the thermophilic bacillus ps3 affects catalytic site cooperativity by destabilizing the closed conformation of the catalytic site. Biochemistry 2002, 41, 14421–14429. [Google Scholar] [CrossRef] [PubMed]
- Jurasekova, Z.; Marconi, G.; Sanchez-Cortes, S.; Torreggiani, A. Spectroscopic and molecular modeling studies on the binding of the flavonoid luteolin and human serum albumin. Biopolymers 2009, 91, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and autodocktools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Gotz, A.W.; Williamson, M.J.; Xu, D.; Poole, D.; Le Grand, S.; Walker, R.C. Routine microsecond molecular dynamics simulations with amber on gpus. 1. Generalized born. J. Chem. Theory Comput. 2012, 8, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Ferrer, R.; Gotz, A.W.; Poole, D.; Le Grand, S.; Walker, R.C. Routine microsecond molecular dynamics simulations with amber on gpus. 2. Explicit solvent particle mesh ewald. J. Chem. Theory Comput. 2013, 9, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Sousa da Silva, A.W.; Vranken, W.F. Acpype-antechamber python parser interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, X.; Liu, S.; Li, G.; Shi, L.; Dong, J.; Li, W.; Deng, X.; Niu, X. Morin hydrate attenuates Staphylococcus aureus virulence by inhibiting the self-assembly of alpha-hemolysin. J. Appl. Microbiol. 2015, 118, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jia, L.; Wang, J.; Wu, X.; Hao, H.; Xu, H.; Wu, Y.; Shi, G.; Lu, C.; Shen, Y. Design, synthesis and biological evaluation of 17-arylmethylamine-17-demethoxygeldanamycin derivatives as potent hsp90 inhibitors. Eur. J. Med. Chem. 2014, 85, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, C.; Wang, T.; Xiang, H.; Chen, F.; Hu, J.; Liu, B.; Cai, H.; Zhong, X.; Deng, X.; et al. A coagulase-negative and non-haemolytic strain of Staphylococcus aureus for investigating the roles of srta in a murine model of bloodstream infection. Pathog. Dis. 2015, 73, ftv042. [Google Scholar] [CrossRef] [PubMed]
| Proteins | WT-SrtA | V193A-SrtA | R197A-SrtA |
|---|---|---|---|
| The binding energy | −24.1 ± 2.3 | −23.8 ± 2.2 | −18.9 ± 2.0 |
| KA (1 × 104) L mol−1 | 44.5 ± 7.2 | 43.9 ± 6.7 | 39.6 ± 4.8 |
| Primer Name | Oligonucleotide (5-3) a |
|---|---|
| PsrtA59F | GCGGGATCCCCGGAATTCCAAGCTAAACCTCAAATTCC |
| PsrtA59R | CCGCTCGAGTTATTTGACTTCTGTAGCTACAA |
| V193A-srtA-F | TGAAAAGACAGGCGCTTGGGAAAAAC |
| V193A-srtA-R | TTCCCAGCGCCTGTCTTTTCATTGTAATCAT |
| R197A-srtA-F | GACAGGCGTTTGGGAAAAAGCGAAAATCTTTGTAGCTACAG |
| R197A-srtA-R | CTGTAGCTACAAAGATTTTCGCTTTTTCCCAAACGCCTGTC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, P.; He, X.; Yuan, Z.-W.; Yin, Z.-Q.; Fu, H.; Lin, J.; He, C.; Liang, X.; Lv, C.; Shu, G.; et al. Erianin against Staphylococcus aureus Infection via Inhibiting Sortase A. Toxins 2018, 10, 385. https://doi.org/10.3390/toxins10100385
Ouyang P, He X, Yuan Z-W, Yin Z-Q, Fu H, Lin J, He C, Liang X, Lv C, Shu G, et al. Erianin against Staphylococcus aureus Infection via Inhibiting Sortase A. Toxins. 2018; 10(10):385. https://doi.org/10.3390/toxins10100385
Chicago/Turabian StyleOuyang, Ping, Xuewen He, Zhong-Wei Yuan, Zhong-Qiong Yin, Hualin Fu, Juchun Lin, Changliang He, Xiaoxia Liang, Cheng Lv, Gang Shu, and et al. 2018. "Erianin against Staphylococcus aureus Infection via Inhibiting Sortase A" Toxins 10, no. 10: 385. https://doi.org/10.3390/toxins10100385
APA StyleOuyang, P., He, X., Yuan, Z.-W., Yin, Z.-Q., Fu, H., Lin, J., He, C., Liang, X., Lv, C., Shu, G., Yuan, Z.-X., Song, X., Li, L., & Yin, L. (2018). Erianin against Staphylococcus aureus Infection via Inhibiting Sortase A. Toxins, 10(10), 385. https://doi.org/10.3390/toxins10100385





